Tobacco-related cancer mortality: projections for different geographical regions in Switzerland
DOI: https://doi.org/10.4414/smw.2013.13771
Verena
Jürgens, Silvia
Ess, Harish C.
Phuleria, Martin
Früh, Matthias
Schwenkglenks, Harald
Frick, Thomas
Cerny, Penelope
Vounatsou
Summary
PRINCIPLES: Switzerland is divided into 26 cantons of variable population size and cultural characteristics. Although a federal law to protect against passive smoking and a national tobacco control programme exist, details of tobacco-related policies are canton-specific. This study aimed to project gender-specific tobacco-related cancer mortality in Switzerland at different geographical levels for the periods 2009–2013 and 2014–2018.
METHODS: In this analysis, data on Swiss tobacco-related cancer mortality from 1984 until 2008 were used. Bayesian age-period-cohort models were formulated to assess past trends of gender-specific tobacco-related cancer mortality and to project them up to 2018 at cantonal and language region levels. Furthermore, estimates are provided on a national scale by age categories of 50–69 and ≥70 years.
RESULTS: Model-based estimates at cantonal level identified regions with low and high tobacco-related cancer mortality rates for the observed and projected periods. Our analysis based on language regions showed the lowest mortality in the German-speaking part. Projections at national level, between younger (age 50–69) and older (age ≥70) males, indicated an ongoing decreasing trend for males but an upward trend for females. The gap in tobacco-related cancer mortality rates between younger and older males seems to be shrinking. In females, a stronger rise was obtained for the younger age group.
CONCLUSION: Our findings indicate region-, sex- and age-related differences in tobacco-related cancer mortality in Switzerland and this could be useful for healthcare planning and for evaluating the impact of canton-specific tobacco-related policies and interventions.
Introduction
Tobacco-smoking is a major cause of premature mortality. In 1990, around three-quarters of a million deaths in the age group 35–69 years were attributable to tobacco smoking in Europe [1]. In fact, smoking accounts for more deaths than traffic-accidents, acquired immunodeficiency syndrome (AIDS), alcohol, cocaine, homicide, suicide and fires together [2]. Smoking cessation reduces the hazard substantially in the younger age group [3]. Smoking behaviour has changed over the last decades. In Switzerland, smoking prevalence decreased during 1992 and 2007 in both genders [4]. Several factors may have contributed to this trend, including prevention programmes and greater sensitivity to mortality from tobacco-related illness in males [5]. Smoking can be supposedly used to balance stress and younger women especially use smoking to control or even suppress appetite. On average, the weight of smokers is 3–4 kg lower than for nonsmokers [6].
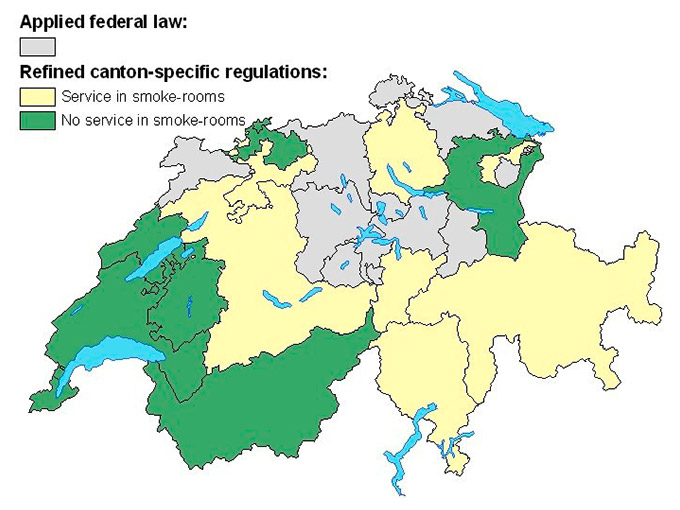
Figure 1
Canton-specific smoking ban regulations indicating application of the federal law (”Bundesgesetz zum Schutz vor Passivrauchen”) (in grey), further regulations covering service in smoke-rooms (in yellow) and no service in smoke-rooms (in green).
