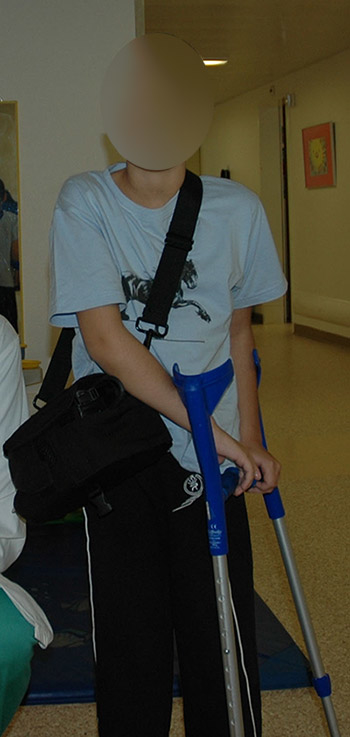
Figure 1
Eight-year old child provided with a Heartware HVAD (third-generation continuous flow device) as a bridge to candidacy.
DOI: https://doi.org/10.4414/smw.2013.13804
Hospitalisation of children suffering from heart failure due to congenital heart disease is increasing [1] and heart transplantation (HTx) remains the treatment of choice although it is limited by the shortage of suitable organ donors.
Various initiatives have been introduced to overcome donor organ shortage [2], but children still remain at an increased risk of death on the waiting list for heart transplantation, as a result of the rarity of small donor hearts in particular [3]. Identifying all potential brain-dead donors and expanding the donor pool on an international level is thus of vital importance, especially for smaller countries like Switzerland. Experiences with these international exchange programmes have been published [4]. All these efforts have, however, not resulted in a decrease in time on the waiting list. As a result of the uncertainty and the time required to obtain a donor heart, prolonged mechanical circulatory support (MCS) has become mandatory to avoid death on the waiting list (bridge to transplantation [BTT]).
Ventricular assist devices (VADs) have evolved owing to the invention of advanced biocompatible materials, versatile technologies and improved patient management algorithms, and are now widely accepted in adult practice [5, 6]. However, their use in children is still limited for several reasons. Firstly, there is limited experience with paediatric VADs, because in most major trials involving VADs children have been excluded. In addition, there are differences between children and adults in the pathophysiology of heart failure. Studies have been published which demonstrate that congenital aetiology is associated with a significantly higher postoperative mortality in paediatric VAD patients [7, 8]. The substantial incidence of single ventricles and Fontan circulations amongst paediatric patients with end-stage heart failure poses a unique challenge for paediatric MCS. This may lead to a less aggressive approach to initiating VADs in children in many institutions; most centres have experience with extracorporeal membrane oxygenation (ECMO), mainly for postcardiotomy failure, as a bridge to recovery (BTR). ECMO has been the mainstay for small children with heart or respiratory failure, with excellent outcomes for short-term support. Evidence shows that after 2 to 3 weeks of support serious complications such as bleeding occur [9], leading to an inferior outcome compared with a shorter support time. A rate of survival to hospital discharge of less than 50% with ECMO support in children has been reported, as well as a reduction of waiting list mortality from 38% on ECMO to 13% with VAD use [10]. Another point to consider is that ECMO use leads to immobilisation of the patient and the patient must remain on the intensive care unit (ICU). Therefore VAD implantation should be considered if long-term support is anticipated and mobilisation of the patient is intended.
Secondly, although a large variety of adult sized VADs have proven to be safe for long-term support [6], only a small number of VADs are available for patients with a body surface area (BSA) of less than 1.2 m2 or weight less than 20 kg [11]. Thirdly, whereas in adult patients the number of biventricular assist device (BVAD) implantations is declining [5, 12], the incidence of biventricular failure among children remains high, ranging from 29% to 43% [9, 11]. BVAD has been identified as independent risk factor for mortality [11].
