DOI: https://doi.org/10.4414/smw.2013.13772
Data from the AMSP drug surveillance programme in Switzerland
The overall consensus is that elderly psychiatric patients are more prone to develop adverse drug reactions (ADRs) than younger patients as a consequence of psychopharmacological medication [1]. This is described in special guidelines on the use of antipsychotics [2] (especially concerning cardiovascular accidents) and of anticonvulsants [3] in the elderly. However, actual scientific data on the frequency of ADRs in relation to age are rather sparse.
Elderly persons with psychiatric disorders frequently suffer from somatic diseases and may receive polypharmacy more than younger patients. Thus, they may tend to develop ADRs more frequently. On the other hand, physicians possibly consider these special requirements of elderly psychiatric patients and reduce the risks of ADRs in these patients. They may monitor their patients more intensively, prescribe lower dosages or avoid high-risk drugs and dangerous combinations. Indeed, several authors have emphasised that psychotropic drugs are in general well tolerated by the elderly [4], particularly lamotrigine [5], olanzapine [6], quetiapine [7], risperidone [7] and valproic acid [8].
The present study aimed to verify the hypothesis that the risk of ADRs increases with the age of patients. For this purpose we used a large data sample from the international AMSP drug surveillance programme (Arzneimittelsicherheit in der Psychiatrie – drug safety in psychiatry) [9]. The programme records severe ADRs in patients from a large age range who are hospitalised in psychiatric settings. It also records the prescriptions of drugs and personal data such as age, sex and diagnosis. The data were taken exclusively from Swiss psychiatric hospitals and were collected from 2001 to 2010.
The data allowed us to evaluate the frequency of ADRs of psychotropic drugs in relation to the age of the patients and to compare patients aged up to 60 years with those older than 60 years. To the best of our knowledge, such an analysis had not previously been conducted.
The ethics committee of the Ludwig Maximilian University of Munich, the location of the AMSP main data centre (up to 2011), had approved the analysis of the AMSP data with a waiver of authorisation. The permission to use the special data set from Swiss hospitals was given by the publication commission consisting of the presidents of the AMSP associations in Germany, Austria and Switzerland.
The data stem from the international AMSP project initiated at the Psychiatric University Hospital in Munich in 1993. Today it runs in 60 psychiatric hospitals in Germany, Austria and the German-speaking part of Switzerland (12 hospitals). The data in the present study were collected between 2001 and 2010 in selected wards (including gerontopsychiatric wards) of psychiatric hospitals in Switzerland. The AMSP method is described in detail elsewhere [9–11].
Briefly, the AMSP project comprises of two differing data sets: One data set includes prescriptions of drugs, the age, sex and primary diagnosis of patients receiving the drugs as well as the number of inpatients monitored per year. The second data set includes detailed information on severe ADRs that were observed in the patients of the participating hospitals during psychotropic drug treatment. The medical description of the ADRs was made by the treating physician, and the association between the ADRs and the medication was assessed by consensus of an expert committee (causality assessment). The present data comprise of only data for severe ADRs with “probable” and “definite” associations.
An ADR is rated as severe in the AMSP project if it (a) has a significant impact on the course of treatment (e.g., if it is life threatening or seriously endangers the patient’s health), (b) considerably impairs everyday functioning, or (c) requires the patient’s transfer to another department or ward where more intensive or specialised care is provided [9, 10] .The AMSP study protocol provides additional guidelines based on each system or organic class and gives precise definitions of ADRs [9, 10], for example increase in liver enzymes >5 times the upper normal limit; allergic skin reaction, if affecting the whole body or more than one body part (e.g. face and arms) or if connected with general symptoms like fever and malaise; oedema if marked, lasting more than one week and affecting special body parts as face, lower or upper eyelid or requiring diuretic treatment; galactorrhoea, if marked or accompanied by pain or tension of the breast or gynecomastia. The definitions for weight gain and extrapyramidal motor system (EPMS) symptoms were changed in 2001, including ADR weight gain: weight gain >10% of patient’s normal weight; EPMS symptoms: malignant and catatonic neuroleptic syndrome, tardive dyskinesia, acute dystonia and Parkinsonism, of severely disabling in everyday functioning; akathisia: grade 4 on the Barnes akathisia rating scale; and all cases of Pisa syndrome and atypical dyskinesias. The AMSP definitions of severe ADRs differ from the definition as given by the Food and Drug Administration [12]. All ADRs analysed in this study had been classified as severe.
Each individual case is reviewed by an expert committee in detail. In national and international case conferences occurring several times per year, disputable cases are discussed by experts of the participating sites, by drug safety experts of pharmaceutical companies and by delegates of a pharmaco-vigilance centre (University of Zurich, Switzerland) and drug regulating authorities (Germany).
The probability of a causal relationship (causality assessment) is graded as follows: Possible: ADR not known or alternative explanation more likely. Probable: ADR known for drug in question, as well as time course and dosage in accordance with previous experience, and alternative explanation less likely. Definite: ADR “probable” plus reappearance after re-challenge with the drug [9, 10]. In cases of drug combinations in which a pharmacodynamic / pharmacokinetic interaction is held responsible for an ADR, each drug is imputed. For the present analysis ADR rates for different drugs or drug groups referred to all cases in which drugs were imputed alone or in combination with other drugs. Only data from hospitals with a minimum frequency of 0.5% of reported ADRs were included in the calculations.
Relative frequencies of ADRs were calculated by dividing the number of patients who experienced ADRs by the number of all patients exposed to this particular psychotropic medication. Two different statistical methods were used:
1. Logistic regression: To analyse the relationship between the relative frequency of ADRs and age, the relative frequency of ADRs for each year of age (15 years – 93 years) of the patients was plotted against age, and logistic regression and cross table analyses were calculated. Linear regression was used for the analysis of the relation of doses of drugs and age.