Reports from the International Agency for Research on Cancer showed that in 2008 21.9% of all male cancer deaths in Switzerland were related to the lung, whereas the proportion was 12.7% for Swiss women. Smoking is the major risk factor for the development of lung cancer, but it also causes cancers at other sites. Smoking may have an effect on cancers of the larynx and the pharynx [7]. Parental smoking may induce childhood cancer, but studies showing evidence of a definite association are still lacking. In addition, there is strong evidence of a causal association between passive smoking and lung cancer [8]. Doll and Peto [9] considered the following cancer sites to be related to tobacco: lung (C33–C34), oesophagus (C15), rectum (C20), pancreas (C25), bladder (C67) and cancer of other respiratory sites (C00–C14) including cancer of the lip, tongue, mouth, larynx, trachea and pharynx (excluding nasopharynx).
Switzerland is divided into 26 cantons, which vary in population size and cultural characteristics. Regulations, for example in health systems, are canton-specific, covering domains which are not controlled by the federal constitution. In 2010 a federal law to protect against passive smoking was enacted, aiming at smoke-free interiors in enclosed spaces that are publicly accessible (i.e. restaurants, bars, schools, hospitals etc.), and workplaces with two or more people. However, smoking-rooms still exist under certain conditions in many cantons as federal law just provides the minimum provisions for protection against passive smoking and stricter smoking-related laws are canton-dependent. Figure 1 shows the Swiss cantons according to their smoking ban regulations in restaurants in May 2012. All cantons applied the national law, which can be seen as a minimal protection against passive smoking, and some defined further regulations. These refinements either allow service only in those rooms indicated for smoking (‘smoke-rooms’) or prohibit any service in smoke-rooms.
Information about the trend of tobacco-related cancer mortality over time, as well as the spatial distribution of the disease, are essential for healthcare planning related to smoking behaviour. This can identify areas with high tobacco-specific cancer mortality. Projections of mortality rates are helpful tools for assessing the future burden of a disease and as a basis for evaluating the impact of interventions carried out. Model-based estimates can then be compared with observed data for the projection periods.
Age-period-cohort (APC) models are commonly used to project cancer mortality and incidence. With this approach, the dataset is stratified into three components separating age, the period when the event occurred and the birth cohort of the person. Typically, projections based on APC models are done at country level [10, 11]; however, estimates at subunits may be useful [12] especially when healthcare planning is decentralised as in the case of Switzerland.
This study aimed to project gender-specific tobacco-related cancer mortality in Switzerland at different geographical levels for the periods 2009–2013 and 2014–2018. Projected estimates at a cantonal level may identify high- or low-risk areas and help evaluate canton-specific smoking-related policies in the future. In addition, mortality data were aggregated over the three language regions (i.e. German, French and Italian) to assess trends among the regions which can be compared with trends in same language neighbouring countries – Germany, France and Italy. Furthermore, countrywide projections were estimated for specific age groups to determine the burden of tobacco-related cancer in the future on a larger scale and possibly identify age-specific differences.
Methods
Data sources and management
Mortality data at an individual level were provided by the Swiss Federal Statistical Office (FSO). Cancer death counts related to tobacco, following the definition by Doll and Peto [9], were used for the analysis. Tobacco-related cancer rarely occurs in persons younger than 50 years. Therefore, we excluded death counts of those of younger age. In 1995, priority given to certain causes of deaths was removed from rules related to coding death certificates. This change affected cause-specific reported deaths, but the FSO provides disease-related correction factors to adjust for the years before 1995 [13]. Estimated rates were (age) standardised directly, using Segi World population [14].