In 1967, DeBakey used a left atrial to axillary artery VAD in a 16-year-old girl suffering from postcardiotomy failure after mitral valve replacement [13]. During the following decades, modifications of the original “heart-lung machine” such as ECMO or extracorporeal centrifugal pumps [14], have been the principal form of cardiac support. As newer indications requiring long-term circulatory support evolved, together with longer waiting times for donor organs, the need for long-term MCS became evident. In 1989, Frazier implanted a mechanical assist device in a nine-year-old boy, who was successfully bridged to heart transplantation with a Biomedicus (Medtronic, Eden Prairie, MN) centrifugal pump; the support time was twelve hours. In 1990, the first Berlin Heart Excor, an adult size 50-ml pump, was implanted in a nine-year-old child for 1 week with an uneventful postoperative period after heart transplantation [15]. Two years later, in 1992, pumps sized 10 ml, 25 ml and 30 ml were devised, and the 10-ml pump was implanted in a 12-month-old child [16]. Two years later, the first Medos VAD (Medos Medizintechnik GmbH, Stolberg, Germany) was implanted successfully as a bridge to transplantation [17]. Availability of implantable continuous-flow left ventricular assist devices (LVADs) such as the Heart Mate II (Thoratec Corp., Pleasanton, CA) led to implantation in intermediate-size children [18]. However, evidence showed that incidences of thromboembolic and haemorrhagic neurological events in children differed from those in adults [7, 19–20], and that patient size, device selection and postimplant anticoagulation regimens needed to be tailored, as paediatric patients are not simply small adults.
Historically, MCS was developed for BTT; however, in patients with myocarditis, or after cardiotomy, where a recovery of ventricular function is expected, MCS for days to weeks during the critical interval may be as necessary as it is for BTT. If a fast recovery is expected, ECMO remains the mainstay of support. If a contraindication for HTx, such as pulmonary hypertension or a successfully treated malignancy with a too short observational period for HTx, is diagnosed, a concept known as bridge to transplantability should be considered. For this indication, a VAD designed for long-term support is necessary. Successful HTx after VAD support in patients diagnosed with fixed pulmonary hypertension [21–22], in children or teenagers with chemotherapy-induced cardiomyopathy (CMP), and successful bridge to transplantability [23] or recovery using long-term VADs have been published [24–26]; in our own experience an eight-year-old child suffering from anthracycline-induced CMP could be provided with an intracoporeal VAD for bridge to transplantability (fig. 1).

Figure 1
Eight-year old child provided with a Heartware HVAD (third-generation continuous flow device) as a bridge to candidacy.
Patient selection and timing remain crucial factors for improving outcomes in VAD recipients. Children who have critical peripheral perfusion (i.e. metabolic acidosis, cardiac index <2.0 l/m2/min, mixed venous oxygen saturation <40%) despite inotropic support and early signs of renal, hepatic or multiorgan failure, and for whom there are no surgical options to correct any residual structural lesions should be considered for MCS. If a fast recovery can be expected, and in most cases of postcardiotomy failure, ECMO support will be the first choice. For children suffering from chronic cardiomyopathy or when a fast recovery cannot be expected, VAD implantation should be evaluated. There are only a few contraindications for MCS, such as ongoing malignant neoplastic diseases with a very limited life expectancy. Further contraindications are advanced multiorgan failure, complex congenital heart lesions involving intracardiac shunts or irreversible pulmonary failure, and severe extracardiac malformations such as chromosomal and genetic syndromes with a poor quality-of-life prognosis. It has to be kept in mind that if VAD implantation is performed the child must be suitable for HTx if weaning off VAD is not possible.
There is evidence that a decision in favour of earlier VAD implantation results in a better outcome, especially in children under 1 year of age [27].
In the our Department for Congenital Cardiovascular Surgery at the Children’s Hospital Zurich, we try to differentiate between acute and chronic heart failure; for children under inotropic support without the possibility to wean them off or in the case of a deterioration of clinical status, MCS is evaluated. If there are no absolute contraindications, MCS will be initiated; once again, if a fast recovery may be expected (i.e. acute myocarditis or postcardiotomy failure) ECMO will be used. In chronic heart failure, a VAD will be implanted; on the basis of the BSA and the right ventricular (RV) function of the child either an intracoporeal VAD (BSA >0.6 m2 and acceptable RV function) or an extracorporeal VAD (BSA <0.6 m2 or severe biventricular failure) will be chosen.
Currently there are only two VADs designed for children with a body surface area below 1.2 m2: the Medos HIA and the Berlin Heart Excor.
Figure 2
The Berlin Heart Excor is suitable for children of all sizes and with biventricular failure.
