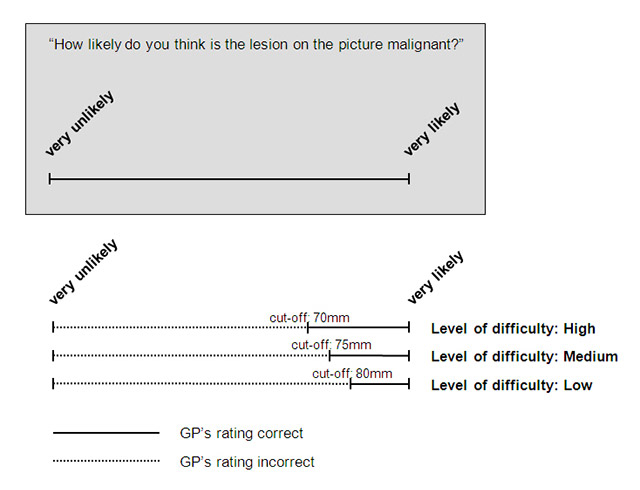
Figure 1
Example of the interpretation (picture with a malignant lesion).
DOI: https://doi.org/10.4414/smw.2013.13834
QUESTIONS UNDER STUDY: In Switzerland, skin cancer is one of the most prevalent neoplasms. General practitioners (GPs) are often faced with suspicious skin lesions in their patients. The aim of our study was to assess GPs’ competence to diagnose skin cancer and to examine whether this can be improved by a one-day dermatologic education programme.
METHODS: Study design:Pre / post-intervention study.Study population:78 GPs in the Canton of Zurich. Intervention:A one day dermatologic education programme provided by a dermatologist. Measurements:Before (T0) and after (T1) the dermatologic education programme, GPs were asked to rate pictures (with a short history) of skin lesions on a visual analogue scale according to their likelihood of malignancy. Analysis:Non-parametric paired Wilcoxon signed rank test was used to compare the sum score of correctly classified skin lesions at T0 and T1.
RESULTS: At T0 GPs classified 63.9% lesions correctly (benign: 51.2%; malignant: 76.6%), while at T1 these figures increased to 75.1% (benign: 67.6%; malignant: 82.7%).
CONCLUSION: A one-day dermatologic education programme led to improvements in GPs’ diagnostic competence in skin cancer, but there remained room for further improvement.
Skin cancer is one of the most common neoplasms in Switzerland [1] and its prevalence is high compared to other countries in Europe [2–5]. Forsea et al. [6] estimated that Switzerland has the highest melanoma incidence in Europe with 19.2 cases per 100,000 residents (compared for example with Germany with around 12 cases per 100,000 residents or Austria with under 8 cases per 100,000 residents). Melanoma is responsible for more than 90% of all skin cancer related deaths. The melanoma lifetime risk at birth in the year 2000 was estimated to be 1:80 in Switzerland [7, 8]. There are currently approximately 1,900 new cases of melanoma per year in Switzerland [1] and the incidence continuously increases. Melanoma is the fourth most frequent cancer in Switzerland in men as well as in women [9]. For men, only prostate cancer, lung/bronchus/trachea cancer and colorectal cancer have a higher incidence than melanoma; for women only breast cancer, colorectal cancer and lung/bronchus/trachea cancer have a higher incidence than melanoma [9]. Potential determinants of this increase in Switzerland are the more frequently intermittent sun exposure for fashion [10] and during recreation as well as a higher detection rate because increasing numbers of nevi get excised and histologically examined [1, 11].
One third of Swiss patients with melanoma are less than 50 years old: melanoma is one of the most frequent cancer diagnoses in young adults [1]. Due to a lack of adequate therapies for metastatic melanoma, the best management option remains early diagnosis and prompt surgical excision of the primary cancer.
Non-melanoma skin cancer (NMSC) including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) are more frequent than melanoma. They are, from a clinical point of view, less aggressive because of their low metastatic potential, but due to their high incidence, local destructive growth pattern, and their tendency to recur after treatment, the morbidity and cost related to these cancers are very high [12].