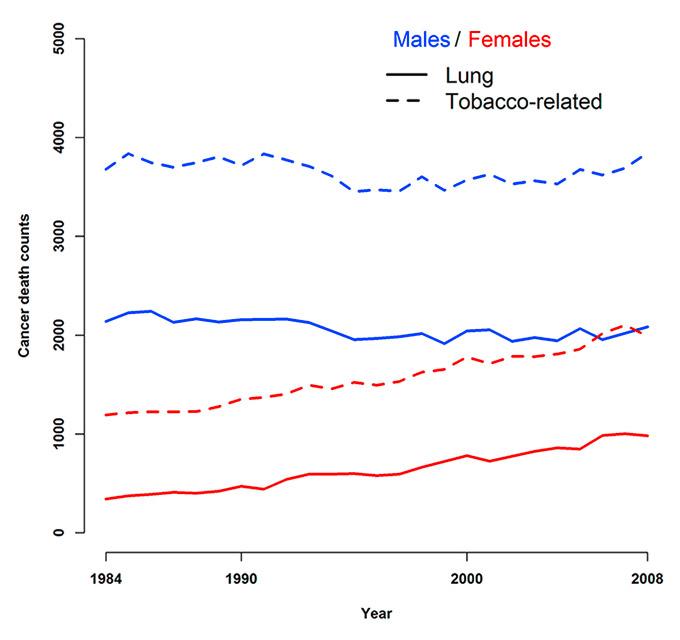
Figure 2
Overall lung and all tobacco-related cancer deaths observed during 1984–2008 at the national level for males and females.
Subgroup-specific population data at a cantonal level were obtained from the webpage of the FSO, covering the five 5-year time periods 1984–1988 to 2004–2008, as well as 2009–2013 and 2014–2018. The data for the latter time periods were estimated by the FSO assuming a mid-way scenario, based on past population trends and assuming no extreme changes (e.g. migration). Age and period were aggregated into five-year groups.
Cantons with a small number of cases, which did not allow for reliable estimates, were merged with their neighbours. These included canton Uri, Obwalden and Nidwalden, as well as Appenzell Ausserrhoden and Appenzell Innerrhoden. A map of Switzerland, as well as the cantonal abbreviations, are provided in Appendix A http://www.smw.ch/fileadmin/smw/pdf/SMW-13771-Appendix_A.pdf . ArcGIS, a geographic information system, was used to create maps illustrating the results. The required shapefile describing the spatial configuration of the cantons was obtained from the FSO and modified.
Statistical analysis
Bayesian Poisson and Negative Binomial APC regression models were formulated and fitted in WinBUGS (Imperial College and MRC, London, UK) using Markov chain Monte Carlo (MCMC) simulations. The mean of the age- and period-specific death counts μij was regressed on the effects of age, period and cohort. The general model applied for projecting at cantonal level reads as follows:
where nijk is the population for the age group (i), period (j) and region (k), α, β and γ account for the effect of age, period and cohort, respectively. Canton-specific random effects φk modelled via conditional autoregressive (CAR) specifications [15] were included to allow for spatial correlation.
Bayesian APC-models were applied to project age- and gender-specific tobacco-related cancer mortality for the periods 2009–2013 and 2014–2018 (1.) at a national level, (2.) for the three language regions, and (3.) at cantonal level. The models for countrywide and language-specific analyses did not consider spatial random effects. Results are presented for each gender and sub-region. In addition, countrywide estimates are given for the age categories 50–69 and ≥70 years. The predictive performance of the models was assessed by comparing model-based and observed data using the sum of squared residuals. In particular, models were fitted during 1984–1998 and predictions were obtained for the remaining period of 1999–2008. Implementation details and model evaluation can be found in Appendix B.
Results
Preliminary analysis suggested similar trends in lung and tobacco-related cancer mortality in Switzerland during 1984 to 2008 (fig. 2). Death counts for males were stable, while for females they increased steadily.
