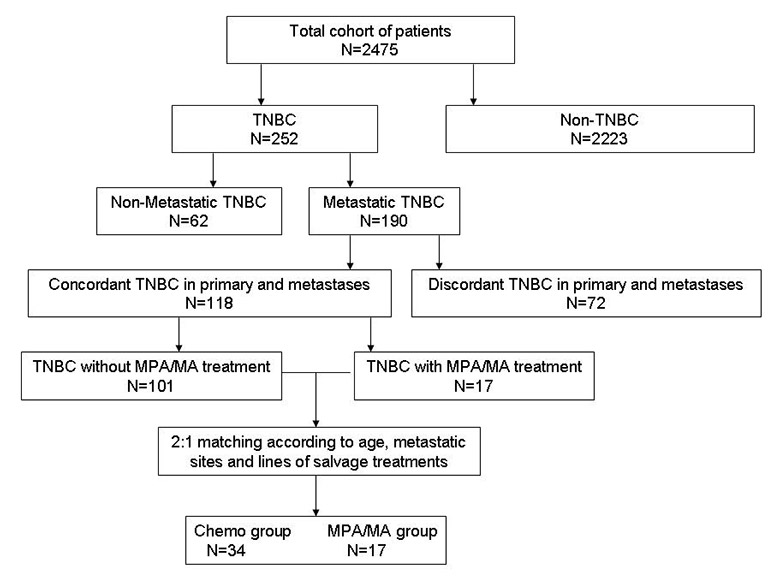
Figure 1
Flow chart of patient selection.
TNBC, triple-negative breast cancer; MPA/MA, medroxyprogesterone acetate/megestrol acetate.
DOI: https://doi.org/10.4414/smw.2013.13765
Tumours without oestrogen receptor (ER), progesterone receptor (PgR), or human epidermal growth factor receptor 2 (HER2) expression are referred to as triple-negative breast cancer (TNBC), representing about 15% of all breast cancers [1–4]. TNBC has an aggressive clinical phenotype with early brain and other distant metastases and a poor prognosis [5–9]. This form of cancer constitutes an important clinical challenge because it is not likely to respond to anti-oestrogen therapy or HER2 antagonists. At present, patients with recurrent TNBC currently have no established treatment option other than chemotherapy [10]. However, the long-term use of chemotherapeutic agents is difficult because of side effects, especially in multi-treated patients. Effective and sustainable therapeutic avenues are greatly needed, as the interruption of treatment may lead to rapid progression.
Endocrine manipulations are generally well tolerated. Selective estrogen response modulators and aromatase inhibitors (AIs) are currently the most commonly used endocrine agents, but such drugs mainly target hormone receptor–positive breast cancers [11, 12]. The progestins megestrol acetate (MA) and medroxyprogesterone acetate (MPA) were used frequently until the early 1990’s as a second-line hormonal therapy for metastatic breast cancer [13]. A previous study showed that MPA and MA achieved comparable median progression-free survival (PFS) [14], and the two agents were more effective in patients with hormone receptor–positive breast cancer than in those with hormone receptor–negative breast cancer. MPA and MA have shown 10%–30% response rates in patients with ER-negative breast cancer [15, 16]. In the 1990‘s, our team reported comparable response rates in patients with ER-negative and ER-positive breast cancer who were treated with progestins [15]. Although the use of progestins declined following the advent of selective estrogen response modulators and AIs, practitioners have recently shown renewed interest in the use of alternative hormonal treatments when conventional therapies fail [16, 17]. Furthermore, MPA/MA is also frequently used in patients with advanced malignancies to improve quality of life [18], especially with respect to appetite [19]; most research on this topic has been published in Chinese.
As TNBC is a relatively newly defined subgroup of breast cancer, older clinical trials did not differentiate breast cancer types in terms of ER, PgR, and HER2 status. While research continues to identify potential new targets based on phenotypic and molecular characteristics of these tumours [20–25], formerly popular agents whose use has declined may remain effective in triple-negative disease. Whether a single progestin is a suitable therapy for multi-treated TNBC should be examined.
To investigate the effect of MPA/MA in patients with recurrent TNBC, we conducted a retrospective observational study to review the outcomes of a sample of patients treated with MPA/MA and chemotherapy at our hospital between 2002 and 2011.
The Ethics Committee and Review Board of the Affiliated Hospital of the Academy of Military Medical Sciences approved this study.

Figure 1
Flow chart of patient selection.
