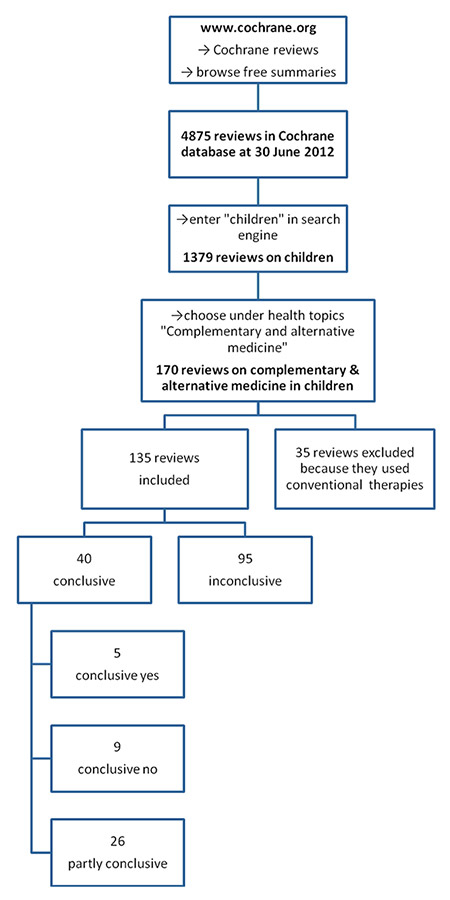
Figure 1
Flow chart of study inclusion.
DOI: https://doi.org/10.4414/smw.2013.13794
Complementary and alternative medicine (CAM) refers to a broad range of healing philosophies, approaches and therapies that exist largely outside the institutions where conventional medicine is taught and provided (exclusive definition). An increasing and generally high prevalence of CAM use by children and adolescents with chronic illnesses has been documented in industrialised countries [1–3]. While there are compelling data concerning increasing CAM use in adults, the use of CAM by children is not only less well studied, but also appears to have a considerable prevalence. CAM use by hospitalised children, as well as in outpatient settings, ranges from 1.8% to 84% [4–7]. A recent trial showed prevalence rates between 36% in general paediatrics and 61.9% in children with epilepsy [8]. Herbal medicine, homeopathy, reflexology and acupuncture are among the most popular treatments [9, 10]. The reasons for this high prevalence of use are diverse and might include dissatisfaction with conventional medicine and positive reports from friends and family [11, 12]. Children whose parents use CAM are almost five times more likely to use CAM than children whose parents do not use it [13].
Conversely, over the last two decades, evidence-based medicine (EBM) has substantially changed the approach to patients in clinical practice. EBM has contributed to improving the quality of medicine in general and in paediatrics in particular [14–16]. This development has led to the publication of a growing number of guidelines in the field of paediatrics [17, 18]. Moreover, with the advent and further development of EBM in modern medicine, clear and ambitious requirements for what is considered acceptable evidence have been defined. Today, the gold standard of EBM is the randomised controlled trial and systematic reviews of randomised controlled trials. The further development and standardisation of EBM – by providing specific tools to assess the level of evidence – is constantly promoted by the Oxford Centre for Evidence-Based Medicine [http://www.cebm.net/].
In recent years, efforts have been made to apply rigorous scientific standards to CAM through various initiatives, one of which is the Cochrane CAM Field [http://www.compmed.umm.edu/cochrane_about.asp]. The Cochrane CAM Field, which was founded in 1996, is dedicated to facilitating the production of systematic reviews of randomised controlled trials in the field of CAM. It is also a member entity of the Cochrane Collaboration. It is noteworthy that a number of Cochrane reviews in the field of CAM [19] have demonstrated a clinical benefit for some aspects of CAM therapy [21].
High-quality research and its perpetual critical assessment in accordance with EBM standards is the best way to clarify the role of CAM in the medical arena (diagnosis, therapy, funding, reimbursement, etc.). To date, no formal overview/synopsis of all published systematic Cochrane reviews in the field of CAM in children has been performed for physicians caring for children. Thus, the aim of this study was to provide the reader with a systematic overview/synthesis of Cochrane reviews assessing the efficacy, and the practical implications and limitations of CAM therapies in children.