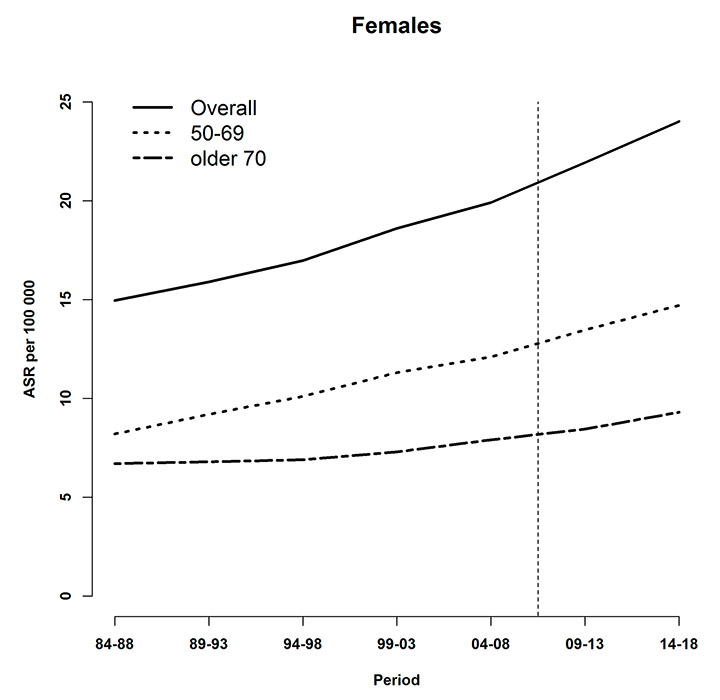
Figure 3
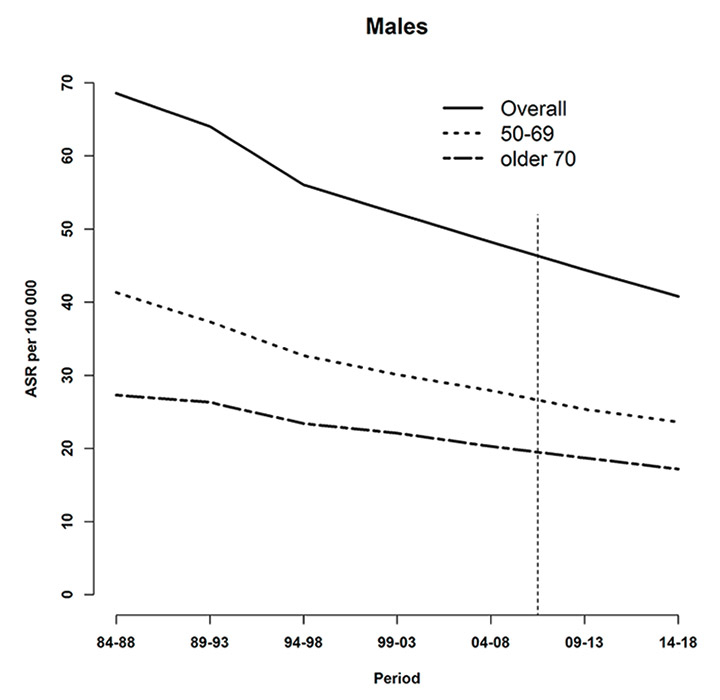
Age-specific and overall age-standardised tobacco-related cancer mortality rates (per 100,000), observed (during 1984–2008) and predicted (posterior median during 2009–2013 and 2014–2018) at the national level for (a) males and (b) females. The vertical line indicates the start of the prediction period.

Figure 4
Relative change in population (in % based on preceding year) for males (red) and females (blue) and age groups 50–69 (dashed) and ≥70 years.
Age- and gender-specific projections at national level
Figure 3 shows observed and predicted tobacco-related cancer mortality rates during 1984–2008 and 2009–2018, respectively, for each gender and age category. For both males and females, higher rates were observed for the younger age group.
For males, a decreasing trend was seen which was less pronounced from 1994–1998 onwards. On the other hand, projected absolute death counts increased during 2009–2013 and 2014–2018 (table 1), reflecting the demographic aging of the population (fig. 4).
The difference in mortality rates between younger and older males narrowed during the study periods. Overall, the mortality rate in males decreased by approximately 30%.
Tobacco-related cancer mortality for Swiss women steadily increased during the study period. In contrast to males, model-based estimates indicated that the difference of mortality rates between younger and older females increased. The projected rates in 2014–2018 indicate that the female tobacco-related cancer mortality rates rose by more than 60% during the whole study period.