Due to the rapidly rising incidence of skin cancer and the rising awareness of the topic in patients, GPs are more and more faced with suspicious skin lesions in their patients, they are usually the first contact person to examine and potentially refer patients to dermatologists. Appropriate knowledge and a high diagnostic competence are crucial in handling suspicious skin lesions correctly. During medical school there is no specific skin cancer training at this point in time. Research data on the dermatological diagnostic competence of GPs in Switzerland is lacking, but clinical experience suggests that diagnosis of skin cancer in primary care may be suboptimal. Embedded in an on-going broader project called “minSKIN” [13] this study aimed to assess GPs’ competence to diagnose skin cancer and to determine whether it was increased by a one-day dermatologic education programme.
This is a pre / post-intervention study.

Figure 1
Example of the interpretation (picture with a malignant lesion).
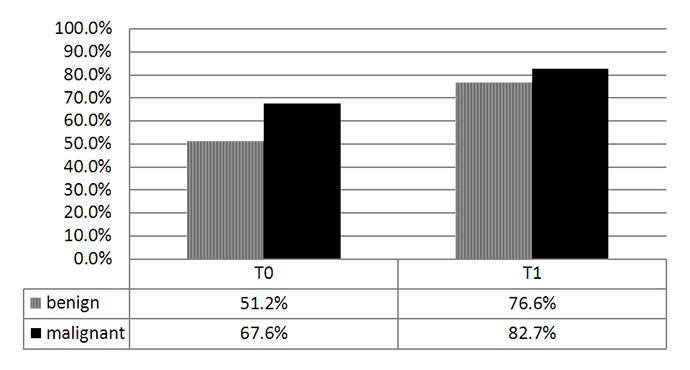
Figure 2
Percentage of correctly classified skin lesions before (T0) and after (T1) the one-day dermatologic education programme.
All of the around 1,000 GPs in the Canton of Zurich [14] were invited to take part in the study by an advertisement, by postal letters and information sessions provided by members of the study centre. Any general practitioner who provided primary medical care with a workload of at least 20 hours per week, who did not plan to retire or to move away during the study period was entitled to participate in the study. Finally, 78 GPs were included in our study. All of these 78 GPs later joined the RCT called “minSKIN” after our one day education programme [13]. The participating GPs did not receive financial compensation.
The dermatologic education programme was embedded in a randomised-controlled study with GPs [13]. The GPs received a full day dermatologic training course, organised by a dermatologist of the University Hospital Zurich. This training contained lectures about current epidemiology of skin cancer in Switzerland, background information on melanoma, basal cell carcinoma and squamous cell carcinoma, diagnosis and differential diagnosis of skin cancer, strategies to handle with suspicious skin lesions and interactive case discussions for the three main types of skin cancer.
For the study, we developed a pool of total 108 case-vignettes of skin lesions with a short history and known histology (benign: 54; malignant: 54), 36 cases at a high difficulty level, 36 cases at a medium difficulty level and 36 at a low difficulty level. The benign case-vignettes included the diagnoses: nevi, dysplastic nevi, seborrhoic keratosis, angioma and other benign lesions. The malignant case-vignettes included the diagnoses: BCC, SCC, melanoma, M. Bowen, keratoacanthoma and other malignant lesions. The pictures had to be of good quality (in focus etc.) and the diagnoses had to be histologically proven. They were randomly chosen from an image collection of the Clinic of Dermatology at the University Hospital Zurich. The levels of difficulty were independently defined by two dermatologists prior to the start of the study.
As primary outcome, we defined the competence in the diagnosis of skin cancer by GPs, measured as the percentage of correctly classified pictures of skin lesions. Prior to the start of the study, we randomly allocated 36 of the clinical case-vignettes (12 at each level of difficulty) for each picture scoring session to assure an equal difficulty of the sessions. Before (T0) and after (T1) the dermatologic education programme, the GPs were asked to rate these pictures of skin lesions according to their likelihood of malignancy on a visual analogue scale (VAS) with length 100 mm. Visual analogue scales were chosen for measurements because they are easy to handle and well known in the study population. Furthermore, they more strongly reflect diagnostic uncertainty in every day’s clinic than a dichotomous outcome.