TNBC, triple-negative breast cancer; MPA/MA, medroxyprogesterone acetate/megestrol acetate.
In this single-institution retrospective study, we used data from a representative sample of 2,475 patients with breast cancer who had been hospitalised between 1 January 2002 and 31 December 2011. The following information was collected from original medical records: patient age and sex, dates of primary invasive breast cancer and metastasis diagnoses, initial tumour stage, metastasis sites, adjuvant therapy, lines and regimens of salvage therapy, PFS, and adverse events. The inclusion criteria were primary breast cancer diagnosis; proven metastatic disease; and ER, PgR and HER2 testing in primary and metastatic tumours. The exclusion criteria were prior salvage therapy (including chemotherapy, hormone therapy, and target therapy) within 3 weeks, and prior treatment using the same regimen. Patient characteristics are presented in table 1.
In this cohort, 252 patients had been diagnosed with primary TNBC, and metastases were diagnosed in 190 of these patients. Triple-negative tumours were diagnosed at primary and metastatic sites in 118 patients. Only 17 patients with TNBC who received progestin (MPA/MA) treatment were included in our database. To create a statistical model in which patients in the MPA/MA and chemotherapy groups were matched at a 1:2 ratio according to age, metastatic sites, and lines of salvage treatment, we selected 34/101 patients in the database who had undergone chemotherapy for inclusion in this study. We believe that the use of this model is more effective than analysis using an unselected model. A flow chart of the cohort is presented in figure 1.
Primary and metastatic ER, PgR, and HER2 data were collected from pathology reports. ER and PgR status were evaluated by immunohistochemistry (IHC) and classified as positive (≥10% cells immunostained) or negative [26]. HER2 status was evaluated by IHC and/or fluorescence in situ hybridisation (FISH) [27]. According to the Hercep Test criteria, the immunoreaction of specimens was scored as 3+, 2+, 1+ or 0 [28]. Tumours scored as 3+ by IHC or FISH (+) were considered HER2 positive, and those scored as 0/1+ by IHC or FISH (–) were designated as HER2 negative.
Each patient in the MPA/MA group was treated with a single progestin agent: 500 mg oral MPA (10/17, 58.82%) or 160 mg oral MA (7/17, 41.18%) daily. In the chemotherapy group, 21 patients (61.76%) were treated with single-agent chemotherapy, and 13 patients (38.24%) underwent combined chemotherapy. Chemotherapy regimens (table 2) included anthracyclines, taxanes, platinum, gemcitabine, vinorelbine, capecitabine, or etoposide; regimens varied among patients because they were selected according to each patient’s prior therapy and general condition. The doses of chemotherapeutic agents and treatment intervals were adjusted according to each patient’s physical condition and adverse effects.
Treatment of patients in both groups continued until disease progression or an unacceptable adverse effect was noted. The therapeutic effect, time to progression, and curative and side effects were recorded. Adverse effects were assessed using the Common Terminology Criteria for Adverse Events, version 3.0 [29]. PFS was defined as the time from the date of administration to the date of disease progression. The Response Evaluation Criteria in Solid Tumours (RECIST; ver. 1.0) were used to classify disease status as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD) [30]. Assessable lesions were deemed to have shown clinical benefit (CB) when objective responses were classified as a CR or PR, or when SD persisted ≥6 months, in accordance with the Union for International Cancer Control criteria [31, 32]. Disease control status was defined as the “best status to date,” specifically whether patients showed CR, PR, or SD [33].
Data were analysed using SAS statistical software (version 9.2; SAS Institute, Cary, NC, USA). Patient characteristics, CB rates, and disease control rates were compared between groups using the χ2 test. The rank-sum test was used to compare therapeutic effects between groups. PFS was estimated using to the Kaplan–Meier product limit method, and groups were compared with the log-rank test.