2. Analysis of group means: The relative frequency of ADRs was sub-divided into two groups (0–60 years and 61–120 years), and 95% confidence intervals (CI) were calculated for these groups using the Clopper Pearson method [13], with α = 0.05; k = number of patients with ADRs, n = number of patients. A statistically significant difference between two relative frequencies was defined as non-overlapping confidence intervals of these two relative frequencies. A marginal overlap was defined as a statistical trend.
The use of two different methods allowed us to generalise the results across both statistical methods.
Table 1 describes the patient population. In the age group up to 60 years, schizophrenia was the most common diagnosis (45%); in the group above 60 years organic disorders and schizophrenia (32% and 27%, respectively) were the most frequent diagnostic categories. Antipsychotics were the preferred class of medication in both age groups (72% and 74%, respectively). Considering the whole group of patients, severe ADRs were assessed in 699 out of 39,728 patients (1.76%, CI 1.63–1.89). Within the group up to 60 years the respective numbers were 517 cases with ADRs out of 28,282 (1.82%; CI 1.68–1.99); in the elderly the percentage of patients with reported ADRs was 1.60% (182 ADR cases out of 11,446 patients, CI 1.37–1.84). The difference between these age groups was not significant.
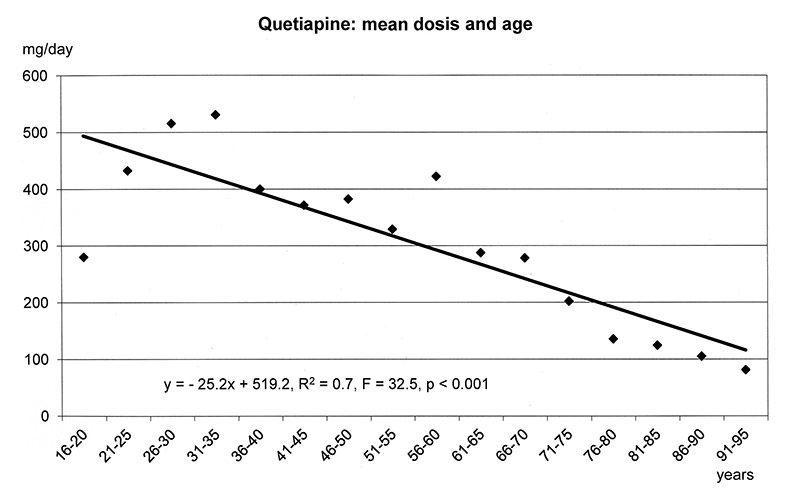
The regression models in figure 1a-f show that the dosages of selected antipsychotics and anticonvulsants decreased significantly with increasing age: lamotrigine (number of patients: n = 1597, mean dose: 143 mg), olanzapine (n = 5248, mean: 16 mg), quetiapine (n = 6339, mean: 355 mg), risperidone (n = 3183, mean: 3.4 mg) and valproic acid (n = 8448, mean: 1298 mg), but not carbamazepine (n = 707, mean: 659 mg). For these substances lower frequencies of ADRs were found in the elderly (cf. fig. 3–5.)
As shown in fig. 2 the relative frequency of severe ADRs due to psychotropic medication decreased with age. According to logistic regression and cross table analyses a significant relation of ADRs and age was found. The risk of developing severe ADRs in clinical settings decreased during lifetime.