Gender-specific projections for the language regions
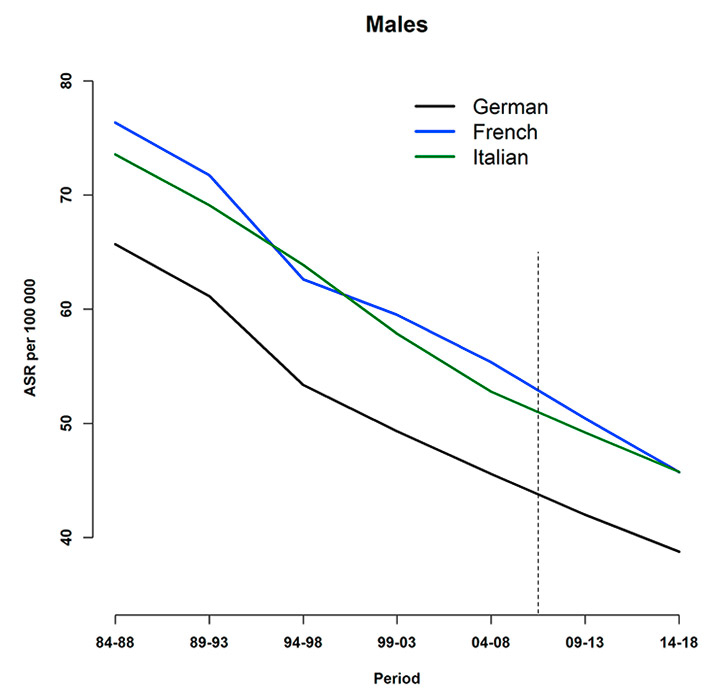
Figure 5 shows the trends for gender-specific tobacco-related cancer mortality rates by language region. For males, the lowest rates were observed for the German-speaking region. No significant differences were observed for the rates in the French- and Italian-speaking part for either sex. Considering cause-specific mortality rates for females, the gap between the lowest (German-speaking) and the highest (French-speaking) region-specific rates clearly decreased during the 30 study years. The German-speaking region did show significantly lower rates up to 2008, but estimates for the projected periods indicate no significant difference between the two regions. In 1984–1988 the rates for the Italian-speaking area were not significantly different from those in the German-speaking one. Subsequently, however, a significantly higher tobacco-related cancer mortality rate for females was observed in the Italian-speaking part. Again, this can not be said for the projected periods.
Gender-specific projections at cantonal level
Figures 6 and 7 show the observed gender-specific (male and female, respectively) tobacco-related cancer mortality (age ≥50) observed for period 5 (2004–2008) and the model-based projections for period 6 (2009–2013) and 7 (2014–2018) at cantonal level. Cantonal boundaries correspond to the updated structure including merged cantons. For males, an overall decreasing trend was observed, whereas rates in the French-speaking cantons still remained the highest, with the highest rates in the cantons of Valais and Neuchâtel.
Lowest tobacco-related cancer mortality rates were observed in the region covering the two cantons Appenzell Ausserrhoden and Appenzell Innerrhoden in Eastern Switzerland. Figure A2 (Appendix A http://www.smw.ch/fileadmin/smw/pdf/SMW-13771-Appendix_A.pdf ) shows less precise estimates for these regions. However, maps of the observed rates (fig. 6(a), fig. 7(a)) confirm low rates.
For males, the highest decrease was observed for the two cantons Zurich and Luzern – a steady reduction of around 12%–16% per period. For females, tobacco-related cancer mortality was projected to remain stable in the cantons Geneva and Graubünden. Furthermore, an increase of approximately 50% from 2004–2008 to 2014–2018 was estimated for the cantons Schaffhausen, Zug and Schwyz.
|
Table 1: Raw age- and gender-specific tobacco-related cancer death counts observed (during 2004–2008) and projected (posterior median during 2009–2013 and 2014–2018) at country level. The numbers in the brackets correspond to 95% credible intervals. |
| |
2004–2008
|
2009–2013
|
2014–2018
|
|
Males
|
|
|
|
| Overall |
17,656 |
18,340 (17,300‒19 820) |
19,270 (17,260‒22,100) |
| 50–69 years |
7,703 |
8,030 (7,522‒8,690) |
8,023 (7,085‒9,333) |
| ≥70 years |
9,953 |
10,310 (9,713‒11,130) |
11,260 (10,080‒12,910) |
|
Females
|
|
|
|
| Overall |
9,349 |
11,080 (10,510‒11,620) |
13,270 (11,960‒14,570) |
| 50–69 years |
3,453 |
4,255 (4,005‒4,534) |
5,010 (4,416‒5,643) |
| ≥70 years |
5,896 |
6,824 (6,456‒7,148) |
8,261 (7,453‒9,026) |
Discussion
This is the first study in Switzerland to project age- and gender-specific tobacco-related cancer mortality at different geographical levels for 2009–2013 and 2014–2018. Gender-specific estimates of age-standardised rates are presented for each canton and language region. In addition, projections were also made on the national scale by the two age categories 50–69 and ≥70 years.