For each level of difficulty, a cut-off on the VAS was defined prior to analysis in order to dichotomise the GPs’ judgement into correct or false as compared with the histology results. These cut-offs are displayed in table 1, an example for the interpretation of a malignant lesion picture and different levels of difficulty is shown in figure 1. We used a statistical test procedure that took into account dependencies in the data: assessment of 36 pictures per participating GP for each moment in time (T0 and T1). For each of the 78 GPs, we summed up the number of “correctly” classified skin lesion pictures according to the above described dichotomisation and obtained a score ranging from 0 (no correctly classified picture) to 36 pictures (all pictures correctly classified), at time T0 and T1. We then compared these two vectors of length 78 with a non-parametric paired Wilcoxon signed rank test. Significance level was set at 5%. For descriptive measures, we call “sensitivity” the malignant lesions (“disease”) that were correctly classified as malignant and “specificity” the benign lesions (“no disease”) that were correctly classified as benign.
The study was embedded in a broader project called “minSKIN” [13], which was approved by the ethics committee of Zurich (KEK-ZH-Nr. 2010-0384/5) and which has been registered at a trial register (current-controlled-trials: ISRCTN29854485).
| Table 1: Cut-offs on the VAS, depending on the difficulty of the pictures. | |
| Picture category | Cut-off on the VAS |
| Malignant lesion, high level of difficulty | 70 mm |
| Malignant lesion, medium level of difficulty | 75 mm |
| Malignant lesion, low level of difficulty | 80 mm |
| Benign lesion, high level of difficulty | 30 mm |
| Benign lesion, medium level of difficulty | 25 mm |
| Benign lesion, low level of difficulty | 20 mm |
Before the dermatologic education program (at T0) GPs classified total 63.9% of the lesions correctly (51.2% of benign lesions and 76.6% of malignant lesions). After the dermatologic education program (at T1) corresponding values significantly increased to total 75.1% (67.6% for benign lesions and 82.7% for malignant lesions).
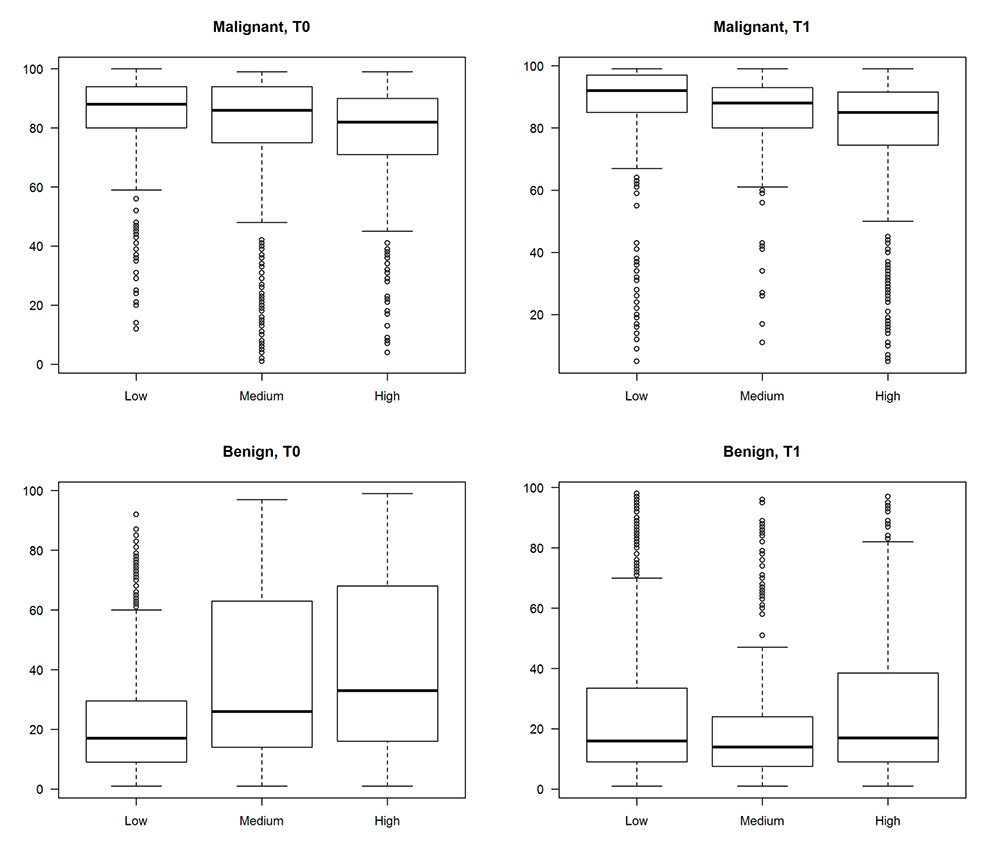
Figure 3
Outcomes by the difficulty level of the pictures and without the pre-defined cut-offs.