Figure 3 shows the frequency of ADRs in relation to the number of patients receiving selected psychotropic medication, in particular the relative frequency of ADRs in the younger patient group (≤60 y) and in the elderly (>60 y). The relative frequency of reported ADRs for antipsychotics and for anticonvulsants was significantly higher in younger patients than in the elderly: 1.8% versus 1.3% and 1.0% versus 0.64% of the patients treated with these drugs, respectively. Within the anticonvulsants, this statistical difference was also found for the substance valproic acid: 0.9% versus 0.42% ADRs. The confidence interval (CI) values are given in the legend. With regard to minor tranquilisers, medicated patients >60 years showed a trend for more ADRs than younger patients. In almost all cases, the tranquilisers were imputed for ADRs in combination with other drugs. Comparisons with other drugs or classes of drugs did not yield significant differences.

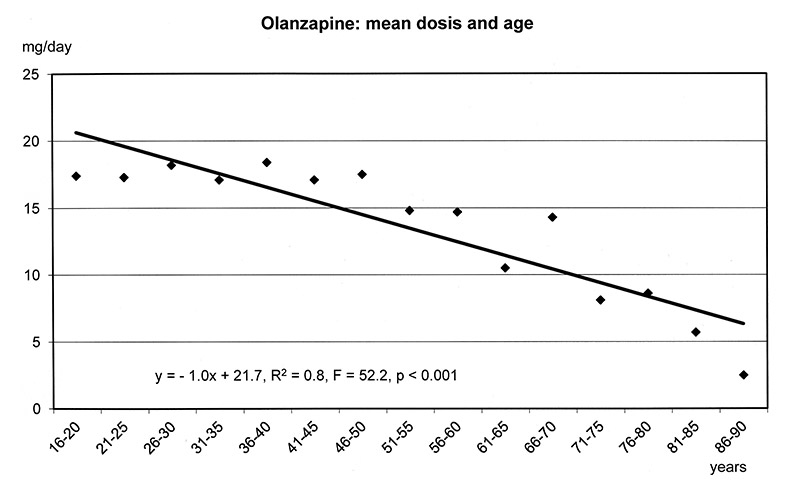
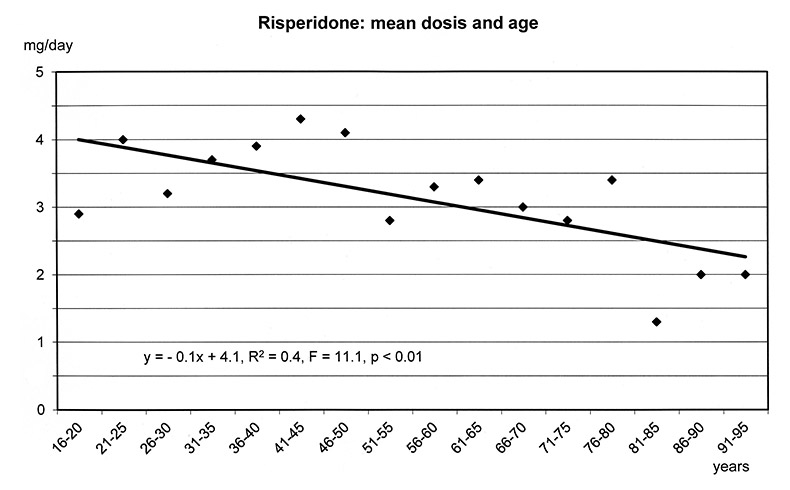
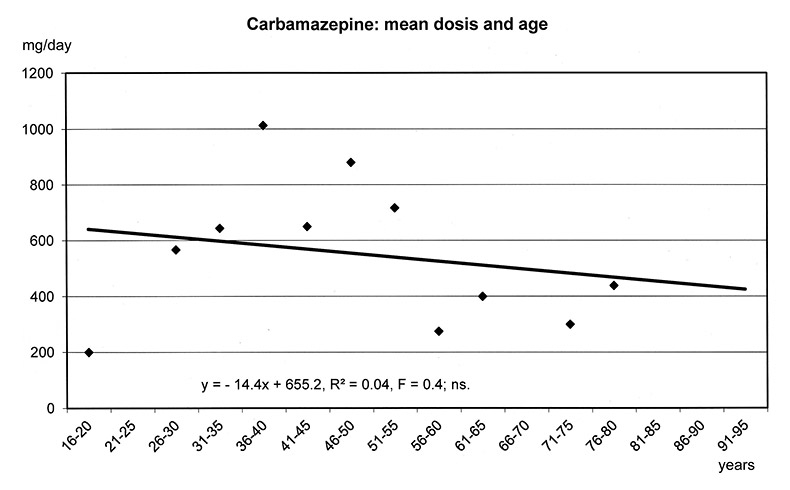
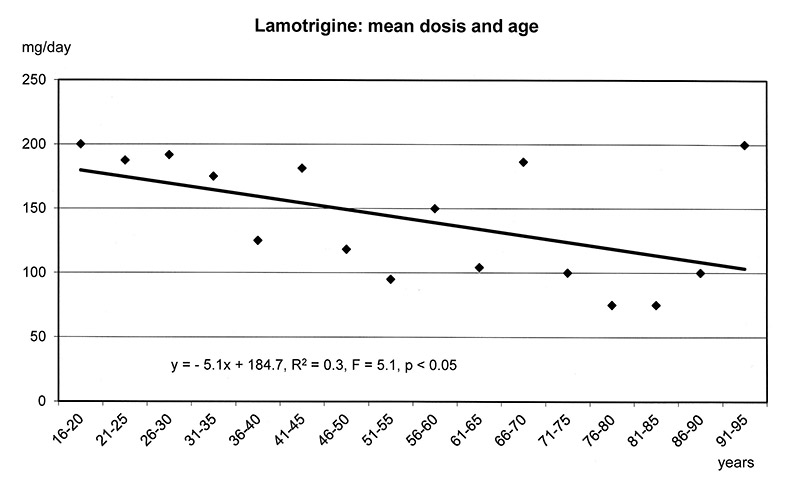
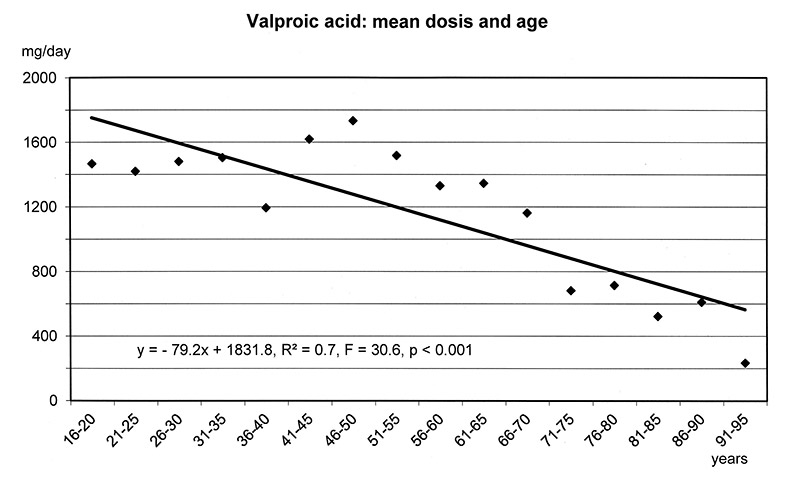
Figure 1a–f
Linear Regression Models of dosages of selected drugs in relation to age.
Table 2 shows the results of logistic regression and cross table analyses for selected ADRs as observed in at least 20 patients. A minimum of n = 20 was chosen to ensure valid results in the regression analyses. Generally, the risk for ADRs decreases significantly with age (cf also fig. 2) and also for some particular ADRs, for example extrapyramidal motor system (EPMS) symptoms, galactorrhoea and weight gain (according to both, regression analysis and cross tables) and increased liver enzymes (according to regression analysis). In contrast, the risk of developing delirium increased with age (according to regression analysis), and the risk of developing oedema also showed a corresponding trend (according to cross table analysis). No relationship was found between age and the risk of developing allergic skin reactions and hypotonia / collapse. Besides the EPMS symptoms (including various single motor system ADRs, e.g. Parkinsonism and tardive dyskinesia), weight gain and allergic skin reactions were the ADRs observed most often. Hence, the age dependency of the latter ADRs will be described more in detail.