| Table 1: Patient characteristics. | ||||
| MPA/MA group (n = 17) | Chemotherapy group (n = 34) | t or χ2 | p | |
| Age, years (mean ± standard deviation) | 53.60 ± 11.00 | 52.10 ± 12.30 | 1.010 | 0.319 |
| Median disease-free survival, months [median (range)] | 17.50 (10, 40) | 27.00 (19, 41) | 0.821 | 0.365 |
| Metastases, % (n) | ||||
| Viscera | 82.35 (14/17) | 73.52 (25/34) | 0.490 | 0.728 |
| Bone | 29.41 (5/17) | 44.12 (15/34) | 1.028 | 0.373 |
| Brain | 5.88 (1/17) | 5.88 (2/34) | 0.000 | 1.000 |
| Lymph node/soft tissue | 70.59 (12/17) | 64.71 (22/34) | 0.177 | 0.760 |
| Number of metastases, % (n) | ||||
| 1–2 | 52.94 (9/17) | 73.52 (25/34) | 2.162 | 0.208 |
| ≥3 | 47.06 (8/17) | 26.47 (9/34) | 2.162 | 0.208 |
| Lines of salvage therapy, % (n) | ||||
| 1–3 | 23.53 (4/17) | 26.47 (9/34) | 0.052 | 1.000 |
| 4–6 | 47.06 (8/17) | 44.12 (15/34) | 0.040 | 1.000 |
| 7–9 | 23.59 (4/17) | 26.47 (9/34) | 0.052 | 1.000 |
| ≥10 | 5.88 (1/17) | 2.94 (1/34) | 0.260 | 1.000 |
| MPA/MA, medroxyprogesterone acetate/megestrol acetate. | ||||
| Table 2: Salvage therapy regimens. | ||
| MPA/MA group (n = 17) | Chemotherapy group (n = 34) | |
| Salvage therapy regimen, % (n) | ||
| Megestrol acetate | 41.18 (7/17) | |
| Medroxyprogesterone | 58.82 (10/17) | |
| Containing anthracyclines | 8.82 (3/34) | |
| Containing taxanes | 26.47 (9/34) | |
| Containing platinum | 20.59 (7/34) | |
| Containing gemcitabine | 14.71 (5/34) | |
| Containing vinorelbine | 17.65 (6/34) | |
| Containing capecitabine | 14.71 (5/34) | |
| Containing etoposide | 17.65 (6/34) | |
| 1–6 lines, % (n) | ||
| Single-agent therapy | 100 (12/12) | 58.33 (14/24) |
| Combination therapy | 41.67 (10/24) | |
| ≥7 lines, % (n) | ||
| Single-agent therapy | 100 (5/5) | 70.00 (7/10) |
| Combination therapy | 30.00 (3/10) | |
| MPA/MA, medroxyprogesterone acetate/megestrol acetate. | ||
The two groups were well balanced in terms of patient age, disease-free survival, metastasis, number of metastases, and lines of salvage therapy (table 1). Mean patient ages in the MPA/MA (n = 17) and chemotherapy (n = 34) groups were 53.60 and 52.10 years, respectively. In the entire study sample, 70.59% of patients received 1st–6th-line therapy for metastatic disease. All patients in the MPA/MA group were treated with a single agent, and 38.23% (13/34) of patients in the chemotherapy group underwent combination chemotherapy.