| Table 1: Characteristics of included reviews with a “positive” recommendation [24–28]. | |||||||
| Year of first publication | Continent | Country | Author | Therapy mode | Title | Studies included | Patients included |
| 2004 | Asia | China | Anna Lee | Traditional Chinese medicine | Stimulation of the wrist acupuncture point P6 for preventing postoperative nausea and vomiting | 40 | 4,858 |
| 2011 | Asia | China | Qiukui Hao | Dietary supplements | Probiotics for preventing acute upper respiratory tract infections | 14 | 3,451 |
| 2010 | Asia | Pakistan | Zohra S Lassi | Dietary supplements | Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months | 6 | 7,850 |
| 2007 | Europe | Germany | Rudolf A Kley | Dietary supplements | Creatine for treating muscle disorders | 14 | 364 |
| 1998 | Africa | South Africa | G Justus Hofmeyr | Dietary supplements | Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems | 13 | 15,730 |
We conducted a systematic literature overview/synopsis including reviews from the Cochrane Review Group (CRG) from 1995 (launch of the Cochrane Database of Systematic Reviews in London by the British Minister for Health) until June 2012 (fig. 1). Initially, all Cochrane reviews in the general field of CAM in children provided by the CRG were screened for potential eligibility (i.e. whether they pertained to the field of CAM), using the operational definition of CAM with the inclusion of dietary supplements as detailed by Wieland and co-workers [22]. Of note, for many of these categories the operationalisation is clear-cut (e.g. any therapy described as ‘homeopathy’ or ‘homeopathic’ would be considered CAM). In some cases, however, the context of the therapy determines whether the therapy itself would be considered CAM or not (e.g. hyperbaric oxygenation is a standard treatment for carbon monoxide poisoning, but is an alternative treatment for multiple sclerosis) [22]. If then considered pertinent to the field of CAM in children, the reviews were subsequently included in the data synopsis (fig. 1).

Figure 1
Flow chart of study inclusion.
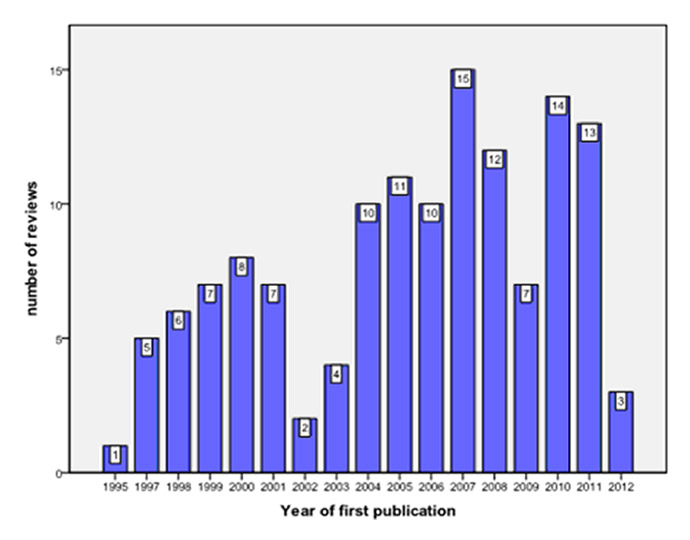
Figure 2
Number of Cochrane reviews published between 1995 and 2012.
The following data were retrieved from the CRG database: (1) date of publication; (2) origin of publication by continent/country (address of corresponding author was used for this purpose); (3) number of included studies/participants; and (4) type of intervention (pharmacological, nonpharmacological, etc.).
The main outcome parameters were:
(a) Number (percentage) of reviews with a definitive recommendation in favour of a certain intervention = review provides clear, unconditional recommendations
(b) Number (percentage) of reviews with a definitive recommendation against a certain intervention = review provides clear, unconditional recommendations
(c) Number (percentage) of inconclusive reviews = no recommendation can be issued (e.g.because of contradictory data)
(d) Number of reviews with conditional recommendations = for example, recommendation for a subgroup of patients only
We subsequently analysed the specific reasons why reviews were considered inconclusive, as provided by the authors. We also evaluated whether differences with regard to the primary outcome parameters were seen between three different, a priori-defined, time periods (1995–2000; 2001–2006; 2007–2012).
All data were retrieved from the CRG and stored in an electronic database, using SPSS 18.0 (SPSS, Chicago, Illinois, USA). If necessary, the original publications / randomised controlled trials were retrieved from PubMed/Medline and hand-searched for missing data relating to our study endpoints. Information was added to the database if indicated. The chi-square test was used for the comparison of categorical variables (e.g. number of conclusive and inconclusive reviews). A p-value <0.05 was considered significant.