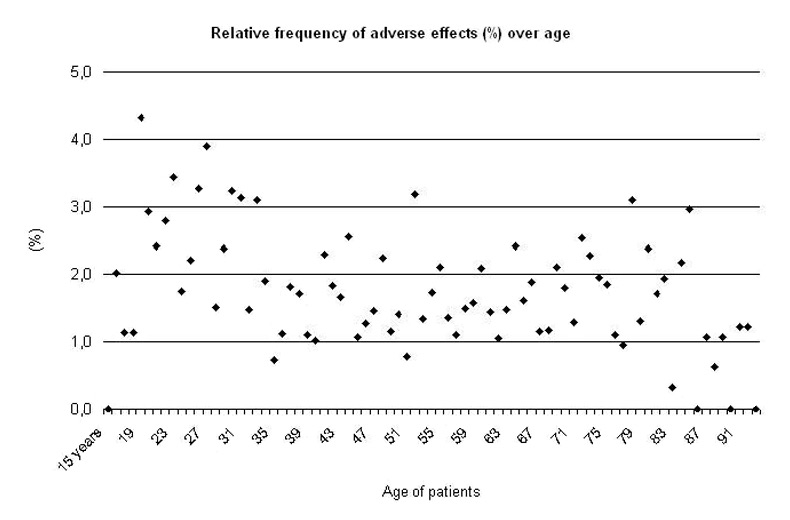
Figure 2
Linear Regression Model of the relative frequency of all ADRs over age of patients (15–93 years).
Regression Model for the subgroup 31–93 years: y = – 0.02x + 2.3, R² = 0.1 F = 8.1, p <0.01; for the subgroup 61–93 years: y = – 0.03 x + 2.0, R² = 0.1, F = 4.7, p <0.05.
Regression analysis (table 2) revealed a highly significant decrease in weight gain with age, to the extent that weight gain was almost never found in patients >60 years. Additional statistical analyses between age groups confirmed that patients <30 years of age had the highest risk of developing weight gain (n = 6493, k = 41, relative frequency = 0.63%,CI (0.45 – 0.86)), while patients aged between 30 and 60 years have a significantly lower risk of weight gain (n = 21789 , k = 35 relative frequency = 0.16%, CI [0.11–0.22]). Patients >60 years had the lowest risk of weight gain (n = 11446, k = 4, relative frequency = 0.03%, CI [0.01–0.09]) compared to both other groups. The use of antipsychotic drugs was at least 75% in all three age groups: 95% (0–30 years) versus 86% (31–60 years) versus 75% (over 60 years).
Differences in weight gain could be found between elderly and younger patients during treatment with psychotropic substances in general, with antipsychotics as a class, and with olanzapine (fig. 4). Weight gain was not found in the elderly at all for quetiapine and risperidone, although 2060 and 953 elderly patients received quetiapine and risperidone, respectively.
Regression analysis showed that weight gain during olanzapine treatment decreased significantly with age. This relationship could not be explained by the differences in dosages which showed no significant relation to age in the group of patients with weight gain under olanzapine.
As the regression analysis in table 2 shows, allergic skin reaction was not related to the age of the patients in general. However, for patients taking drugs out of the group of mood-stabilising anticonvulsants, that is carbamazepine, lamotrigine, and valproic acid, elderly patients had a statistically significant lower incidence of reported allergic skin reactions (fig. 5): 0.31% for patients up to 60 years and 0.07% for those over 60 years. Furthermore, a respective trend was found for lamotrigine: 1.15% compared to 0.24%.
| Table 1: Patient population. Number of patients (n), percentage of patients in a particular subgroup and number of patients with (probable or definite) severe adverse drug reactions (ADRs), psychiatric diagnoses and the most important classes of psychotropic medication. | |||||
| Overall | ≤60 years | >60 years | |||
| n | n | % | n | % | |
| Number of patients | 39728 | 28282 | 100% | 11446 | 100% |
| Female | 19838 | 12956 | 46% | 6882 | 60% |
| Patients with ADRs | 699 | 517 | 1.8% | 182 | 1.6% |
| Addiction (ICD 10: F1) | 3462 | 2931 | 10% | 531 | 5% |
| Affective disorders (ICD 10: F3) | 9288 | 6367 | 23% | 2921 | 26% |
| Neuroses / Personality dis. (ICD 10: F4.6) | 4076 | 3364 | 12% | 712 | 6% |
| Organic disorder (ICD 10: F0) | 5167 | 1551 | 6% | 3616 | 32% |
| Schizophrenia (ICD10: F2) | 15692 | 12602 | 45% | 3090 | 27% |
| Antidepressants | 18744 | 12421 | 44% | 6323 | 54% |
| Antipsychotics | 28715 | 20283 | 72% | 8432 | 74% |
| Anticonvulsants | 12125 | 8032 | 28% | 4093 | 36% |
| Antiparkinsonian drugs | 5457 | 3633 | 13% | 1824 | 16% |
| Hypnotics | 3972 | 2482 | 9% | 1490 | 13% |
| Lithium-Salts | 2170 | 1726 | 6% | 444 | 4% |
| Nootropics | 1063 | 148 | 1% | 915 | 8% |
| Tranquilisers | 11407 | 7835 | 28% | 3572 | 31% |
| Table 2:Logistic Regression and Cross Table Analyses of selected ADRs | |||
| Adverse effect | k | Significance Logistic regression and cross tables | Change |
| All ADRs | 699 | p < 0.01 | Decreasing |
| Allergic skin reactions | 53 | p > 0.05 ns | None |
| Delirium | 34 | p < 0.05 (regression analysis) | Increasing |
| EPMS | 126 | p < 0.05 | Decreasing |
| Galactorrhoea | 22 | p < 0.01 | Decreasing |
| Hypotonia / Collapse | 23 | p > 0.05 ns | None |
| Increased liver enzymes | 22 | p < 0.05 (regression analysis) | Decreasing |
| Oedema | 27 | p = 0.07 trend (cross table analysis) | Increasing |
| Weight gain | 80 | p < 0.01 | Decreasing |
| Statistical significance relates to both statistical methods, logistic regression and cross table analysis. When the data are significant according to one of the methods only it is given in brackets. | |||
The present data provide evidence that the incidence of severe ADRs due to psychotropic medication decreases in hospitalised psychiatric patients with increasing age of patients. The reported severe ADRs in clinical routine appear to be less frequent in patients older than 60 years than in younger patients, especially those up to 30 years. At first glance, this finding is surprising and also counterintuitive. However, several factors may contribute to this effect, and thus it is difficult to draw any causal inferences.