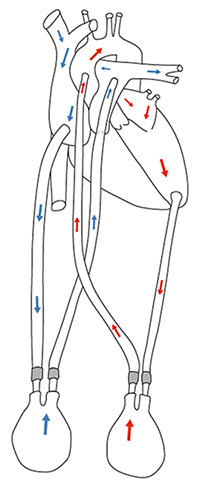
Figure 3
Schema of the BVAD Berlin Heart (blue arrows = venous blood flow, red arrows = arterial blood flow). (Property of Julia Schrempf, Graz, Austria.)
The Medos HIA (Medos Medizintechnik AG, Stolberg, Germany) is a miniaturised, extracorporeal, pneumatically driven system suitable for children and adults for left, right or biventricular support. Support times of over 30 days have been reported with good results, but this device does not allow the patient to be discharged home [17, 28].
Most data are available for the Berlin Heart Excor system (Berlin Heart AG, Berlin, Germany) (figs 2 and 3); it was specifically designed for small children. It is a paracorporeal, pulsatile, pneumatically driven VAD for univentricular or biventricular support. The system offers a range of pumps with valves divided into a blood and air chamber and silicone cannula, for every body size between 3 kg and adult size. The pump consists of a translucent, semi-rigid housing of polyurethane. In 1990, the system was first successfully used in a 8-year-old child at the Berlin Heart Institute, Germany [15]. The Berlin group gained great experience with the EXCOR even in neonates, and reported 18 patients less than 1 year of age supported by EXCOR (9 LVADs, 9 BVADs) with a survival rate of 70% [29]. Although the EXCOR paediatric VAD has been approved in Europe and used since the 1990s, thanks to regulatory hurdles experience in the USA was limited until the beginning of 2000. Morales and colleagues reported the initial North American experience in 73 patients from 2000 to 2004; the majority of patients were suffering from dilated cardiomyopathy (58%) or congenital heart disease (26%); age ranged from 12 days to 17.8 years; the lowest patient weight was 3 kilograms. Extracorporeal membrane oxygenation was used as a bridge to EXCOR in 22 patients (30%); a LVAD was implanted in 57% and a BVAD in 43%. Overall mortality on the EXCOR was 23%. The authors concluded that younger age and BVAD use were significant risk factors for death while on the EXCOR [11]. Recently Fraser et al. published the results of the US investigational device exemption (IDE) multicentre trial, which examined the safety and efficacy of the device [30]. The study group compared the EXCOR with a historical ECMO group and found that survival rates were significantly higher with the EXCOR. Serious adverse events, including infection, stroke and bleeding, were reported, with 0.07 events per patient-day in the VAD group and with 0.08 events per patient-day in the ECMO group [9].
Several VADs designed for adults have been adopted for use in children. The Heartmate II has been used in teenagers between 12 and 18 years old in various institutions [18]. The extracorporeal Thoratec VADs (Thoratec Corporation, Pleasanton, USA) can be implanted in adults and children. Like the Excor, it is a pneumatically driven pulsatile VAD available for patients with a body-surface area down to 0.7 m2 [31]. In adults, 69% of Paracorporeal Ventricular Assist Device (PVAD) patients were successfully supported to HTx or device explantation (http://www.thoratec.com/vad-trials-outcomes/clinical-outcomes/thoratec-pvad.aspx). When using the Thoratec TLC-II portable drive for these patients, successful home discharge was reported [32]. There are no major trials investigating the safety and effectiveness of Thoratec VADs in the paediatric population. Reinhartz and colleagues reported PVAD support for up to 120 days in a patient population with a BSA <1.3 m2. In this study congenital heart disease was associated with increased mortality [33].
The worldwide first clinically implanted axial flow pump was the MicroMed DeBakey VAD [34]. This gained FDA approval as DeBakey VAD Child (MicroMed Technology Inc., Houston, TX) for children older than 5 years or a with body surface area between 0.7 and 1.5 m2. The first successful BTT after 56 days on support with the Debakey VAD Child was in a 14-year-old boy [35]; further implantations were reported, with the smallest child being 6 years old [36]. Very aggressive anticoagulation was necessary to prevent pump thrombosis in the DeBakey VAD Child [37]. Today the DeBakey VAD Child is known as the HeartAssist 5 Paediatric VAD (fig. 4).