To ensure the transparency and objectivity of this review, we adhered to the PRISMA statement [23] and checklist of items, in order to include as much as possible, for a systematic overview rather than a meta-analysis. Moreover, we decided to include only systematic reviews / meta-analyses provided by the Cochrane Collaboration since these publications are considered to be scientific reports/analyses of the highest quality. Reports including a singular randomised controlled trial were not included in this study.
| Table 2:Characteristics of included reviews with a “negative” recommendation [29–37]. | |||||||
| Year of first publication | Continent | Country | Author | Therapy mode | Title | Studies included | Patients included |
| 2007 | Asia | Israel | Dan Turner | Dietary supplements | Omega 3 fatty acids for maintenance of remission in Crohn's disease | 6 | 1,063 |
| 2008 | Asia | China | Hengxi Chen | Dietary supplements | Vitamin A for preventing acute lower respiratory tract infections in children up to 7 years | 10 | 33,179 |
| 1995 | Africa | South Africa | Charles Shey Wiysonge | Dietary supplements | Vitamin A supplementation for reducing the risk of mother-to-child transmission of HIV infection | 5 | 7,528 |
| 2000 | Europe | Italy | Francis CK Thien | Dietary supplements | Dietary marine fatty acids for asthma in adults and children | 9 | 187 |
| 1998 | Australia | Australia | Karen Simmer | Dietary supplements | Longchain polyunsaturated fatty acid supplementation in infants born at term | 15 | 1,889 |
| 1999 | Europe | Switzerland | Sven M Schulzke | Dietary supplements | Longchain polyunsaturated fatty acid supplementation in preterm infants | 17 | 2,217 |
| 2011 | Asia | India | Siddhartha Gogia | Dietary supplements | Vitamin A supplementation for the prevention of morbidity and mortality in infants six months of age or less | 18 | 155,605 |
| 2005 | Australia | Australia | Alice Rumbold | Dietary supplements | Vitamin supplementation for preventing miscarriage | 28 | 96,674 |
| 2008 | Australia | Australia | Robert J Boyle | Dietary supplements | Probiotics for treating eczema | 12 | 781 |
Between 1995 and 2012, 170 reviews (from the field of CAM as provided by the Cochrane data base) were considered eligible and 135 reviews were subsequently included (figs 1 and 2). Thirty-five Cochrane reviews were excluded because they dealt with conventional therapies (fig. 1).
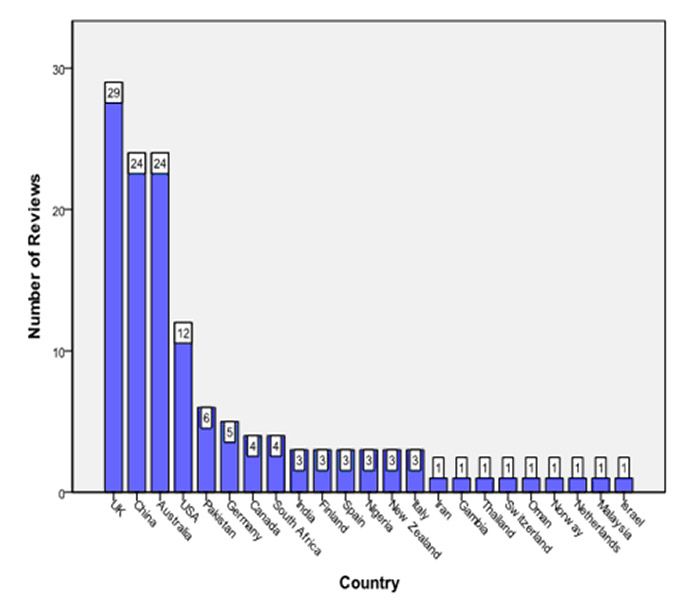
Figure 3
Origin of Cochrane reviews by country.
The origin of Cochrane reviews by continent was as follows: Europe (45), Asia (40), Australia (26), North America (16) and Africa (8). Figure 3 depicts the country of origin of the included reviews. A wide range in the number of included studies/patients was noticed (fig. 4). Eighty-six reviews assessed dietary supplements; other fields included traditional Chinese medicine (16/135), physical therapy (12/135), and various other forms of CAM (figs 5 and 6).
Only 5/135 (3.7%) reviews gave a clear recommendation in favour of a certain intervention (table 1 [24–28]), 26/135 (19.4%) issued a conditional positive recommendation, while 9/135 (6.6%) reviews concluded that certain interventions should not be performed (table 2 [29–37]). Ninety-five reviews (70.3%) were inconclusive. Conclusive reviews were significantly associated with the number of included studies (p < 0.01; table 3). The proportion of inconclusive reviews increased during three, a priori-defined, time intervals (1995–2000: 15/27 [55.6%]; 2001–2006: 33/44 [75%]; and 2007–2012: 47/64 [73.4%]).