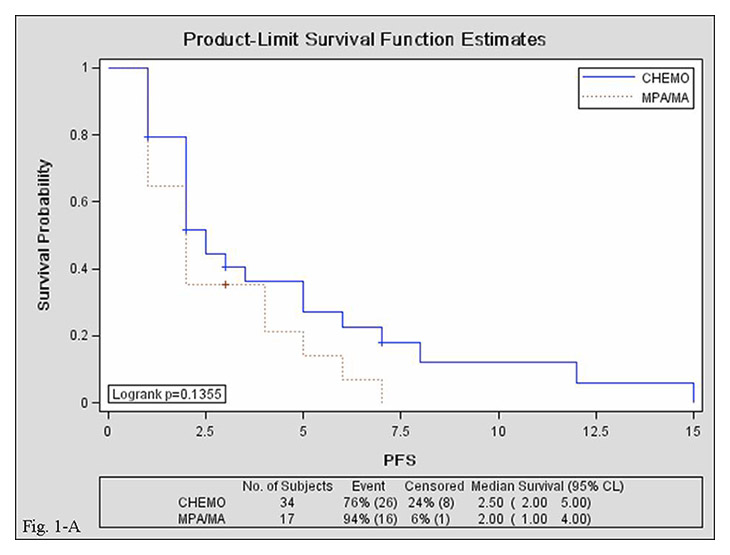
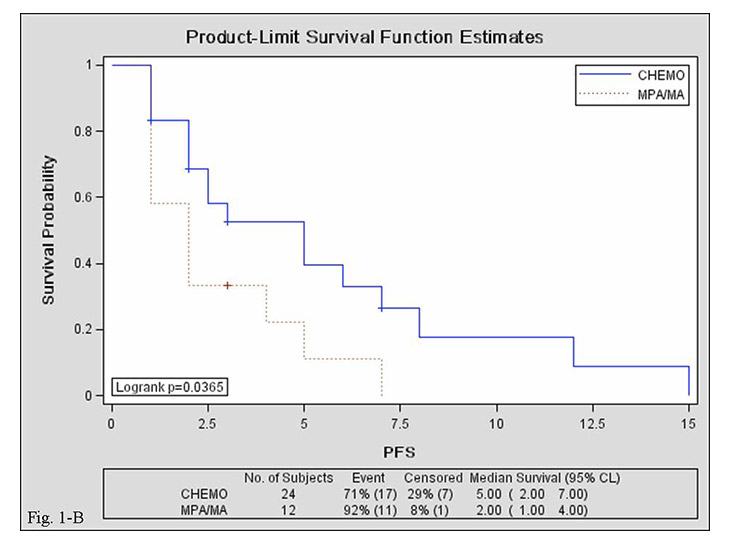
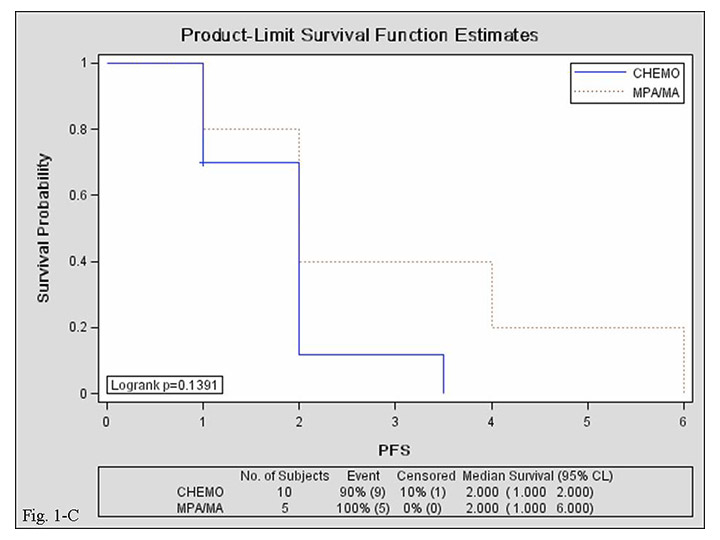
Figure 2
Kaplan–Meier curves for progression-free survival in the MPA/MA and chemotherapy groups. A: overall; B: 1–6 lines of salvage therapy; C: ≥7 lines of salvage therapy.
CHEMO, chemotherapy group; MPA/MA, medroxyprogesterone acetate/megestrol acetate group; PFS, progression-free survival; CI, confidence interval.
All patients had target lesions, allowing the use of RECIST to evaluate objective responses. Therapeutic effects are presented in table 3. One case of CR and three cases of PR were observed in the chemotherapy group, but no such cases were documented in the MPA/MA group. More SD cases and fewer PD cases occurred in the chemotherapy group than in the MPA/MA group, but overall efficacy did not differ significantly between groups (rank-sum test, p = 0.076). Disease control rates were 52.94% (9/17) in the MPA/MA group and 73.53% (25/34) in the chemotherapy group (χ2test, p = 0.208). CB rates (CR + PR + SD ≥ 6 months) showed no significant difference between the MPA/MA and chemotherapy groups (11.76% vs. 29.41%; χ2test, p = 0.263). More than 35% of patients in each group showed at least 3 months of CB (CR + PR + SD ≥3 months). Patients in the MPA/MA group showed a marked response to treatment that continued for more than 7 months.
Median PFS was comparable in the MPA/MA and chemotherapy groups {2 [95% confidence interval (CI), 1–4] vs. 2.5 [95% CI, 2–5] months; log-rank test, p = 0.135}. Median PFS of 1st–6th-line salvage treatments was shorter in the MPA/MA group than in the chemotherapy group [2 (95% CI, 1–4) vs. 5 (95% CI, 2–7) months; log-rank test, p = 0.036], but median PFS of ≥7th-line salvage treatments was comparable between groups [2 (95% CI, 1–2) vs. 2 (95% CI, 1–6) months; log-rank test, p = 0.139]. Median PFS was comparable between patients treated with MPA (n = 7) and those treated with MA [n = 10; 2 (95% CI, 2–4) vs. 1.5 (95% CI, 1–4) months; log-rank test, p = 0.921]. Survival curves are presented in figure 2.