Figure 3
Relative frequency of ADRs for different classes of psychotropic drugs in patients ≤60 years and patients >60 years of age.
* significant difference
(k = number of patients with ADRs). Significant differences for
Antipsychotics:
Patients ≤60 years: n = 20283, k = 362; 1.78% CI (1.61 – 1.98)
Patients >60 years: n = 8432, k = 111; 1,32% CI (1.08 – 1.58)
Anticonvulsants:
Patients ≤60 years: n = 8032, k = 81; 1.00% CI (0.80 – 1.25)
Patients >60 years: n = 4093, k = 26; 0.64% CI (0.42 – 0.80)
Valproic acid:
Patients ≤60 years: n = 5320, k = 48; 0.90% CI (0.71 – 1.19)
Patients >60 years: n = 3128, k = 13; 0.42% CI (0.22 – 0.71)
A trend for patients with tranquilisers: patients ≤60 years: n = 7835, k = 17; 0.22% CI (0.13–0.35), patients >60 years: n = 3572, k = 20; 0.56% CI (0.34–0.86).
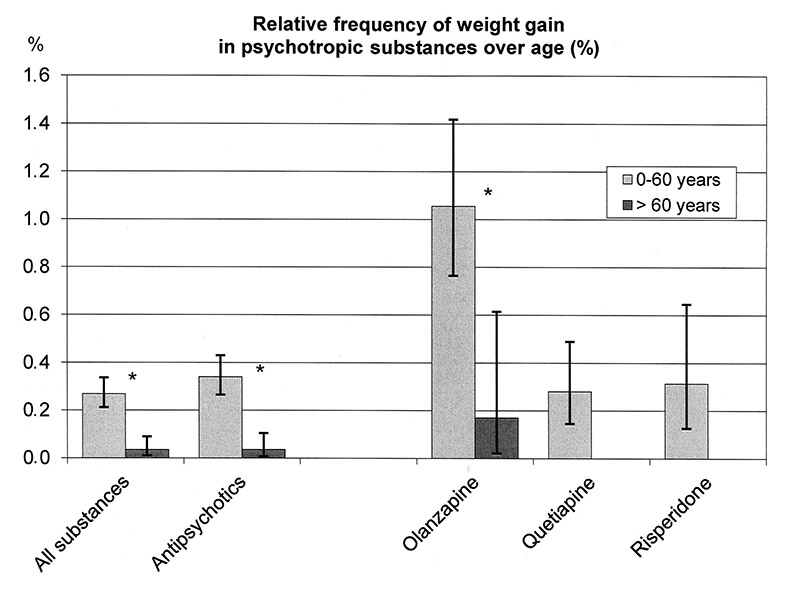
Figure 4
Relative frequency of weight gain for patients ≤60 years and patients >60 years medicated with different psychotropic substances.
* significant difference
Significant differences for all psychotropic substances (patients ≤60 years: n = 28282, k = 97, 0.26%, CI (0.21–0.34); patients >60 years: n = 11446, k = 7, 0.06%, CI [0.01–0.09]), for antipsychotics (patients ≤60 years: n = 20283, k = 69, 0.34% CI (0.26 – 0.43); patients >60 years: n = 8432, k = 3, 0.04% CI [0.01–0.10]), for olanzapine (patients ≤60 years: n = 4076, k = 43, 1.05% CI (0.76–1.42); patients >60 years: n = 1172, k = 2, 0.17% CI (0.02–0.61)), quetiapine (patients ≤60 years: n = 4279, k = 12; patients >60 years: n = 2060, k = 0) and risperidone (patients ≤60 years: n = 2230, k = 7; patients >60 years: n = 953, k = 0).
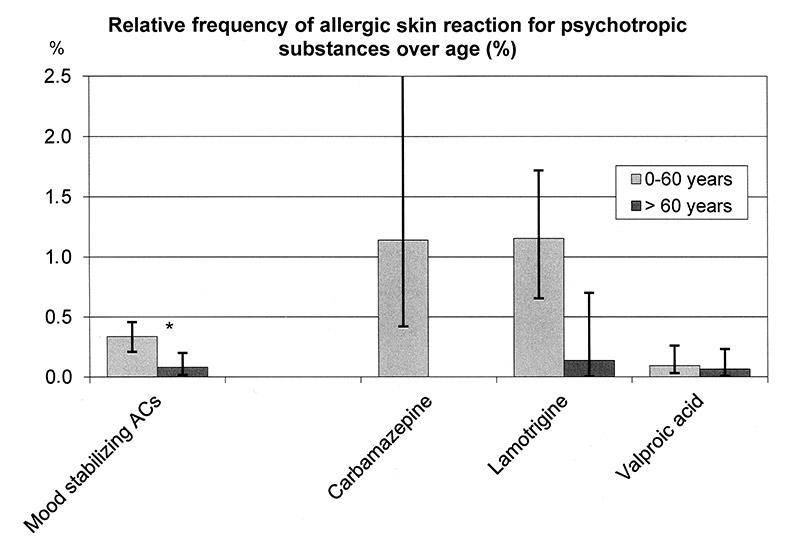
Figure 5
Relative frequency of allergic skin reactions for patients ≤60 years and patients >60 years medicated with mood stabilizing anticonvulsants (ACs).