The three most common criticisms with regard to quality of the included studies were: more research needed (82/135), low methodological quality (57/135) and small number of study participants (48/135; fig. 7).
| Table 3:Number of studies included in Cochrane reviews and conclusiveness of the review. | |||||
| Number of included studies | |||||
| 0–10 | 11–150 | Total | |||
| Recommendation: | Conclusive | Number | 17 | 23 | 40 |
| % of total number | 12.6% | 17.0% | 29.6% | ||
| Inconclusive | Number | 76 | 19 | 95 | |
| % of total number | 56.3% | 14.1% | 70.4% | ||
| Total | Number | 93 | 42 | 135 | |
| % of total number | 68.9% | 31.1% | 100.0% | ||
The results of our study do not provide consistent evidence to suggest that CAM is effective for a variety of paediatric conditions. In our study, only a minority of systematic reviews provided data to answer the question as to whether a certain intervention should or should not be performed (5 positive, 9 negative, 26 partly conclusive). The results of these reviews will provide the physician at the bedside with invaluable information about both optimal and unnecessary or potentially harmful treatment modalities, as detailed in table 1 and table 2. Moreover, and of note, our study also demonstrated that by far the largest proportion of systematic Cochrane reviews were inconclusive (95/135), and failed to provide any recommendation with regard to a specific intervention / clinical question. In fact, previous reports have also demonstrated that for some conditions, CAM seems to worsen symptoms (e.g. in children with stridor treated with acupuncture [38]).
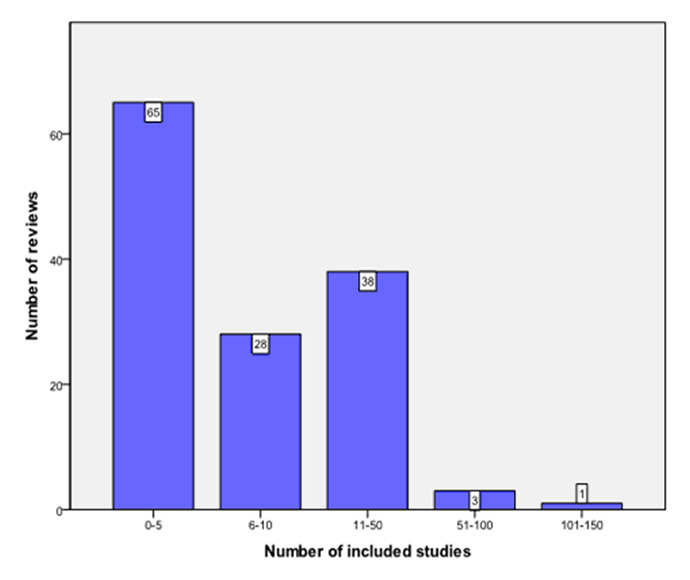
Figure 4
Number of studies included in Cochrane reviews.
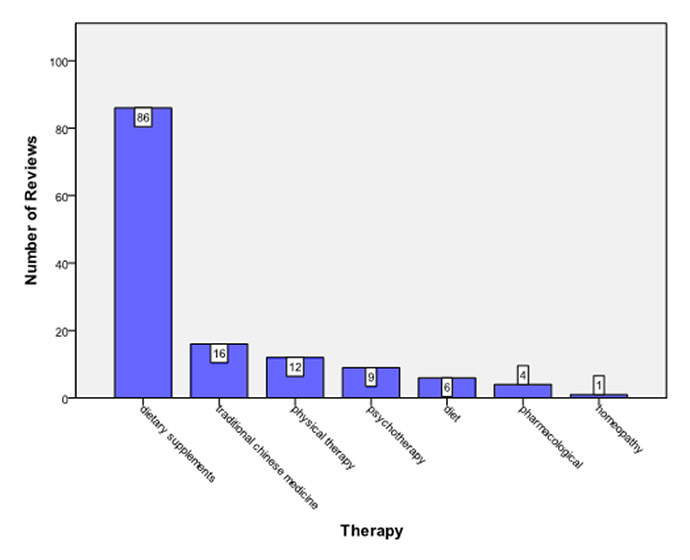
Figure 5
Types of intervention assessed in Cochrane reviews.
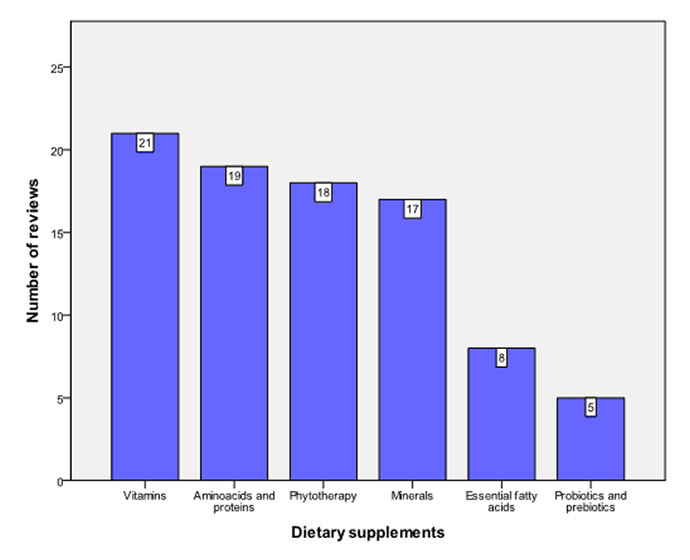
Figure 6
Types of dietary supplements assessed in Cochrane reviews.