Due to the high interest from the GPs during recruitment, more than the initially planned number of 60 GPs wanted to take part in the study, so we were finally able to include 78 GPs. All GPs completed the pre-intervention picture scoring session at T0 as well as the post-intervention picture scoring session at T1. One GP did not provide information about his or her demographic data. The mean age of the participating GPs was 50.9 years with a range from 34 to 75 years. As shown in table 2, 71.8% of the GPs were male and 28.2% female. 55.8% worked fulltime while 44.2% worked part-time. 31.2% worked in a single handed practice, 26.0% in a double handed practice and 42.9% in a group practice with three or more physicians. 18.2% of the participating GPs stated that they have had additional dermatologic training while 81.8% did not. Compared to all GPs in the Canton of Zurich [14], our study population was slightly younger. Furthermore, we had rather more male GPs in the study population compared to all GPs in the Canton of Zurich.
As shown in figure 2, GPs classified 51.2% of benign and 76.6% of malignant lesions correctly before any intervention (at T0). After the dermatologic education programme (at T1) GPs classified 67.6% of benign and 82.7% of malignant lesions correctly. The increase in the percentage points of correctly classified skin lesions was 16.4% for benign lesions and 6.1% for malignant lesions. With our dermatologic education programme, GPs’ sensitivity increased from 76.6% to 82.7% without any loss in specificity.
We calculated the sum score as number of correctly classified pictures per participating GP before and after the intervention. The paired Wilcoxon signed rank test showed a significant increase of the sum score after the intervention (p <0.001).
It could be argued that the cut-offs based on the pre-specified levels of difficulty were arbitrary. In a secondary analysis we re-evaluated the outcome of the participating GPs with respect to difficulty level of the pictures and without the pre-defined cut-offs, the results can be found in figure 3. It turns out that with increasing level of difficulty, the mean outcome clearly deviates further from 100 for the malignant pictures. For benign pictures, the increasing deviation from 0 was clear at T0 but inconsistent at T1. Corresponding interquartile ranges increase for both malignant pictures at T0 and T1 and benign pictures at T0 with increasing difficulty, indicating increasing uncertainty of the participants. These findings support and justify our original assumptions.
As also shown in figure 3, the mean VAS values shift from T0 to T1 nearer to 100 (for malignant lesions) or nearer to 0 respectively (for benign lesions) and the interquartile ranges were reduced (except for low difficulty benign lesions) from T0 to T1.
| Table 2: Demographic data of the participating GPs. | |
| Gender (n = 78) Male Female | 56 (71.8%) 22 (28.2%) |
| Age (n = 77) <= 40 years 41–50 years >= 51 years | 9 (11.7%) 29 (37.7%) 39 (50.6%) |
| Years in practice (n = 77) <= 10 years 11–20 years >= 21 years | 35 (45.5%) 21 (27.3%) 21 (27.3%) |
| Workload (n = 77) Fulltime Part-time | 43 (55.8%) 34 (44.2%) |
| Practice type (n = 77) Single handed Double handed Group practice | 24 (31.2%) 20 (26.0%) 33 (42.9%) |
| Additional training in Dermatology (n = 77) Yes No | 14 (18.2%) 63 (81.8%) |
The diagnostic competence of the GPs’ in our study was relatively high at baseline, compared to GPs in other studies [15–21]. Nevertheless, after our one-day dermatologic education programme, the GPs significantly increased their diagnostic competence in skin cancer regarding lesions’ dignity.