Significant difference for the group of ACs (carbamazepine, lamotrigine, valproate): 0.31% CI (0.21–0.51) for patients ≤60 years (n = 6712, k = 21) and 0.07% CI (0.02–0.20) for those >60 years (n = 4040, k = 3). A trend for lamotrigine (patients ≤60 years: 1.16 %; CI (0.65–2.1), n = 866, k = 10; patients >60 years: 0.14%, CI (0.01–0.7), n = 422, k = 1).
It is possible that elderly patients are administered different medications than younger patients. The present data, however, show that lower rates of ADRs in the elderly were found for the same classes of psychotropic drugs (antipsychotic drugs and anticonvulsants for ADRs in general and antipsychotic drugs for weight gain) and for the same psychotropic substances (valproic acid for ADRs in general and olanzapine for weight gain).
Furthermore, elderly patients could already have had experience with psychopharmacological treatment and its ADRs. Their physicians might have avoided medication that was not tolerated. In contrast, younger patients might have received certain substances for the first time and thus have reacted with various ADRs. This might be a factor contributing to the lower rate of allergic skin reaction observed in the older age group with mood-stabilising anticonvulsants.
In addition, differences in dosages of medication could be a relevant factor. Young patients received higher doses that were associated with a higher frequency of ADRs. Indeed, our results clearly demonstrate that physicians reduce the dosages with increasing age of the patients (e.g., for olanzapine, quetiapine, and risperidone). The differences in the dosages of antipsychotics are presumably responsible for the decrease in EPMS symptoms with increasing age, an adverse effect known to be dose-dependent [14].
In contrast, allergic skin rashes appear to be independent of the recommended dosages of medication [15, 16]. Therefore, the observation in our study that allergic skin rashes occur more often in younger patients on mood-stabilising anticonvulsants can hardly be explained by differences in dosages between the age groups.
The relationship between weight gain and dosage of antipsychotics is controversial. Some studies have not reported any relationship [17], while others found a positive relationship for risperidone [18], clozapine [19], and olanzapine, respectively [20]. However, clinically significant metabolic changes seem to be rather independent of dosage [20, 21]. In the current study, a weight gain of 10% and more while on olanzapine and antipsychotics in general was found more frequently in the group of younger patients up to 60 years of age. A regression analysis of our data showed that weight gain during medication with olanzapine significantly decreases with age. This relationship appears to be independent of dosage.
The method of the present study, which was that physicians noted and reported ADRs and experts established whether there was a relationship between ADRs and medication, may have produced a bias in the data. Physicians may tend to notice ADRs more easily in younger patients and more readily assign unexpected somatic reactions to particular psychotropic substances. In contrast, it can be difficult to establish an association between a psychotropic substance and somatic symptoms in elderly patients with a variety of somatic troubles. Such perceptual biases in the causality assessment (with selective reporting and general underreporting of ADRs) can hardly be avoided in the present naturalistic study. However, the influence of age was found to differ for various ADRs. For example, the relative frequency of weight gain was lower in the elderly, whereas the relative frequency of delirium increased with age and for oedema a respective trend was found.
Our method of relating the frequency of reported ADRs to the frequency of prescribed drugs has yielded plausible results from the AMSP project. Higher rates of the ADR increased liver enzymes were found for clozapine in comparison to other substances [22] in agreement with the literature [23–26], as well as of EPMS symptoms for risperidone and haloperidol [27–29], and of weight gain for olanzapine [29–33]. Another AMSP study [34] reported delirium during treatment with tricyclics [35, 36] and low rates of ADRs due to SSRIs [37–39]. Higher rates of allergic skin reactions during treatment with carbamazepine and lamotrigine [10] were also reported [40, 41]. Although the method has some important shortcomings, it still corroborates well-known effects and thus has proven to be a valid method for measuring and quantifying ADRs.
Although it may be considered a caveat that ours was a naturalistic and not a controlled study, a strength of the study is that the data represent the clinical reality of many different psychiatric hospitals (university, state and private hospitals) and various kinds of wards, including gerontopsychiatric wards. Moreover, some of the results are based on group means with multiple comparisons, which is at risk of overestimating significant differences. However, since two statistical methods were applied, linear regression and comparisons of group means, it is possible to generalise the results across statistical methods. To note, severe ADRs according to the definition in the AMSP project differ from ADRs according to other definitions, such as the FDA definition [12].
There might be objective physiological differences in response to psychopharmacological medication, such as an altered tolerability of psychotropic medication in the elderly or a slower and less obvious onset of ADRs. For example, the neural responses of several receptors might decline with age, for example pain receptors or dopaminergic receptors. The ADRs weight gain and EPMS symptoms were increased in schizophrenic adolescents on atypical antipsychotics compared to adults [42, 43]. To the best of our knowledge, similar data comparing adults and elderly patients are not available.
Only a few studies present data on age and ADRs of psychopharmacological treatment. A good tolerability of lamotrigine in elderly patients was found in several studies [5, 16, 44]. Valproic acid was also observed to be well tolerated by patients over 60 years [8, 45], and weight gain was not found [8]. One study comparing young and elderly patients, which did not include the middle age group of 37–54 year olds, reported very low rates of ADRs for lamotrigine in the young group and higher values in the elderly [46]. Our data showed that severe ADRs during treatment with lamotrigine were not found in patients up to 30 years of age, but were particularly frequent in the middle age range (31–60 years) and relatively low in the elderly group treated (0% versus 1.41% versus 0.14%, data not shown).
Two studies reported only a few ADRs of olanzapine in elderly patients, and in particular no extrapyramidal side effects [6, 47]. One epidemiologic study on elderly patients found a general risk of metabolic effects with olanzapine [48]. A good tolerability was reported for quetiapine and risperidone in elderly psychiatric patients [4, 7]. In about 600 psychiatric patients aged over 65 years, Curran et al. [4] found that antipsychotic drugs were the class of medication mostly used in the elderly – in agreement with our data – and that serious ADRs were rare. Cerebrovascular events in elderly patients suffering from dementia and treated with antipsychotic drugs as reported in the literature [2] are very rare and were not observed in the present study.