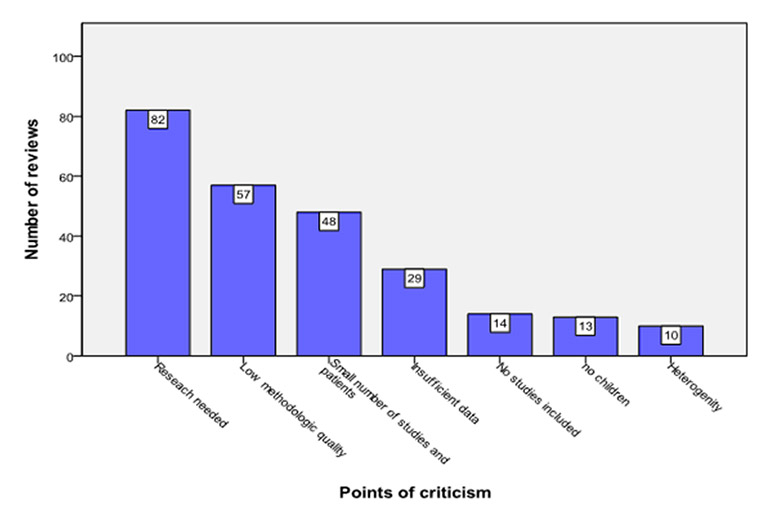
Figure 7
Most common criticisms in Cochrane reviews.
The disproportionately high number of inconclusive reviews, when compared with other paediatric subspecialities [39, 40], strongly emphasises that CAM needs to undergo the same rigorous evaluation process with standardised techniques as conventional medicine (i.e. randomised controlled trials), in order to substantiate scientifically its role in modern medicine [2]. Inconclusive reviews usually conclude that, after an extensive literature search and appraisal, “insufficient trial evidence was found to guide clinical practice”. One possible and important way to increase research quality and overcome common reasons for failure to generate specific recommendations (most importantly low number of study participants) will be the formation of national and international research networks.
Although defining clinical uncertainty and thereby generating new research questions is a fundamental driving force for EBM, clinicians at the bedside will find this frustrating and unhelpful. However, by identifying important gaps in the evidence, Cochrane reviews have the potential to promote high-quality randomised controlled trials in the field of CAM (as has been demonstrated in other fields of medicine, e.g. collaborative quality improvement initiatives such as the WOMBAT collaboration in Australasia (see: http://www.wombatcollaboration.net http://www.wombatcollaboration.net/ /) [41, 42]).
If the above outlined approach is bound to be successful, one would expect an increasing number of high quality (and probably conclusive) reviews that are based on sophisticated research data. However, the number of inconclusive reviews over the 18-year study period increased (from 15/27 [55.6%] to 33/44 [75%], and finally to 47/64 [73.4%]; a relative increase from 1995 to 2012 of 32.3%). Interestingly, a positive association was seen between the number of included studies/reviews and conclusiveness, which is line with reports from other specialities (surgery) [43].
It is also noteworthy that the vast majority of reviews published in the CRG database originate from western, industrialised countries (fig. 2), with the largest proportion of Cochrane reviews originating from the United Kingdom. Thus, the recommendations issued in these reviews are most applicable to the field of CAM as practised in industrialised countries, and will only in part be useful for the majority of children being cared for in the developing world. The substantial proportion of reviews from the United Kingdom may in part be attributed to the fact that the Centre for Evidence-Based Medicine is located in Oxford, England, and that the use of EBM plays an important role in the provision of healthcare in the United Kingdom. However, other underlying factors – both political and administrative ones – related to the British National Health Service may also contribute to this phenomenon.
Also of note, the majority of reviews evaluated interventions assessing “dietary supplements”. This may in part be attributable to the fact that large randomised controlled trials require adequate funding, which in many circumstances can best be provided by pharmaceutical companies. Importantly, recent research by the Cochrane collaboration has demonstrated that results of drug and device studies may be distorted through sponsorship by manufacturing and pharmaceutical companies (i.e. more favourable results and conclusions than with sponsorship by other sources) [44]. Other inventions in the field of CAM that may merit critical assessment in a randomised clinical trial may not find adequate sponsorship. Therefore, adequate funding from independent organisations will be vital to tackle and solve this issue.