No serious side effect occurred in the MPA/MA group; moderate side effects included body weight gain, hyperglycaemia, vaginal bleeding, and blurred vision. One patient withdrew from MPA treatment because of weight gain. Eight patients (23.52%) in the chemotherapy group withdrew because of adverse effects. The most frequent grade 3 or 4 adverse events in the chemotherapy group were neutropenia, leucopenia, thrombocytopenia, anaemia, fatigue or asthenia, and increased alanine aminotransferase level. Adverse effects and withdrawal data are presented in table 4.
| Table 3: Therapeutic effects. | ||||
| MPA/MA group (n = 17) | Chemotherapy group (n = 34) | χ2 | p | |
| Therapeutic effect, % (n) | ||||
| CR | 2.94 (1/34) | 3.157 | 0.076 | |
| PR | 8.82 (3/34) | |||
| SD | 52.94 (9/17) | 61.76 (21/34) | ||
| PD | 47.06 (8/17) | 26.47 (9/34) | ||
| Clinical control rate (CR + PR + SD) | 52.94 (9/17) | 73.53 (25/34) | 2.162 | 0.208 |
| CR + PR + SD ≥6 months | 11.76 (2/17) | 29.41 (10/34) | 1.962 | 0.293 |
| CR + PR + SD ≥3 months | 35.29 (6/17) | 41.18 (14/34) | 0.165 | 0.767 |
| CR + PR + SD ≥4 months | 29.41 (5/17) | 32.35 (11/34) | 0.046 | 1.000 |
| CR + PR + SD ≥5 months | 21.43 (3/17) | 32.35 (11/34) | 1.231 | 0.334 |
| CR: complete remission; PR: partial remission; SD: stable disease; PD: progressive disease; MPA/MA: medroxyprogesterone acetate/megestrol acetate. | ||||
| Table 4:Adverse effects and withdrawal from treatment. | ||
| MPA/MA group (n = 17) | Chemotherapy group (n = 34) | |
| Adverse effects,* % (n) | ||
| Grade 3–4 haematological toxicity | ||
| Neutropenia | 35.29 (12/34) | |
| Leucopenia | 44.12 (15/34) | |
| Thrombocytopenia | 5.88 (2/34) | |
| Anaemia | 14.71 (5/34) | |
| Grade 3–4 non-haematological toxicity | ||
| Fatigue or asthenia | 55.89 (19/34) | |
| Increased alanine aminotransferase level | 5.88 (1/17) | 47.06 (16/34) |
| Body weight gain | 47.06 (8/17) | |
| Hyperglycaemia | 17.65 (3/17) | 11.76 (4/34) |
| Vaginal bleeding | 47.06 (8/17) | |
| Blurred vision | 5.88 (1/17) | 5.88 (2/34) |
| Hand–foot syndrome | 5.88 (2/34) | |
| Withdrawal, % (n) | 5.88 (1/17) | 23.53 (8/34) |
| *Assessed using the Common Terminology Criteria for Adverse Events, version 3.0 MPA/MA, medroxyprogesterone acetate/megestrol acetate. | ||
The results of this retrospective observational study demonstrate that MPA/MA has a comparable CB but lower toxicity than chemotherapy for patients with multi-treated (>7th-line salvage therapy) recurrent TNBC. These findings will be useful for clinicians in the management of patients with recurrent TNBC. Because this disease is incurable, sustainable systemic therapy is needed to prolong survival. The long-term use of chemotherapeutic agents is difficult due to side effects, and treatment interruption may lead to rapid progression. MPA/MA may have relevant clinical implications for patients with recurrent TNBC, especially for multi-treated patients with poor general conditions.
TNBC is currently the subject of active research, and several novel classes of drug are under investigation or clinical development [20–25]. A previous study showed that these novel agents, such as histone deacetylase inhibitor [25], may have a curative effect on breast cancer, but none are currently routinely used in clinical practice. Several studies have shown that MPA/MA has a curative effect on breast cancer, even on ER-negative tumours [15, 16]. A literature search found that no previous study examined the treatment of recurrent TNBC with a single progestin agent (MPA or MA); this study is thus, the first to do so.