So far, the evidence in the literature indicates that psychopharmacological treatment seems to be rather well tolerated by elderly patients.
In conclusion, the present data suggest that under clinical routine conditions in psychiatric hospitals, younger patients rather than elderly are at a higher risk of developing common, severe ADRs (e.g., EPMS symptoms and weight gain) due to treatment with psychotropic drugs. However, this conclusion must still be proven by epidemiological and controlled clinical studies in a large population over a wide age range.
1 Alexopoulos GS. Using Antipsychotic Agents in Older Patients: Introduction: Methods, Commentary, and Summary. J Clin Psychiat. 2004;65:5–20.
2 Consensus Group: Using Antipsychotic Agents in Older Patients: Indication for Using Antipsychotics in Older Patients. J Clin Psychiat. 2004;65:21–41.
3 Fenn HH, Sommer BR, Ketter TA, Alldredge B. Safety and tolerability of mood-stabilising anticonvulsants in the elderly. Expert Opin Drug Saf. 2006;5:401–16.
4 Curran S, Turner D, Musa S, Wattis J. Psychotropic drug use in older people with mental illness with particular reference to antipsychotics: a systematic study of tolerability and use in different diagnostic groups. Int J Geriatr Psychiatry. 2005;20:842–7.
5 Sajatovic M, Gildengers A, Al Jurdi RK, Gyulai L, Cassidy KA, Greenberg RL, et al. Multisite, open-label, prospective trial of lamotrigine for geriatric bipolar depression: a preliminary report. Bipolar Disord. 2011;13:294–302.
6 Ciudad A, Montes JM, Olivares JM, Gomez JC. Safety and tolerability of olanzapine compared with other antipsychotics in the treatment of elderly patients with schizophrenia: a naturalistic study. Eur Psychiatry. 2004;19:358–65.
7 Rainer M, Haushofer M, Pfolz H, Struhal C, Wick W. Quetiapine versus risperidone in elderly patients with behavioural and psychological symptoms of dementia: efficacy, safety and cognitive function. Eur Psychiatry. 2007;22:395–403.
8 Sajatovic M, Coconcea N, Ignacio RV, Blow FC, Hays RW, Cassidy KA, et al. Adjunct extended-release valproate semisodium in late life schizophrenia. Int J Geriatr Psychiatry. 2008;23:142–7.
9 Grohmann R, Engel RR, Rüther E, Hippius H. The AMSP drug safety program: methods and global results. Pharmacopsychiatry. 2004;37(Suppl 1):S4–S11.
10 Lange-Asschenfeldt C, Grohmann R, Lange-Asschenfeldt B, Engel RR, Rüther E, Cordes J. Cutaneous adverse reactions to psychotropic drugs: data from a multicenter surveillance program. J Clin Psychiatry. 2009;70:1258–65.
11 Stübner S, Grohmann R, von Stralendorff I, Rüther E, Möller HJ, Müller-Oerlinghausen B, et al. Suicidality as Rare Adverse Event of Antidepressant Medication: Report from the AMSP Multicenter Drug Safety Surveillance Project. J Clin Psychiat. 2010;71:1293–307.
12 Moore TJ, Cohen MR, Furberg CD. Serious Adverse Drug Events Reported to the Food and Drug Administration, 1998–2005. Arch Internal Med. 2007;167:1752–9.
13 Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–13.
14 Barr AM, Honer WG, Williams G, Johnson JL, Wu TKY, Procyshyn RM. A comparison of antipsychotic drug-defined daily doses versus chlorpromazine equivalent doses in patients with or without extrapyramidal motor symptoms. J Clin Psychopharmacol. 2010;30:741–3.
15 Wong IC, Mawer GE, Sander JW. Factors influencing the incidence of lamotrigine-related skin rash. Ann Pharmacother. 1999;33:1037–42.
16 Malik S, Arif H, Hirsch LJ. Lamotrigine and its applications in the treatment of epilepsy and other neurological and psychiatric disorders. Expert Rev Neurother. 2006;6:1609–27.
17 Henderson DC, Nguyen DD, Copeland PM, Hayden DL, Borba CP, Louie PM, et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. J Clin Psychiatry. 2005;66:1116–21.
18 Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151:825–35.
19 de Leon L, Diaz FJ, Josiassen RC, Cooper TB, Simpson GM. Weight gain during a double-blind multidosage clozapine study. J Clin Psychopharmacol. 2007;27:22–7.
20 Kinon BJ, Volavka J, Stauffer V, Edwards SE, Liu-Seifert H, Chen L, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder: a randomized, double-blind, fixed-dose study. J Clin Psychopharmacol. 2008;28:392–400.
21 Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–30.
22 Bender S, Grohmann R, Engel RR, Degner D, Dittmann-Balcar A, Rüther E. Severe adverse drug reactions in psychiatric inpatients treated with neuroleptics. Pharmacopsychiatry. 2004;37(Suppl 1):S46–S53.
23 Gaertner HJ, Fischer E, Hoss J. Side effects of clozapine. Psychopharmacology. (Berl) 1989;99(Suppl):S97–100.
24 Hummer M, Kurz M, Kurzthaler I, Oberbauer H, Miller C, Fleischhacker WW. Hepatotoxicity of clozapine. J Clin Psychopharmacol. 1997;17:314–7.
25 Gaertner I, Altendorf K, Batra A, Gaertner HJ. Relevance of liver enzyme elevations with four different neuroleptics: a retrospective review of 7,263 treatment courses. J Clin Psychopharmacol. 2001;21:215–22.
26 Kelly DL, Conley RR, Richardson CM, Tamminga CA, Carpenter WT, Jr.: Adverse effects and laboratory parameters of high-dose olanzapine vs. clozapine in treatment-resistant schizophrenia. Ann Clin Psychiatry. 2003;15:181–6.