Recently an Italian physician team implanted a tiny, 11-gram implantable pump, invented by Dr. Jarvik and previously only tested on animals, in a 16-month-old baby, keeping the baby alive for 13 days before a switch back to the Berlin Heart was necessary because of technical problems (http://abcnews.go.com/blogs/health/2012/05/24/baby-saved-by-smallest-artificial-heart/ http://abcnews.go.com/blogs/health/2012/05/24/baby-saved-by-smallest-artificial-heart/ ).
One of the newest devices is the Heartware HVAD (HeartWare Inc., Framingham, MA, USA) (see fig. 1). It is a third generation rotary blood pump with a magnetically levitated rotor. It was initially designed for left ventricular support [38], but owing to its small size is also used as implantable BVAD [39]. Because of its size it is suitable for children to support congenital or acquired systemic circulation [40]. Miera et al. reported their experience in seven patients, the youngest being 6 years of age, with a median support time of 75 days and a 86% success rate of bridging to HTx [41]. First use of this VAD in children in the USA has already been reported also [42].
Alongside the paracorporeal VADs, total artificial hearts (TAHs) have been developed for biventricular failure. In contrast to adults, in whom the use of BVADs is declining [5, 12], the need for BVADs in children remains high [9, 11]. When implanting a TAH, the native heart is completely excised. The Cardiowest (SynCardia, Tucson, AZ, USA) (fig. 5) was approval for BTT [43] but cannot be used in small children owing to its size. Nevertheless it remains an option for larger children; Leprince reported good results in patients with a BSA below 1.7 m2 [44]. To date, worldwide there are 21 Cardiowest implants in patients ranging from 13 to 17 years of age (personal communication). Besides the Cardiowest, the AbioCor artificial heart (Abiomed, Danvers, MA, USA) has evolved as TAH and human implants have been successfully used, including in a paediatric patient (18 years) [45] (http://www.youtube.com/watch?v=oixUVlf5h9U). Even if TAHs are not a valid option for small children, intracorporeal VAD implantation is tried to achieve better quality of life.
Thromboembolic events in children supported with VAD remain serious adverse events and differ from those in adults [7, 19–20]. No standard anticoagulation protocol has been developed so far and anticoagulation is tailored to different types of VAD and individualised by different centres. To achieve a balance between minimising thromboembolic events and causing bleeding complications, an anticoagulation strategy involving the international normalised ratio (INR), the thrombocyte aggregation test (TAT) and thrombelastography (TEG) has been proposed. The USA investigational device trial for the EXCOR agreed on the Edmonton protocol, which proposes a three-drug regimen involving aspirin, persantine and enoxaparin or oral anticoagulation [11]. In the immediate postoperative period, unfractionated heparin is given guided by activated partial thromboplastin time (aPTT; target approximately 1.5 times the baseline) or anti-factor Xa. After removal of invasive lines and drains, long-term anticoagulation with warfarin (target INR from 2.5 to 3.5 depending on device type) and additional antiplatelet therapy can be started. Antiplatelet therapy including aspirin and persantine is monitored with TAT and TEG, respectively.
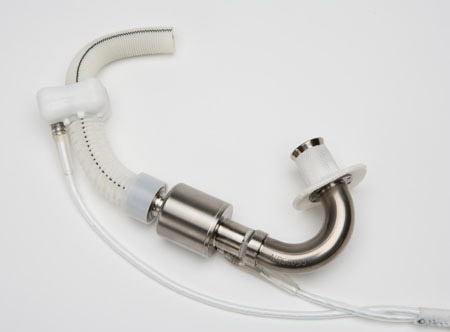
Figure 4
The HeartAssist 5 (MicroMed Technology Inc, Houston, TX) formerly known as DeBakey Child, usable in children down to 5 years, is the only ventricular assist device measuring the actual flow using a flow probe. (http://www.micromedcv.com/united_states/heart-assist-5/small_and_light.html).
Children supported by VAD and not showing signs of cardiac recovery (see also below “Bridge to recovery”) while on support will undergo paediatric HTx. Time on the HTx waiting list is increasing in all European countries regardless of patient age. Within the last decade, VADs in adults have evolved dramatically with the introduction of continuous flow devices, and permanent support, also known as destination therapy, has become real for many adults owing to the excellent 2- year survival rates on LVAD support [46]. Permanent support is still not a valid option in children; nevertheless VAD support times are increasing. Results of paediatric HTx are excellent [47–48], even after VAD support [8, 49]. Jeewa et al. reported that survival during the period from HTx to hospital discharge was better after VAD support (92%) than with ECMO (80%) [8, 10].