At baseline, GPs scored a higher proportion of malignant lesions correctly than benign lesions. This may reflect a GPs’ “fear of missing” of a suspicious skin lesion. From a clinical point of view it is less severe to overrate a benign lesion as malignant than to underrate a malignant lesion as benign because of the lack of therapeutic options for a metastatic melanoma for example. On the other hand, inadequate diagnosis of benign lesions may increase distress in patients and use of healthcare services at the expense of other, clinically potentially more effective services. It is therefore also important to reduce the number of inappropriately diagnosed benign skin lesions. The effect of our dermatologic education programme demonstrated a higher impact when diagnosing benign lesions as compared to malignant lesions, implying that it is feasible to enhance diagnostic competence.
In figure 3, the shift of the mean VAS values from T0 to T1 displayed the effect of our dermatologic education programme on the GPs’ diagnostic competence. The reduction of the interquartile ranges from T0 to T1 may indicate an increasing dermatological diagnostic security in the participating physicians. This reduction can especially been seen in two subgroups: benign lesions at a medium difficulty level and benign lesions at a high difficulty level. This may indicate that a dermatologic education programme could lead to a reduction of unnecessarily removed benign skin lesions without affecting the removal rate of malignant skin lesions. A reduction of unnecessarily removed benign skin lesions can have a considerable impact on health care costs.
Because the recruiting procedure was done partially by an advertisement in a GP journal, we are not able to give a numerical amount of the response rate. However, the GPs’ interest to participate in this study was considerably higher than in our previous comparable projects. Due to this high interest, we were able to include many more GPs than initially planned. From our point of view, this interest shows the importance and frequency of patients with suspicious skin lesions in daily practice and the GPs’ relative uncertainty on how to handle all these patients. During their vocational training, most of the GPs did not attend further dermatological training until now, because it was almost impossible to get short-term positions in a dermatological institution as GP trainee.
With this study setting, we were not able to evaluate the long-term effect of our intervention. Because we did not ask for specific diagnoses but for their likelihood of malignancy, it may be that GPs’ diagnostic competence was overestimated. Nevertheless, from the clinical point of view, the very specific diagnosis is less important for the GP because he acts mainly as first contact person for further referral. Furthermore, the pool of our case-vignettes did not reflect the distribution of malignant skin cancer in primary care, so the pre-test probability for a malignant lesion in our study was much higher than in “real life”. As an additional weakness, we did not ask for the clinical procedure (excision yes/no) of the lesions, but just for their likelihood of malignancy. Aside from these limitations, we had a relatively high number of GPs participating in this study. Furthermore, by discriminating the skin cancer example cases into three levels of difficulty, we used a very advanced approach in the picture scoring session, considering the heterogeneity of skin lesions.
With a one-day dermatologic education programme, GPs’ diagnostic competence in skin cancer can be significantly increased. Taking into account the high interest of GPs in the topic and considering the effect of our intervention, skin cancer diagnostic skills should be integrated more often into GPs’ continuing medical education.
Acknowledgement:We would like to thank all GPs for their participation in our study. Furthermore, we are very grateful to Anke Schickel and Barbara Portner from the Institute for General Practice for their logistic support. We thank Nikon AG, MEDA Pharma GmbH and Spirig AG for their support of this study.
1 Bouchardy C, Lutz JM, Kühni C. Cancer in Switzerland. Situation and Development from 1983 up to 2007. http://www.nicer.org/Editor/files/Krebs_in_der_Schweiz_e_web.pdf. http://www.nicer.org/Editor/files/Krebs_in_der_Schweiz_e_web.pdf In.; 2011.
2 Lutz JM, Francisci S, Mugno E, Usel M, Pompe-Kirn V, Coebergh JW, Bieslka-Lasota M. Cancer prevalence in Central Europe: the EUROPREVAL Study. Ann Oncol. 2003;14(2):313–22.