Figure 5
Language-region‒specific age-standardised tobacco-related cancer mortality rates (per 100,000), observed (during 1984–2008) and predicted (posterior median during 2009–2013 and 2014–2018) for (a) males and (b) females. The vertical line indicates the start of the prediction period.
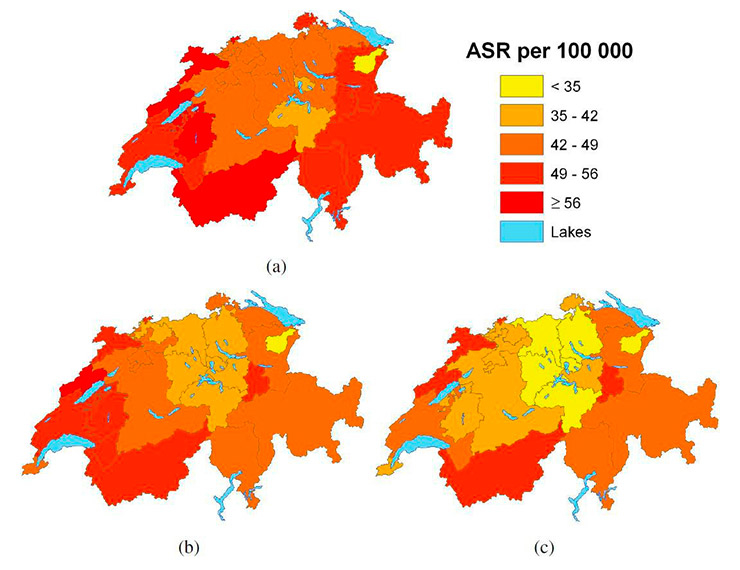
Figure 6
Age-standardised tobacco-related cancer mortality rates (per 100,000) for males age ≥50 years, (a) observed during 2004–2008 and predicted posterior median (b) during 2009–2013 and (c) 2014–2018.
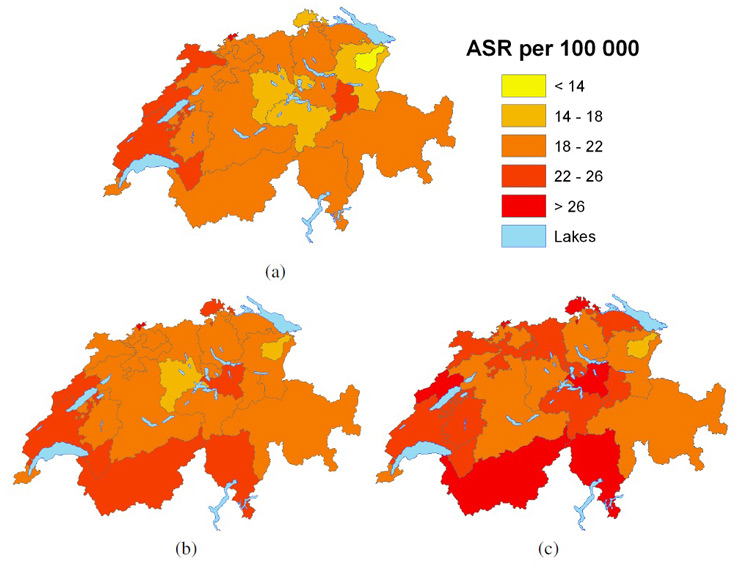
Figure 7
Age-standardised tobacco-related cancer mortality rates (per 100,000) for females age ≥50years, (a) observed during 2004–2008 and predicted posterior median (b) during 2009–2013 and (c) 2014–2018.