Given the limited financial and human resources that are nowadays available in the medical arena, future emphasis must be on conditions for which new treatment modalities would have an important impact on the health system, children, families and healthcare workers. While some therapeutic modalities, such as homeopathy and reiki, are based on beliefs that lie outside the scope of science and may lack scientific plausibility [45], it is noteworthy that lack of scientific plausibility is not always and necessarily equivalent to a lack of efficacy. Moreover, it is important to take into consideration the clinical context of using certain therapies [22]: while vitamin preparations may be considered CAM therapies under certain circumstances, they may represent standard therapy in other clinical situations (vitamin K administration to prevent haemorrhagic events in neonates), they may have profound immunological properties (probiotics), or may be characterised as strong growth factors (vitamin A for lung development) [46].
It will be important that future research addresses safety issues, as well as assessing effectiveness of CAM in children. This is of great importance since CAM is commonly considered by its users as a safe medical method, and this contributes to its widespread prevalence. Therefore, prior to issuing recommendations for or against the use of some CAM in children, more rigorous testing of therapies using standardised techniques and systematic reporting of adverse events in practice and research needs to be put in place [47], as is the case for conventional medicine [48]. For children, this is a particularly relevant because they are unable to give informed consent to treatment, and therefore rely upon appropriate decisions about their care being made on their behalf [38].
Of note, adverse events with CAM may extend beyond the actual treatments themselves. It has been demonstrated that children who consult naturopathy and chiropractic practitioners are significantly less likely to receive the recommended vaccinations and are significantly more likely to suffer from a vaccine-preventable disease [49]. Also, the use of CAM products may lead to unintentional household poisoning, although symptoms of toxicity are usually mild [50].
However, it is important to note that some inherent weaknesses may be applicable to our data synopsis. First, Cochrane reviews themselves may be at risk of suffering from major weaknesses with regard to both clinical relevance (e.g. inclusion of primary trials that do not reflect clinical or “best” practice) and biased data [19, 51].
Second, a further possible shortcoming of our study was that we did not include data from sources other than the Cochrane database(e.g. randomised controlled trials and meta-analyses published in another form in the medical literature), thus possibly failing to provide a larger perspective on the current status of EBM in the field of CAM in children. Nevertheless, it is important to note that systematic reviews / meta-analyses as provided by the Cochrane Collaboration are considered to be scientific reports of the highest quality. Moreover, by performing this comprehensive analysis of all relevant Cochrane reviews in this field, we most likely have included the vast majority of all relevant randomised controlled trials. Although we used an up-to-date definition of CAM as provided by Wieland and co-workers [22], this operationalisation cannot be considered to be exhaustive, and will be subject to expansion over time.
In conclusion, based on our findings and in line with previous studies [2, 38], there is a need for more high quality research in children to assess whether there is a potential role for CAM in children. This will probably result in more systematic reviews that will come to clear conclusions (i.e. in favour or against a certain intervention or treatment modality, etc.). Moreover, the use of systematic reviews as provided by the CRG plays an important role in providing and disseminating the best available evidence in the field of CAM, thus contributing to the provision of good medical care at the bedside. The provision of high-quality data on the efficacy of CAM therapies in children will enable the physician to inform both patients and parents about potentially beneficial CAM options, especially if they involve less risk or fewer adverse effects than conventional therapy [21].
Acknowledgements:We thank Holger Nunold with technical help with the manuscript.
1 Zuzak TJ, Zuzak-Siegrist I, Simões-Wüst AP, Rist L, Staubli G. Use of complementary and alternative medicine by patients presenting to a Paediatric Emergency Department. Eur J Pediatr. 2009;168(4):431–7. Epub 2008 Jul 3
2 Snyder J, Brown P. Complementary and alternative medicine in children: an analysis of the recent literature. Curr Opin Pediatr. 2012;24:539–46.
3 Gottschling S, Gronwald B, Schmitt S, Schmitt C, Längler A, Leidig E, et al. Use of complementary and alternative medicine in healthy children and children with chronic medical conditions in Germany. Complementary Therapies in Medicine 2012 (in press).
4 Davis MP, Darden PM. Use of complementary and alternative medicine by children in the United States. Arch Pediatr Adolesc Med. 2003;157:393–6.
5 Hanson E, Kalish LA, Bunce E, Curtis C, McDaniel S, Ware J, et al. Use of complementary and alternative medicine among children diagnosed with autism spectrum disorder. J Autism Dev Disord. 2007; 37:628–36.
6 Lim A, Cranswick N, Skull S, South M. Survey of complementary and alternative medicine use at a tertiary children’s hospital. J Paediatr Child Health. 2005;41:424–7.
7 Kelly KM, Jacobson JS, Kennedy DD, Braudt SM, Mallick M, Weiner MA. Use of unconventional therapies by children with cancer at an urban medical center. J Pediatr Hematol Oncol. 2000;22:412–6.