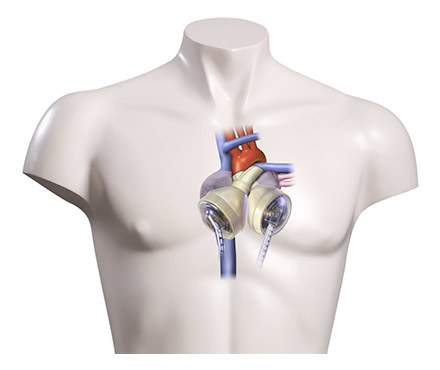
Figure 5
The Cardiowest is a Total Artificial Heart provided by Snycardia (Tucson, AZ, USA). (http://www.syncardia.com/Medical-Professionals/compare-to-bivads.html).
Some patients supported with long-term VAD show marked improvement of ventricular function during MCS, but only a small number of patients undergo device explantation. The process of myocardial recovery is still not completely understood and is dependent on various factors, including age, initial diagnosis, time from diagnosis to VAD implantation, and pulsatile or continuous-flow VAD [50]. The basic mechanism of myocardial recovery seems to be the unloading of the left ventricle leading to molecular reverse remodelling, decreased neurohumonal and cytokine activation, as well as normalisation of cytoskeletal integrity, a reduction in the size of myocytes and a decrease in the extracellular matrix [51, 52]. Additional pharmacological treatment with a selective β2-agonist such as clenbuterol [53] or intraoperative application of stem cells may enhance myocardial recovery. Protocols for long-term device weaning and explantation have been developed for adult patients [54–56]. During VAD support frequent echocardiography studies examining the effects of unloading on LV size, shape and contractility should be evaluated. If the LV wall motion and diameters show improvement so-called “pump-stop echocardiography” and if needed “pump-stop heart catheterisation” (continuous LVAD: blocking the outflow graft of the LVAD) may be carried out [54]. Under sufficient anticoagulation (check before “pump stop”!) and additional heparin bolus if possible VAD support is reduced and finally stopped for some minutes (3–5 minutes). Criteria for long-lasting myocardial recovery and possible LVAD explantation have been reported: sinus rhythm, absence of or trivial mitral regurgitation, left ventricular ejection fraction (LVEF) >45% and left ventricular end-diastolic diameter (LVEDD) <55 mm [57]. These “pump-stop” examinations may be repeated a few times but involve in-hospital observation for three days (one day prior to pump-stop, next day pump-stop, one further day observation). In children, experience with explantation after long-term VAD support is very limited.
In children of up to 0.7 m2 body surface area, the Berlin Heart Excor paediatric ventricular assist device remains the only VAD for long-term support. Improvements in the existing paediatric VADs, as well as technical developments and miniaturisation leading to third-generation VADs, offer new perspectives. Third-generation continuous flow devices like the HVAD achieved excellent results even in support times of over 365 days. As anticoagulation regimens evolve, minimising thromboembolic events and bleeding complications remains one of the core issues of a paediatric VAD programme.
1 Adachi I, C.D. Fraser, Jr., Mechanical circulatory support for infants and small children. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2011;14(1):38–44.
2 Schweiger M, et al. Improving the rate of organ donation. Transplant Proc. 2004;36(9):2543–5.
3 Almond CS, et al. Waiting list mortality among children listed for heart transplantation in the United States. Circulation. 2009;119(5):717–27.
4 Schneider S, et al. Assessing the potential of international organ exchange – the Swiss experience. Eur J Cardiothorac Surg. 2011;40(6):1368–73.
5 Krabatsch T, et al. Mechanical circulatory support-results, developments and trends. J Cardiovasc Transl Res. 2011;4(3):332–9.
6 Krabatsch T, et al. Improvements in implantable mechanical circulatory support systems: literature overview and update. Herz. 2011;36(7):622–9.
7 Reinhartz O, et al. Multicenter experience with the thoratec ventricular assist device in children and adolescents. J Heart Lung Transplant. 2001;20(4):439–48.