Random effects at cantonal level were considered to account for the spatial correlation in mortality which arises from similar exposures (e.g. environmental factors, cultural characteristics, health policies) in neighbouring areas. The models were formulated within the Bayesian framework of inference, with CAR priors adopted for the random effects. The Bayesian approach enables computation via the use of MCMC and smooths the rates so that estimates from areas with small populations do not dominate [16].
Overall, tobacco-related cancer mortality rates in males are decreasing, whereas female mortality is increasing. Estimates at the national level show that mortality rates for younger (age 50–69) females increased more than the rates for older (age ≥70) ones. On the other hand, a steeper downward trend was seen for young males than for older ones. The less pronounced trend for males observed from 1994 onwards might be due to the change in death certificate coding introduced in 1995. Early Swiss cancer mortality projections on the national scale estimated a plateau of male lung cancer mortality and a rising trend for females for the end of the last century [17].
Maps of the projected rates can greatly help in identifying cantons with high or low mortality rates. However, the accuracy of estimates needs to be considered carefully. In this study some cantons were merged to allow for more precise estimates. Figure A2 (Appendix A http://www.smw.ch/fileadmin/smw/pdf/SMW-13771-Appendix_A.pdf ) shows that estimates for these regions have the lowest accuracy; however, they seem reasonable compared to the trends of preceding periods and neighbouring cantons.
Important differences in cancer-related mortality between the language regions were estimated for the observed, but not the projected, periods. The latter may be explained by the wide Bayesian credible intervals of the estimates. Results for language regions could be compared with projections in Germany, France and Italy to assess the hypothesis that Swiss language regions reflect the situation in the corresponding neighbouring countries.
When comparing figure 1 with figures 6 and 7, it is interesting to note that the highest rates occurred in the French-speaking part, which applied the strictest smoking ban regulations. For males’ tobacco-related cancer mortality rates in 2014–2018 (fig. 6(c)), cantons with the lowest rates in the middle of the country resemble those only applying minimal smoking-ban regulations (i.e. the federal law). However, this finding may have arisen by chance because it does not take into account the time lag of 20–30 years between smoking (exposure) and disease/death.
In this study, cancer deaths below the age of 50 were excluded, as lung cancer in this age group is rarely due to tobacco smoking.
A decreasing prevalence of smoking in Switzerland has been reported for 1992–2007 [4]. Marques-Vidal et al. reported a stronger trend, corresponding with stage 4 of the smoking epidemic model proposed by Lopez et al. [18]. This model describes different stages that indicate changes in prevalence of smoking, tobacco consumption and mortality due to smoking. Stage 4 is characterised by an overall declining trend in smoking prevalence and a peak in male smoking-related mortality. As a consequence of rapid changes in female smoking behaviour during the mid-twentieth century, mortality for women has increased. However, a peak in female tobacco-related cancer mortality has not yet been observed. These findings confirm European trends in gender-specific lung cancer mortality. Based on observations in 2007, rates in Europe were estimated to decrease for males and increase for females in 2012, which is consistent with our results [19]. However studies in the United States showed a declining trend in female lung cancer mortality rates [20].
It should be noted that we only account for cancer mortality and did not capture overall mortality due to smoking. Furthermore, we did not account for smoking behaviour and related changes over time. Model predictions could be optimised by using additional information. For example, Held et al. [12] introduced information on tobacco consumption into their analysis, estimated from numbers of cigarette packages sold, to project lung cancer mortality in Western Germany based on the effects of age and cohort. In Switzerland, obtaining temporal as well as spatial information on smoking patterns remains a challenge, especially for earlier years.
The current analysis cannot take into account the impact of recent interventions, such as the Swiss federal law which was introduced in 2010, because there are no mortality data to estimate the intervention effect. This may be considered as a limitation; however, our analysis will allow the assessment of the impact of the smoking ban by comparing our projections with the observed rates in the future.
Demographic changes have an impact on incidence and mortality, especially for diseases mainly occurring in elderly people, as the fraction of older people in the population is rising. Among others, improved healthcare is one reason for the increasing longevity.
Stable rates may be estimated despite the fact that the absolute number of deaths is increasing because the elderly population is increasing. In this study, a downward trend was observed for male cause-specific cancer mortality rates, although raw death counts increased for these periods.
