The impact of emotion on perception, attention, memory, and decision-making
DOI: https://doi.org/10.4414/smw.2013.13786
Tobias
Brosch, Klaus
Scherer, Didier
Grandjean, David
Sander
Summary
Reason and emotion have long been considered opposing forces. However, recent psychological and neuroscientific research has revealed that emotion and cognition are closely intertwined. Cognitive processing is needed to elicit emotional responses. At the same time, emotional responses modulate and guide cognition to enable adaptive responses to the environment. Emotion determines how we perceive our world, organise our memory, and make important decisions. In this review, we provide an overview of current theorising and research in the Affective Sciences. We describe how psychological theories of emotion conceptualise the interactions of cognitive and emotional processes. We then review recent research investigating how emotion impacts our perception, attention, memory, and decision-making. Drawing on studies with both healthy participants and clinical populations, we illustrate the mechanisms and neural substrates underlying the interactions of cognition and emotion.
Introduction
The functioning of the human mind has often been characterised as a battle between opposing forces: reason, rational and deliberate, versus emotion, impulsive and irrational. This way of thinking can be traced back to Plato, who described the human soul as divided into cognition (what we know), emotion (what we feel), and motivation (what we want), and has been further developed by philosophers such as René Descartes (“Les passions de l’âme”) and David Hume (“A treatise of Human Nature”). For a long time, the notion of the opposition of cognition and emotion has been guiding much research in psychology. Cognitive functions – such as perception, attention, memory, or decision-making – have been investigated without taking into account emotion, which was considered as interference that is counterproductive for the correct functioning of the cognitive system.
After a long time of neglect, the last two decades have seen an enormous increase in research on emotion, highlighting the importance of emotional processes for a successful functioning of the human mind. For example, neuropsychological studies have shown that patients with emotional dysfunctions due to brain lesions can be highly impaired in everyday decision-making and social interactions. Neuroimaging studies have demonstrated how brain regions previously thought to be purely “emotional” (e.g., amygdala) or “cognitive” (e.g., frontal cortex) closely interact to make complex behaviour possible. Psychology experiments have illustrated how emotion can change our perception, attention, and memory by focusing them on important aspects of the environment. This research has revealed to what extent emotion and cognition are related, or even inseparable.
In this review, we provide an overview of current theorising and research in the Affective Sciences. We begin by describing how current psychological theories of emotion conceptualise the intertwined functioning of cognitive and emotional processes. We then review recent research on how emotion impacts our perception, attention, and memory, and on the role of emotion in decision-making. Drawing on studies with both healthy participants and clinical populations, we illustrate the psychological mechanisms and the neural substrates that underlie the interactions of cognition and emotion.
What is an emotion?
Donald Hebb wrote that man is the most emotional of all animals, referring to the fact that the degree of emotionality increases across species with the development of more sophisticated nervous systems [1]. This observation suggests that emotion may perform an adaptive function that requires a certain degree of processing complexity. We have previously theorised that the function of emotion is to decouple stimulus and behavioural response, thus allowing for a flexible adaptation to environmental contingencies [2]. A reflex or a fixed action pattern inflexibly links a certain stimulus to a response, whereas an emotional response creates a latency time during which physiological responses can be initiated and several appropriate action tendencies can be prepared while the situation is further analysed. Thus, the organism benefits from a more thorough appraisal of the situation, but does not lose time as potential responses are already prepared and can be executed rapidly.
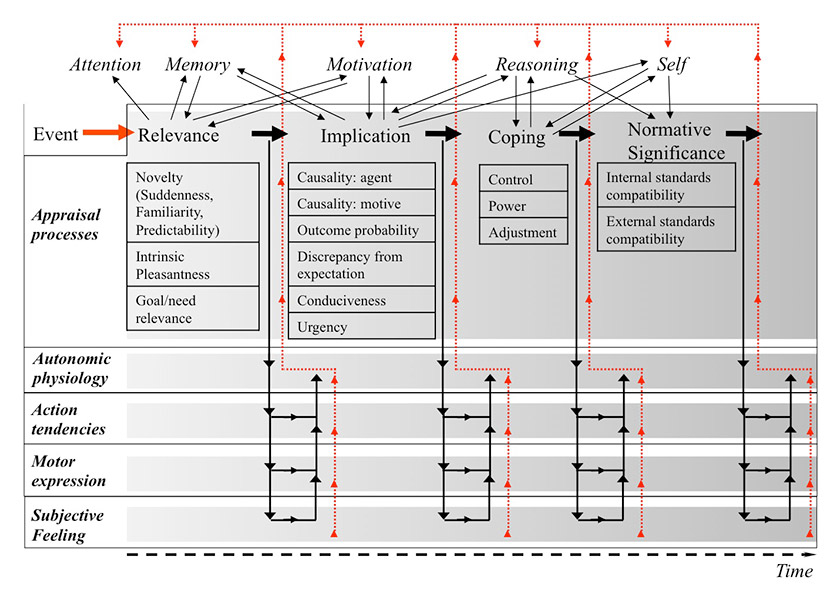
Figure 1
Comprehensive illustration of the Component Process Model. The model assumes that the appraisal process is organised in a sequence of four appraisal objectives. Each appraisal outcome leads to changes in the other emotion components (physiology, action tendency, motor expression, and subjective feeling, as illustrated by the descending arrows). The ongoing appraisal process furthermore interacts with other cognitive functions. For example, minimal attention needs to be given for appraisal to start, but a relevance outcome will immediately deploy further attention to the stimulus. The model thus allows a detailed consideration of the effects of emotional processes on attention, memory, and other cognitive processes (as illustrated by the arrows in the upper part of the figure). Finally, changes in the other emotion components may also influence cognitive functions (as illustrated by the red ascending arrows), for example by increased memory consolidation via increases in physiological arousal, which may in turn influence the processing of appraisal criteria.