8 Blume ED, et al. Outcomes of children bridged to heart transplantation with ventricular assist devices: a multi-institutional study. Circulation. 2006;113(19):2313–9.
9 Fraser CD, Jr., et al. Prospective trial of a pediatric ventricular assist device. N Engl J Med. 2012;367(6):532–41.
10 Jeewa A, et al. Outcomes with ventricular assist device versus extracorporeal membrane oxygenation as a bridge to pediatric heart transplantation. Artif Organs. 2010;34(12):1087–91.
11 Morales DL, et al. Bridging children of all sizes to cardiac transplantation: the initial multicenter North American experience with the Berlin Heart EXCOR ventricular assist device. J Heart Lung Transplant. 2011;30(1):1–8.
12 Kirklin JK, et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant. 2012;31(2):117–26.
13 DeBakey ME. Left ventricular bypass pump for cardiac assistance. Clinical experience. Am J Cardiol. 1971;27(1):3–11.
14 Karl TR, Horton SB, Brizard C. Postoperative support with the centrifugal pump ventricular assist device (VAD). Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006: p. 83–91.
15 Warnecke H, et al. Mechanical left ventricular support as a bridge to cardiac transplantation in childhood. Eur J Cardiothorac Surg. 1991;5(6):330–3.
16 Hetzer R, et al. Mechanical cardiac support in the young with the Berlin Heart EXCOR pulsatile ventricular assist device: 15 years’ experience. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006: p. 99–108.
17 Konertz W, et al. Clinical experience with the MEDOS HIA-VAD system in infants and children: a preliminary report. Ann Thorac Surg. 1997;63(4):1138–44.
18 Owens WR, et al. Initial clinical experience with the HeartMate II ventricular assist system in a pediatric institution. Artif Organs. 2010;34(7):600–3.
19 Fan Y, et al. Outcomes of ventricular assist device support in young patients with small body surface area. Eur J Cardiothorac Surg. 2011;39(5):699–704.
20 Reinhartz O, et al. Thoratec ventricular assist devices in pediatric patients: update on clinical results. ASAIO J. 2005;51(5):501–3.
21 Mikus E, et al. Reversibility of fixed pulmonary hypertension in left ventricular assist device support recipients. Eur J Cardiothorac Surg. 2011;40(4):971–7.
22 Martin J, et al. Implantable left ventricular assist device for treatment of pulmonary hypertension in candidates for orthotopic heart transplantation-a preliminary study. Eur J Cardiothorac Surg. 2004;25(6):971–7.
23 Potapov EV, et al. Bridging to transplantability with a ventricular assist device. J Thorac Cardiovasc Surg. 2005;130(3):930.
24 Castells E, et al. Recovery of ventricular function with a left ventricular axial pump in a patient with end-stage toxic cardiomyopathy not a candidate for heart transplantation: first experience in Spain. Transplant Proc. 2009;41(6):2237–9.
25 Freilich M, et al. Recovery from anthracycline cardiomyopathy after long-term support with a continuous flow left ventricular assist device. J Heart Lung Transplant. 2009;28(1):101–3.
26 Khan N, et al. Remission of chronic anthracycline-induced heart failure with support from a continuous-flow left ventricular assist device. Tex Heart Inst J. 2012;39(4):554–6.
27 Potapov EV, Stiller B, Hetzer R. Ventricular assist devices in children: current achievements and future perspectives. Pediatr Transplant. 2007;11(3):241–55.
28 Kaczmarek I, et al. Mechanical circulatory support in infants and adults with the MEDOS/HIA assist device. Artif Organs. 2005;29(10):857–60.
29 Stiller B, et al. Pneumatic pulsatile ventricular assist devices in children under 1 year of age. Eur J Cardiothorac Surg. 2005;28(2):234–9.
30 Almond CS, et al. Berlin Heart EXCOR Pediatric ventricular assist device Investigational Device Exemption study: study design and rationale. Am Heart J. 2011;162(3):425–35 e6.
31 Hill JD, Reinhartz O. Clinical outcomes in pediatric patients implanted with Thoratec ventricular assist device. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006: p. 115–22.