27 Kurz M, Hummer M, Oberbauer H, Fleischhacker WW. Extrapyramidal side effects of clozapine and haloperidol. Psychopharmacology. (Berl) 1995;118:52–6.
28 Kasper S, Lerman MN, McQuade RD, Saha A, Carson WH, Ali M, et al. Efficacy and safety of aripiprazole vs. haloperidol for long-term maintenance treatment following acute relapse of schizophrenia. Int J Neuropsychopharmacol. 2003;6:325–37.
29 Crossley NA, Constante M, McGuire P, Power P. Efficacy of atypical v. typical antipsychotics in the treatment of early psychosis: meta-analysis. Br J Psychiatry. 2010;196:434–9.
30 Meltzer HY, Bobo WV, Roy A, Jayathilake K, Chen Y, Ertugrul A, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69:274–85.
31 Leucht S, Komossa K, Rummel-Kluge C, Corves C, Hunger H, Schmid F, et al. A Meta-Analysis of Head-to-Head Comparisons of Second-Generation Antipsychotics in the Treatment of Schizophrenia. Am J Psychiatry. 2009;166:152–63.
32 Lindenmayer JP, Khan A, Iskander A, Abad MT, Parker B. A randomized controlled trial of olanzapine versus haloperidol in the treatment of primary negative symptoms and neurocognitive deficits in schizophrenia. J Clin Psychiatry. 2007;68:368–79.
33 Choong E, Bondolfi G, Etter M, Jermann F, Aubry JM, Bartolomei J, et al. Psychotropic drug-induced weight gain and other metabolic complications in a Swiss psychiatric population. J Psychiatr Res. 2012;46:540–8.
34 Degner D, Grohmann R, Kropp S, Rüther E, Bender S, Engel RR, et al. Severe adverse drug reactions of antidepressants: results of the German multicenter drug surveillance program AMSP. Pharmacopsychiatry. 2004;37(Suppl 1):S39–S45.
35 Preskorn SH, Jerkovich GS. Central nervous system toxicity of tricyclic antidepressants: phenomenology, course, risk factors, and role of therapeutic drug monitoring. J Clin Psychopharmacol. 1990;10:88–95.
36 Peretti S, Judge R, Hindmarch I. Safety and tolerability considerations: tricyclic antidepressants vs. selective serotonin reuptake inhibitors. Acta Psychiatr Scand Suppl. 2000;403:17-25.
37 Bezchlibnyk-Butler K, Aleksic I, Kennedy SH. Citalopram – a review of pharmacological and clinical effects. J Psychiatry Neurosci. 2000;25:241–54.
38 Llorca PM, Fernandez JL. Escitalopram in the treatment of major depressive disorder: clinical efficacy, tolerability and cost-effectiveness vs. venlafaxine extended-release formulation. Int J Clin Pract. 2007;61(4):702–10.
39 Garnock-Jones KP, McCormack PL. Escitalopram: a review of its use in the management of major depressive disorder in adults. CNS Drugs. 2010;24:769–96.
40 Arif H, Buchsbaum R, Weintraub D, Koyfman S, Salas-Humara C, Bazil CW, et al. Comparison and predictors of rash associated with 15 antiepileptic drugs. Neurology. 2007;68:1701–9.
41 Yang CY, Dao RL, Lee TJ, Lu CW, Yang CH, Hung SI, et al. Severe cutaneous adverse reactions to antiepileptic drugs in Asians. Neurology. 2011;77:2025–33.
42 Kumra S, Oberstar JV, Sikich L, Findling RL, McClellan JM, Vinogradov S, et al. Efficacy and tolerability of second-generation antipsychotics in children and adolescents with schizophrenia. Schizophr Bull. 2008;34:60–71.
43 Lewis R. Typical and atypical antipsychotics in adolescent schizophrenia: efficacy, tolerability, and differential sensitivity to extrapyramidal symptoms. Can J Psychiatry. 1998;43:596–604.
44 Sajatovic M, Ramsay E, Nanry K, Thompson T. Lamotrigine therapy in elderly patients with epilepsy, bipolar disorder or dementia. Int J Geriatr Psychiatry. 2007;22:945–50.
45 Kando JC, Tohen M, Castillo J, Zarate CA, Jr. The use of valproate in an elderly population with affective symptoms. J Clin Psychiatry. 1996;57:238–40.
46 Arif H, Svoronos A, Resor SR, Jr., Buchsbaum R, Hirsch LJ. The effect of age and comedication on lamotrigine clearance, tolerability, and efficacy. Epilepsia. 2011;52:1905–13.
47 Madhusoodanan S, Brenner R, Suresh P, Concepcion NM, Florita CD, Menon G, et al. Efficacy and tolerability of olanzapine in elderly patients with psychotic disorders: a prospective study. Ann Clin Psychiatry. 2000;12:11–8.
48 Kisely S, Cox M, Campbell LA, Cooke C, Gardner D. An epidemiologic study of psychotropic medication and obesity-related chronic illnesses in older psychiatric patients. Can J Psychiatry. 2009;54:269–74.
Funding / potential competing interests: The authors declare that they have no competing interests. The AMSP Drug Safety Programme is organised by the non-profit associations, the German, Austrian and Swiss Society of Drug Safety in Psychiatry. Almost all pharmaceutical companies involved in CNS research contribute financial support to the three associations, but they have no influence on the publication.
Authors’ contributions:
WG initiated the study, developed the concept and revised the various versions of the manuscript.
AH drew the data out of the AMSP data pool, performed the statistical analyses and drafted the various versions of the manuscript.
TS performed a pre-study and described the results concerning age and adverse effects, and checked the final version of the manuscript.
RG is the project coordinator of AMSP and counselled the analysis and the interpretation of the data.
PB supported and supervised the various versions of the manuscript.