8 Post-White J, Fitzgerald M, Hageness S, Sencer SF. Comple-mentary and alternative medicine use in children with cancer and general and specialty pediatrics. J Pediatr Oncol Nurs. 2009;26:7–15.
9 Gottschling S, Reindl TK, Meyer S, Berrang J, Henze G, Graeber S, et al. Acupuncture to alleviate chemotherapy-induced nausea and vomiting in pediatric oncology – a randomized multicenter crossover pilot trial. Klin Padiatr. 2008;220(6):365–70. Epub 2008 Oct 23.
10 Gottschling S, Meyer S, Gribova I, Distler L, Berrang J, Gortner L, et al. Laser acupuncture in children with headache: a double-blind, randomized, bicenter, placebo-controlled trial. Pain. 2008;137(2):405–12. Epub 2007 Nov 19
11 Robinson N, Blair M, Lorenc A, et al. Complementary medicine use in multi-ethnic paediatric outpatients. Complement Ther Clin Pract. 2008;14:17–24.
12 Jean D, Cyr C. Use of complementary and alternative medicine in a general pediatric clinic. Pediatrics. 2007;120:e138–41.
13 Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;10:1–23.
14 Upshur RE. Looking for rules in a world of exceptions: reflections on evidence-based practice. Perspect Biol Med. 2005;48:477–89.
15 McGuire W, Fowlie PW, Soll RF. What has the Cochrane Collaboration ever done for newborn infants? Arch Dis Child Fetal Neonatal Ed. 2010; 95:F2–F6.
16 Dodd JM, Crowther CA. Cochrane reviews in pregnancy: the role of perinatal randomized trials and systematic reviews in establishing evidence. Semin Fetal Neonatal Med. 2006;11:97–103.
17 Dammann O. Evidence-based child neurology. Developmental Medicine and Child Neurology. 2006;48:624.
18 Procopis PG. Evidence-based medicine. Developmental Medicine and Child Neurology 2002; 44:435.
19 Fønnebø V. Cochrane CAM reviews commentary: is there more to quality than the research method itself? Explore (NY). 2011;7(1):53–4. doi: 10.1016/j.explore.2010.10.010
20 Ezzo JM, Richardson MA, Vickers A, Allen C, Dibble SL, Issell BF, et al. Acupuncture-point stimulation for chemotherapy-induced nausea or vomiting. Cochrane Database of Systematic Reviews. 2006;(2):CD002285.
21 Gilmour J, Harrison C, Asadi L, Cohen MH, Vohra S. Informed consent: advising patients and parents about complementary and alternative medicine therapies. Pediatrics. 2011;128(Suppl 4):S187–92. doi: 10.1542/peds.2010-2720H.
22 Wieland LS, Manheimer E, Berman BM. Development and classification of an operational definition of complementary and alternative medicine for the Cochrane collaboration. Altern Ther Health Med. 2011;17(2):50–9.
23 Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
24 Lee A, Fan LTY. Stimulation of the wrist acupuncture point P6 for preventing postoperative nausea and vomiting. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No.: CD003281. doi: 10.1002/14651858.CD003281.pub3
25 Hao Q, Lu Z, Dong BR, Huang CQ, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database of Systematic Reviews 2011, Issue 9. Art. No.: CD006895. DOI: 10.1002/14651858.CD006895.pub2.
26 Lassi ZS, Haider BA, Bhutta ZA. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database of Systematic Reviews 2010, Issue 12. Art. No.: CD005978. doi: 10.1002/14651858.CD005978.pub2.
27 Kley RA, Tarnopolsky MA, Vorgerd M. Creatine for treating muscle disorders. Cochrane Database of Systematic Reviews 2011, Issue 2. Art. No.: CD004760. doi: 10.1002/14651858.CD004760.pub3
28 Hofmeyr GJ, Lawrie TA, Atallah ÁN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database of Systematic Reviews 2010, Issue 8. Art. No.: CD001059. doi: 10.1002/14651858.CD001059.pub3.
29 Turner D, Zlotkin SH, Shah PS, Griffiths AM. Omega 3 fatty acids (fish oil) for maintenance of remission in Crohn’s disease. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No.: CD006320. doi: 10.1002/14651858.CD006320.pub3.
30 Chen H, Zhuo Q, Yuan W, Wang J, Wu T. Vitamin A for preventing acute lower respiratory tract infections in children up to seven years of age. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No.: CD006090. doi: 10.1002/14651858.CD006090.pub2.
31 Wiysonge CS, Shey M, Kongnyuy EJ, Sterne JAC, Brocklehurst P. Vitamin A supplementation for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database of Systematic Reviews 2011, Issue 1. Art. No.: CD003648. doi:10.1002/14651858.CD003648.pub3.