We define emotion as an event-focused process consisting of (a) specific elicitation mechanisms based on the relevance of a stimulus that (b) shape an emotional response instantaneously across several organismic subsystems, including motivational changes (changes in action tendency, such as approach versus withdrawal), physiological changes (e.g., heart rate, skin conductance), changes in motor expression (in face, voice, and body), and changes in subjective feeling [3, 4]. Emotions are elicited as the individual continuously evaluates objects, events and situations with respect to their relevance for his/her needs, goals, values, and general well-being (appraisal). The detection of a relevant event elicits an adaptive emotional response that mobilises resources that allow the individual to cope with the situation.
Psychological appraisal theories of emotionmore specifically describe the evaluation process that underlies the elicitation of emotion. For example, the Component Process Model of Emotion[4–7] organises appraisal into four sequential objectives that concern the major types of information required to adaptively react to a salient event (fig. 1): (1) Relevance: How relevant is this for me? Does it directly affect me or my social reference group? (2) Implications: What are the implications or consequences of this event and how do these affect my well-being and my immediate or long-term goals? (3) Coping potential: How well can I cope with or adjust to these consequences? (4) Normative significance: What is the significance of this event with respect to my self-concept and to social norms and values? The appraisal outcome organised according to these criteria drives the response pattern consisting of physiological reactions, motor expressions, and action preparation. Importantly, the appraisal of a given event may be highly subjective and a function of individual characteristics and the specific situation, which explains that different people may react to the same situation with different emotions, and the same person may react with a different emotion each time when exposed to similar situations [8, 9].
This model illustrates the rich interactions of emotion and cognition, as the computation of the different appraisal criteria requires the contribution of many cognitive processes for the comparison of stimulus features to stored affective schemata, memory representations, expectations and motivational urges. At the same time, the outcome of the appraisal computation modulates cognitive functions. For example, the appraisal of a stimulus as “relevant” causes rapid allocation of attention toward this stimulus [10]. Similarly, emotionally relevant information is prioritised in memory, potentially via increases in autonomic physiology (arousal) as a consequence of stimulus appraisal [11]. Thus, an emotional episode is characterised by a high interdependence between the organismic subsystems that constitute an emotional response and important cognitive functions with their underlying neural circuits [12, 13]. We will now illustrate in more detail how cognitive functions such as perception, attention, memory and decision-making are modulated during the course of an emotional response.
The impact of emotion on perception and attention
In our everyday environment, we are constantly confronted with large amounts of incoming sensory information. As the capacity of our brain is limited, we cannot process all information entering our senses thoroughly, but have to select a subset to prioritise its processing at the cost of other information. The competition for neural processing resources, in-depth analysis and preferential access to conscious awareness is organised by dedicated attention systems [14]. Distinct functional subprocesses of attention have been put forward, and their respective properties have been isolated using both behavioural and brain-imaging methods. Low-level properties such as the physical intensity of a stimulus may trigger an automatic, reflexive orienting, referred to as exogenous attention. In contrast, stimuli that are important to the current behaviour of the organism (e.g., when searching for one’s keys, or trying to find a friend in a crowd) are selected by a voluntary top-down deployment of endogenous attention, driven by implicit or explicit expectations for a specific object or location. According to a recent neurocognitive model of attention, both endogenous and exogenous attention primarily implicate frontoparietal networks of cortical regions [15], with endogenous attention control being exerted by interactions of dorsal regions such as the intraparietal sulcus and the frontal eye fields, and the exogenous reorienting of the attentional focus mediated by ventral regions in the right hemisphere such as the right ventral frontal cortex and temporoparietal junction.