A previous study conducted in our hospital showed that MPA/MA treatment achieved a comparable response rate in patients with hormone receptor–positive and –negative breast cancer [15]. Although MPA was designed to bind with high affinity to the PgR, androgenic side effects observed in women taking MPA suggested that the androgen receptor (AR) may contribute to its activity in vivo. This hypothesis is supported by the high binding affinity of MPA to the AR [34], and clinical studies have shown that the response of breast tumours to high-dose MPA therapy is dependent on the expression of AR, but not ER or PgR [35, 36]. AR expression has been observed in about 50% of patients with TNBC [37], and may explain the efficacy of MPA/MA treatment in these patients.
This study had several limitations. First, the study was retrospective. Second, because selective oestrogen response modulators and AIs are more effective than progestin in patients with hormone receptor–positive breast cancer, the use of progestins has declined, so our patient sample was small and the results of this study are not generalisable to larger populations. Third, the statistical approach used in this study, including the matching of patients at a 1:2 ratio, is typically employed in randomised trials rather than in retrospective observational studies. However, only 17 patients with TNBC who received progestin treatment were included in our database, and the chemotherapy group was also selected from a very small cohort (34/101). Considering that patients were matched according to age, metastatic sites, and lines of salvage treatment, we believe that the use of a 1:2-matched statistical model was more effective than an unselected model.
Our data provide clinical evidence that MPA/MA may be an alternative treatment for patients with recurrent TNBC, especially for multi-treated patients with poor physical conditions. However, our study was observational and the sample was small. Further studies with larger datasets and prospective research are needed to provide confirmatory evidence for or against the feasibility of MPA/MA treatment for recurrent TNBC.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.
1 Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107.
2 Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist. 2011;16:1–11.
3 Adamo V, Ricciardi GR, De Placido S, Colucci G, Conte P, Giuffrida D, et al. Management and treatment of triple-negative breast cancer patients from the NEMESI study: an Italian experience. Eur J Cancer. 2012;48(5):642–7.
4 Yao-Lung K, Dar-Ren C, Tsai-Wang C. Clinicopathological features of triple-negative breast cancer in Taiwanese women. Int J Clin Oncol. 2011;16(5):500–5.
5 Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic Markers in Triple-Negative Breast Cancer. Cancer. 2007;109(1):25–32.
6 Lin C, Chien SY, Kuo SJ, Chen LS, Chen ST, Lai HW, et al. A 10-year follow-up of triple-negative breast cancer patients in Taiwan. Jpn J Clin Oncol. 2012;42(3):161–7.
7 Lara-Medina F, Pérez-Sánchez V, Saavedra-Pérez D, Blake-Cerda M, Arce C, Motola-Kuba D, et al. Triple-negative breast cancer in Hispanic patients: high prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer. 2011;117(16):3658–69.
8 Jang G, Lee SS, Ahn JH, Jung KH, Lee H, Gong G, et al. Clinical features and course of brain metastases in triple-negative breast cancer: comparison with human epidermal growth factor receptor 2-positive and other type at single institution in Korea. Breast Cancer Res Treat. 2011;128(1):171–7.
9 Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–48.
10 Carey LA. Directed therapy of subtypes of triple-negative breast cancer. Oncologist. 2011;16(Suppl. 1):71–8.
11 Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–2.
12 Coates AS, Keshaviah A, Thürlimann B, Mouridsen H, Mauriac L, Forbes JF, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007;25(5):486–92.
13 Parazzini F, Colli E, Scatigna M, Tozzi L. Treatment with tamoxifen and progestins for metastatic breast cancer in postmenopausal women: a quantitative review of published randomized clinical trials. Oncology. 1993;50:483–9.
14 Willemse PH, van der Ploeg E, Sleijfer DT, Tjabbes T, van Veelen H. A randomized comparison of megestrol acetate (MA) and medroxyprogesterone acetate (MPA) in patients with advanced breast cancer. Eur J Cancer. 1990;26(3):337–43.
15 Jiang Z, Song S, Li J. Clinical trial of high-dose medroxyprogesterone acetate in advanced breast Cancer. Zhonghua Zhong Liu Za Zhi. 1995;17(1):71–3. (article in Chinese)
16 Pannuti F, Malpighi M. Home care for advanced and very advanced cancer patients: the Bologna experience. J Palliat Care. 1988;4(3):54–7.
17 Focan C, Beauduin M, Majois F, et al. High-dose oral medroxyprogesterone acetate or tamoxifen as adjuvant hormone therapy for node-negative early-stage breast cancer: randomized trial with 7-year update. Clin Breast Cancer. 2004;5:136–41.