32 Thien FCK, De Luca S, Woods RK, Abramson MJ. Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochrane Database of Systematic Reviews 2002, Issue 2. Art. No.: CD001283. DOI: 10.1002/14651858.CD001283.
33 Simmer K, Patole SK, Rao SC. Longchain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database of Systematic Reviews 2011, Issue 12. Art. No.: CD000376. doi: 10.1002/14651858.CD000376.pub3.
34 Schulzke SM, Patole SK, Simmer K. Longchain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database of Systematic Reviews 2011, Issue 2. Art. No.: CD000375. doi: 10.1002/14651858.CD000375.pub4.
35 Gogia S, Sachdev HS. Vitamin A supplementation for the prevention of morbidity and mortality in infants six months of age or less. Cochrane Database of Systematic Reviews 2011, Issue 10. Art. No.: CD007480. doi: 10.1002/14651858.CD007480.pub2.
36 Rumbold A, Middleton P, Pan N, Crowther CA. Vitamin supplementation for preventing miscarriage. Cochrane Database of Systematic Reviews 2011, Issue 1. Art. No.: CD004073. doi: 10.1002/14651858.CD004073.pub3.
37 Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, Murrell DF, Tang MLK. Probiotics for treating eczema. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No.: CD006135. doi: 10.1002/14651858.CD006135.pub2.
38 Hunt K, Ernst E. The evidence-base for complementary medicine in children: a critical overview of systematic reviews. Arch Dis Child. 2011;96(8):769–76. Epub 2010 Jul 6. Review.
39 Girisch W, Willhelm C, Gottschling S, Gortner L, Meyer S. Role of Cochrane reviews in pediatric neurology. Pediatr Neurol. 2012;46(2):63–9. Review.
40 Willhelm C, Girisch W, Gortner L, Meyer S. Evidence-based medicine and Cochrane reviews in neonatology: quo vadis? Acta Paediatr. 2012;101(4):352–3. doi: 10.1111/j.1651-2227.2011.02559.x. Epub 2012 Jan 9. Review. No abstract available.
41 Askie LM, Henderson-Smart DJ. Restricted versus liberal oxygen exposure for preventing morbidity and mortality in preterm or low birth weight infants. Cochrane Database of Systematic Reviews 2001, Issue 4. Art. No.: CD00107
42 Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al; Caffeine for Apnea of Prematurity Trial Group. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–902.
43 Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No.: MR000033. doi: 10.1002/14651858.MR000033.pub2.
44 Atwood K. The ongoing problem with the National Center for Complementary and Alternative Medicine. Skeptical Inquirer. 2003;27:3–11.
45 Diener MK, Wolff RF, von Elm E, Rahbari NN, Mavergames C, Knaebel HP, et al. Can decision making in general surgery be based on evidence? An empirical study of Cochrane Reviews. 2009;146(3):444–61. doi: 10.1016/j.surg.2009.02.016. Epub 2009 Jun 3.
46 Massaro D, Massaro GD. Lung development, lung function, and retinoids. N Engl J Med. 2010;362(19):1829–31.
47 Toupin April K, Moher D, Stinson J, Byrne A, White M, Boon H, Duffy CM, et al. Measurement properties of questionnaires assessing complementary and alternative medicine use in pediatrics: a systematic review. PLoS One. 2012;7(6):e39611. Epub 2012 Jun 29.
48 Wolff RF, Forbes CA. Can complementary medicine be based on evidence? Swiss Med Wkly 201;140:w13113.
49 Downey L, Tyree PT, Huebner CE, Lafferty WE. Pediatric vaccination and vaccine preventable disease acquisition: associations with care by complementary and alternative medicine providers. Matern Child Health J. 2010;14:922–30.
50 Zuzak TJ, Rauber-Lüthy C, Simões-Wüst AP. Accidental intakes of remedies from complementary and alternative medicine in children – analysis of data from the Swiss Toxicological Information Centre. Eur J Pediatr. 2010;169(6):681–8. Epub 2009 Oct
51 Higgins JP, Altman DG, Gotsche P, Jüi P, Moher D, Oxman AD, et al, Cochrane Bias Methods Group Cochrane Statistical Methods Group. BMJ. 2011;343; d5928
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.
Authors’ contribution: SM and NS contributed equally to the manuscript. Sascha Meyer and Nicole Schroeder were chief investigators. They were responsible for the study design, data analysis and data synthesis. Sascha Meyer was responsible for writing the manuscript. Ludwig Gortner, Sven Gottschling, Alexander Larsen and Georg Kutschke were involved in data retrieval and data analysis. They contributed to writing the manuscript. Stefan Gräber provided statistical expertise.