Maps of observed and projected tobacco-related cancer mortality highlighted cantons with low or high rates. Estimates for language regions showed lowest mortality in the German-speaking part of the country but appeared to become less pronounced over time especially for women. Countrywide projections indicated an ongoing declining trend for males, while for females a continuing rise was predicted, which is more pronounced in the younger age group.
Appendix
Appendix A: Details of the Swiss cantons
Appendix B: Bayesian APC model formulations
Acknowledgement:We would like to thank the Swiss Federal Statistical Office for providing the data.
References
1 Boyle P. Cancer, cigarette smoking and premature deaths in Europe: a review including the Recommendations of European Cancer Experts Consensus Meeting, Helsinki, October 1996. Lung Cancer. 1997;17(1):1–60.
2 Chaturvedi P. Tobacco is a “weapon of mass destruction”. Should western countries be invaded for that? Postgrad Med J. 2004;80:477.
3 Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4 Marques-Vidal P, Cerveira J, Paccaud F, Cornuz J. Smoking trends in Switzerland, 1992–2007: a time for optimism? J Epidemiol Commun H. 2011;65:281–6.
5 Flandorfer P, Wegner C, Buber I. Gender Roles and Smoking Behaviour. Vienna Institute of Demographic. Working Papers, 2010.
6 Heishman S. Behavioral and cognitive effects of smoking relationship to nicotine addiction. Nicotine Tob Res. 1999;1:143–7.
7 Lee Y, Boffetta P, Sturgis E, Wei Q, Zhang Z, Muscat J, et al. Involuntary smoking and head and neck cancer risk: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidem Biomar. 2008;17(8):1974–81.
8 Taylor R, Najafi F, Dobson A. Meta-analysis of studies of passive smoking and lung cancer: effects of study type and continent. Int J Epidemiol. 2007;36(5):1048–59.
9 Doll R, Peto R: Mortality in relation to smoking: 20 years observations on male British doctors. BMJ. 1976;2:1525–36.
10 Clèries R, Ribes J, Esteban L, Martinez J, Borràs J. Time trends of breast cancer mortality in Spain during the period 1977–2001 and Bayesian approach for projections during 2002–2016. Ann Oncol. 2006;17(12):1783–91.
11 Eilstein D, Uhry Z, Lim T, Bloch J. Lung cancer mortality in France. Trend analysis and projection between 1975 and 2012, using a Bayesian age-period-cohort model. Lung cancer. 2008;59(3):282–90.
12 Knorr-Held L, Rainer E. Projections of lung cancer mortality in West Germany: a case study in Bayesian prediction. Biostatistics. 2001;2:109–29.
13 Berrut S, Junker J. Von Generation zu Generation: Entwicklung der Todesursachen 1970 bis 2004. Bundesamt für Statistik (BFS), Neuchâtel. Schweiz, 2008.
14 Segi M. Cancer mortality for selected sites in 24 countries (1950–57). Department of Public Health, Tohoku University of Medicine, Sendai, Japan, 1960.
15 Bernardinelli L, Montomoli C. Empirical Bayes versus fully Bayesian analysis of geographical variation in disease risk. Stat Med. 1992;11:983–1007.
16 Clayton D, Kaldor J. Empirical Bayes estimates of age-standardized relative risks for use in disease mapping. Biometrics. 1987;43:671–81.
17 Negri E, LaVecchia C, Levi F, Randriamiharisoa A, Decarli A, Boyle P. The application of age, period and cohort models to predict Swiss cancer mortality. J Cancer Res Clin. 1990;116(2):207–14.
18 Lopez AD, Collishaw NE, Piha T. A descriptive model of the cigarette epidemic in developed countries. Tobacco Control. 1995;3:242–7.
19 Malvezzi M, Bertuccio P, Levi F, LaVecchia C, Negri E. European cancer mortality predictions for the year 2012. Ann Oncol. 2012;23(4):1044–52.
20 Kohler B, Ward E, McCarthy B, Schymura M, Ries L, Eheman C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2007, Featuring tumors of the brain and other nervous system. J Natl Cancer I. 2011;103:1–23.