18 Effectiveness of megestrol acetate in patients with advanced cancer: a randomized, double-blind, crossover study. Bruera E, Ernst S, Hagen N, Spachynski K, Belzile M, Hanson J, Summers N, Brown B, Dulude H, Gallant G Cancer Prev Control. 1998;2(2):74–8.
19 Megestrol acetate for anorexia in patients with far-advanced cancer: a double-blind controlled clinical trial. De Conno F, Martini C, Zecca E, Balzarini A, Venturino P, Groff L, Caraceni A Eur J Cancer. 1998;34(11):1705–9.
20 Anders CK, Winer EP, Ford JM, Dent R, Silver DP, Sledge G, et al. Poly(ADP-Ribose) polymerase inhibition: “targeted” therapy for triple-negative breast cancer. Clin Cancer Res. 2010;16(19):4702–10.
21 Thomssen C, Pierga JY, Pritchard KI, Biganzoli L, Cortes-Funes H, Petráková K, et al. First-line bevacizumab-containing therapy for triple-negative breast cancer: analysis of 585 patients treated in the ATHENA study. Oncology. 2012;82(4):218–27.
22 Fojo T, Amiri-Kordestani L, Bates SE. Potential pitfalls of crossover and thoughts on iniparib in triple-negative breast cancer. J Natl Cancer Inst. 2011;103(23):1738–40.
23 Finn RS, Bengala C, Ibrahim N, Roché H, Sparano J, Strauss LC, et al. Dasatinib as a single agent in triple-negative breast cancer: results of an open-label phase 2 study. Clin Cancer Res. 2011;17(21):6905–13.
24 Domagala P, Lubinski J, Domagala W. Iniparib in metastatic triple-negative breast cancer. N Engl J Med. 2011;364(18):1780.
25 Breast Cancer Res. 2012; 14(3): R79. Published online 2012 May 21. Targeting triple-negative breast cancer cells with the histone deacetylase inhibitor panobinostat.
26 Lower EE, Blau R, Gazder P, Stahl DL. The effect of estrogen usage on the subsequent hormone receptor status of primary breast cancer. Breast Cancer Res Treat. 1999;58(3):205–11.
27 Ludovini V, Gori S, Colozza M, Pistola L, Rulli E, Floriani I, et al. Evaluation of serum HER2 extracellular domain in early breast cancer patients: correlation with clinicopathological parameters and survival. Ann Oncol. 2008;19:883–90.
28 Ludovini V, Gori S, Colozza M, Pistola L, Rulli E, Floriani I, et al. Evaluation of serum HER2 extracellular domain in early breast cancer patients: correlation with clinicopathological parameters and survival. Ann Oncol. 2008;19:883–90.
29 Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–81.
30 Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16.
31 British Breast Group. Assessment of response to treatment in advanced breast cancer. Lancet. 1974;2:38–9.
32 Hayward JL, Carbone PP, Heuson JC, Kumaoka S, Segaloff A, Rubens RD. Assessment of response to therapy in advanced breast cancer: a project of the Programme on Clinical Oncology of the International Union Against Cancer, Geneva, Switzerland. Cancer. 1977;393:1289–94.
33 Primo N. Lara Jr, Mary W. Redman, et al. Disease Control Rate at 8 Weeks Predicts Clinical Benefit in Advanced Non-Small-Cell Lung Cancer: Results From Southwest Oncology Group Randomized Trials. J Clin Oncol. 2008;26(3):463–7.
34 Bentel JM, Birrell SN, Pickering MA, Holds DJ, Horsfall DJ, Tilley WD. Androgen receptor agonist activity of the synthetic progestin, medroxyprogesterone acetate, in human breast cancer cells. Mol Cell Endocrinol. 1999;154:11–20.
35 Teulings FA, van Gilse HA, Henkelman MS, Portengen H, Alexieva-Figusch J. Estrogen, androgen, glucocorticoid, and progesterone receptors in progestin-induced regression of human breast cancer. Cancer Res. 1980;40:2557–61.
36 Birrell SN, Roder DM, Horsfall DJ, Bentel JM, Tilley WD. Medroxyprogesterone acetate therapy in advanced breast cancer: the predictive value of androgen receptor expression. J Clin Oncol. 1995;13:1572–7.
37 McNamara K, Yoda T, Takagi K, et al. Androgen receptor in triple negative breast cancer. J Steroid Biochem Mol Biol. 2012 Sep 5.