3 de Vries E, Coebergh JW. Cutaneous malignant melanoma in Europe. Eur J Cancer. 2004;40(16):2355–66.
4 de Vries E, Bray FI, Coebergh JW, Parkin DM. Changing epidemiology of malignant cutaneous melanoma in Europe 1953–1997: rising trends in incidence and mortality but recent stabilizations in western Europe and decreases in Scandinavia. Int J Cancer. 2003;107(1):119–26.
5 Garbe C, Leiter U. Melanoma epidemiology and trends. Clin Dermatol. 2009;27(1):3–9.
6 Forsea AM, Del Marmol V, de Vries E, Bailey EE, Geller AC. Melanoma incidence and mortality in Europe: new estimates, persistent disparities. Br J Dermatol. 2012;167(5):1124–30.
7 Dummer R, Panizzon R, Bloch PH, Burg G. Updated Swiss guidelines for the treatment and follow-up of cutaneous melanoma. Dermatology. 2005;210(1):39–44.
8 Dummer R, Guggenheim M, Arnold AW, Braun R, von Moos R. Updated Swiss guidelines for the treatment and follow-up of cutaneous melanoma. Swiss Med Wkly. 2011;141:w13320.
9 Switzerland - Statistics of Cancer Incidence [http://www.nicer.org/assets/files/statistics/i5y8409ch.pdf]
10 Reinau D, Meier C, Gerber N, Hofbauer GF, Surber C. Sun protective behaviour of primary and secondary school students in North-Western Switzerland. Swiss Med Wkly. 2012;142:w13520.
11 Gass R, Bopp M: [Mortality from malignant melanoma: epidemiological trends in Switzerland]. Praxis. (Bern 1994) 2005;94(34):1295–300.
12 Stang A, Stausberg J, Boedeker W, Kerek-Bodden H, Jockel KH. Nationwide hospitalization costs of skin melanoma and non-melanoma skin cancer in Germany. J Eur Acad Dermatol Venereol. 2008;22(1):65–72.
13 Badertscher N, Rosemann T, Tandjung R, Braun RP. minSKIN does a multifaceted intervention improve the competence in the diagnosis of skin cancer by general practitioners? Study protocol for a randomised controlled trial. Trials. 2011;12:165.
14 [http://aerztestatistik.myfmh2.fmh.ch/]
15 Brochez L, Verhaeghe E, Bleyen L, Naeyaert JM. Diagnostic ability of general practitioners and dermatologists in discriminating pigmented skin lesions. J Am Acad Dermatol. 2001;44(6):979–86.
16 De Gannes GC, Ip JL, Martinka M, Crawford RI, Rivers JK. Early detection of skin cancer by family physicians: A pilot project. J Cutan Med Surg. 2004;8(2):103–9.
17 Gerbert B, Bronstone A, Wolff M, Maurer T, Berger T, Pantilat S, McPhee SJ. Improving primary care residents’ proficiency in the diagnosis of skin cancer. J Gen Intern Med. 1998;13(2):91–7.
18 Girgis A, Clarke P, Burton RC, Sanson-Fisher RW. Screening for melanoma by primary health care physicians: a cost-effectiveness analysis. J Med Screen. 1996;3(1):47–53.
19 Raasch BA, Hays R, Buettner PG: An educational intervention to improve diagnosis and management of suspicious skin lesions. J Contin Educ Health Prof. 2000;20(1):39–51.
20 Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143(5):1016–20.
21 Youl PH, Raasch BA, Janda M, Aitken JF. The effect of an educational programme to improve the skills of general practitioners in diagnosing melanocytic/pigmented lesions. Clin Exp Dermatol. 2007;32(4):365–70.
Authors‘ contribution and competing interests:All authors made a substantial contribution to this manuscript i.e. study conception, data collection, data analysis or manuscript drafting. The authors declare that they have no competing interests. This study was funded by the Swiss Cancer League, the RRMA (subgroup of the Swiss Academy of Medical Sciences), Nikon AG, MEDA Pharma GmbH and Spirig AG. None of the sponsors had any influence on the study design, content or evaluation of results.