32 Slaughter MS, et al. Home discharge experience with the Thoratec TLC-II portable driver. ASAIO J. 2007;53(2):132–5.
33 Reinhartz O, Copeland JG, Farrar DJ. Thoratec ventricular assist devices in children with less than 1.3 m2 of body surface area. ASAIO J. 2003;49(6):727–30.
34 Potapov EV, et al. Pulsatile flow in patients with a novel nonpulsatile implantable ventricular assist device. Circulation. 2000;102(19 Suppl 3): p. III183–7.
35 Imamura M, et al. The first successful DeBakey VAD child implantation as a bridge to transplant. ASAIO J. 2005;51(5):670–2.
36 Morales DL, et al. Lessons learned from the first application of the DeBakey VAD Child: an intracorporeal ventricular assist device for children. J Heart Lung Transplant. 2005;24(3):331–7.
37 Fraser CD, Jr., et al. Preliminary experience with the MicroMed DeBakey pediatric ventricular assist device. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006: p. 109–14.
38 Wieselthaler GM, et al. Initial clinical experience with a novel left ventricular assist device with a magnetically levitated rotor in a multi-institutional trial. J Heart Lung Transplant. 2010;29(11):1218–25.
39 Krabatsch T, et al. Biventricular circulatory support with two miniaturized implantable assist devices. Circulation. 2011;124(11 Suppl):S179–86.
40 Huebler M, et al. Mechanical circulatory support of systemic ventricle in adults with transposition of great arteries. ASAIO J. 2012;58(1):12–4.
41 Miera O, et al. First experiences with the HeartWare ventricular assist system in children. Ann Thorac Surg. 2011;91(4):1256–60.
42 D’Alessandro D, et al. First reported use of the heartware HVAD in the US as bridge to transplant in an adolescent. Pediatr Transplant. 2012.
43 Copeland JG, et al. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med. 2004;351(9):859–67.
44 Leprince P, et al. Patients with a body surface area less than 1.7 m2 have a good outcome with the CardioWest Total Artificial Heart. J Heart Lung Transplant. 2005;24(10):1501–5.
45 Morreim EH, et al. Innovation in human research protection: the AbioCor artificial heart trial. Am J Bioeth. 2006;6(5):W6–16.
46 Kirklin JK, et al. Long-term mechanical circulatory support (destination therapy): on track to compete with heart transplantation? J Thorac Cardiovasc Surg. 2012;144(3):584–603; discussion 597–8.
47 Rossano JW, et al. Effect of body mass index on outcome in pediatric heart transplant patients. J Heart Lung Transplant. 2007;26(7):718–23.
48 Aurora P, et al. The Registry of the International Society for Heart and Lung Transplantation: thirteenth official pediatric lung and heart-lung transplantation report – 2010. J Heart Lung Transplant. 2010;29(10):1129–41.
49 Alba AC, et al. The effect of ventricular assist devices on long-term post-transplant outcomes: a systematic review of observational studies. Eur J Heart Fail. 2011;13(7):785–95.
50 Krabatsch T, et al. Is bridge to recovery more likely with pulsatile left ventricular assist devices than with nonpulsatile-flow systems? Ann Thorac Surg. 2011;91(5):1335–40.
51 Bruggink AH, et al. Reverse remodeling of the myocardial extracellular matrix after prolonged left ventricular assist device support follows a biphasic pattern. J Heart Lung Transplant. 2006;25(9):1091–8.
52 Mohapatra B, et al. Short-term mechanical unloading and reverse remodeling of failing hearts in children. J Heart Lung Transplant. 2010;29(1):98–104.
53 Birks EJ, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355(18):1873–84.
54 Potapov EV, Schweiger M, Krabatsch T. Percutaneous balloon occlusion of a left ventricular assist device outflow cannula to facilitate evaluation of myocardial recovery. J Heart Lung Transplant. 2011;30(11):1300–1.
55 Dandel M, et al. Prediction of cardiac stability after weaning from left ventricular assist devices in patients with idiopathic dilated cardiomyopathy. Circulation. 2008;118(14 Suppl):S94–105.
56 Liang H, et al. Prediction of cardiac function after weaning from ventricular assist devices. J Thorac Cardiovasc Surg. 2005;130(6):1555–60.
57 Dandel M, et al. Long-term results in patients with idiopathic dilated cardiomyopathy after weaning from left ventricular assist devices. Circulation. 2005;112(9 Suppl):I37–45.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.