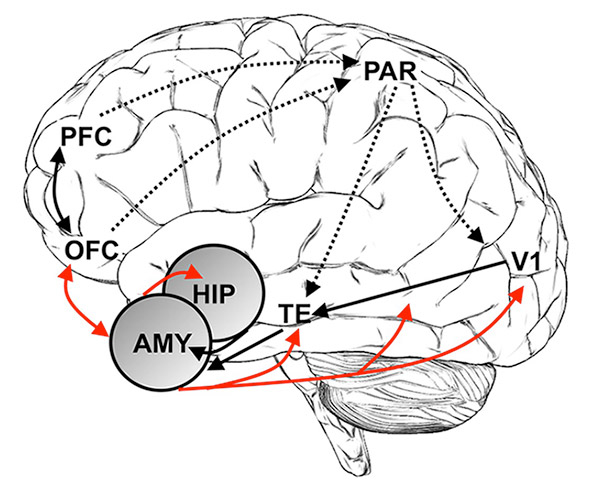
Figure 2
Schematic illustration of brain areas involved in selected cognition-emotion interactions. The amygdala (AMY) has widespread reciprocal connections with many cortical regions, including perceptual pathways (primary visual cortex, V1, inferior temporal cortex, TE), memory-related regions (hippocampus, HIP), and prefrontal regions (orbitofrontal cortex, OFC, prefrontal cortex, PFC). This connectivity enables the amygdala to receive rich sensory information and to strengthen the neural representation of emotional stimuli via feedback to sensory pathways, attention-related regions (PFC and parietal lobe, PAR), and memory regions. In addition, multiple connections to prefrontal areas relay amygdala input to regions involved in more deliberate forms of decision-making and allow for modulations of amygdala activity based on more complex motivational contingencies. Thus, following amygdala activation, multiple brain systems can dynamically reorganise to appropriately deal with the current environment.
In addition to endogenous and exogenous attention mechanisms, the emotional relevance of a stimulus constitutes another important feature influencing attentional selection and prioritisation [emotional attention, see 16]. Behavioural findings across many different tasks and paradigms indicate that perception is facilitated and attention prioritised for emotional information. Emotional stimuli may draw attention more rapidly and impede attentional disengagement for a longer time than neutral stimuli. For example, in visual search tasks, the detection of a target among distractors is faster when the target is emotional, as opposed to neutral [17]. Once attention has been drawn to and engaged by emotional stimuli, it may dwell longer at their location and facilitate the processing of subsequent non-emotional target stimuli appearing at the same location. Such emotional orienting effects have been demonstrated using the dot probetask, where participants respond to a target stimulus that replaces one of two simultaneously presented cues – one being emotionally significant (e.g., a fearful face) and the other neutral. Typical results show faster responses to targets replacing the emotional rather than the neutral cue [18]. Emotional cueing may not only lead to faster detection times, but also directly augment our perceptual capacity by increasing contrast sensitivity for the subsequent target [19]. Thus, emotion processing does not only enrich our experiences with affective flavour, but can directly shape the content of our percepts and awareness [20]. These effects operate not only in the visual, but also in the auditory modality [21] and even across sensory modalities, suggesting that the prioritisation of emotionally relevant stimuli is organised supramodally across multiple sensory channels [22]. Attentional biases can be dysfunctionally exaggerated in clinical populations, for example in patients with anxiety disorders who show especially strong attentional capture by threatening information [23].
Brain imaging studies using fMRI have consistently revealed increased neural responses to many different emotional stimuli, both in early sensory areas [24] and in higher-level regions associated with body [25], face [26] or voice [21] recognition. Thus, emotional stimuli are represented by more robust neural signatures than neutral ones, and can consequently profit from preferential access to further cognitive processing, behaviour control and awareness. It has been suggested that the prioritisation of emotional information is driven by dedicated neural circuits, centred on the amygdala, and partially separate from the frontoparietal networks involved in endogenous and exogenous attention allocation [16, 27]. The amygdala is a limbic region that is critically involved in the processing of emotional information. Whereas the amygdala has long been thought of as a module specialised for the processing of threat [28], more recent proposals have suggested that the amygdala subserves the rapid detection of the relevance of a stimulus for the needs, goals, and values of the organisms [29, 30]. Once the amygdala has determined the relevance of incoming sensory stimuli, it may then modulate the processing of these stimuli through direct feedback projections to sensory cortex and biasing signals to fronto-parietal attention regions [27, 31].
Consistent with this suggestion, several brain imaging studies have reported that cortical increases to emotional stimuli were significantly correlated with amygdala responses; i.e., the more the amygdala was sensitised to the emotional meaning, the more modulation was observed in sensory areas [24]. Patients with medial temporal lobe sclerosis leading to amygdala damage do not show the cortical increases to emotional information, which supports a causal role of the amygdala in the boosting of the neural representation of emotional information [32]. The amygdala may not only have a direct impact on sensory cortices, thus augmenting the neural representation of the emotional stimulus, but it may also recruit fronto-parietal attention networks toward the location of the stimulus, so that subsequent information arising at the same location as emotional cues will benefit from enhanced processing resources. Thus, emotion modulates our perception and attention by privileging stimuli that are especially emotionally relevant [10]. This mechanism may help us organise the perception of our environment depending on our current needs, goals and values. The automatic detection of emotionally relevant events allows unexpected, but emotionally relevant events to be noticed readily and, once detected, become the focus of attention, evaluation, and action.
The impact of emotion on memory
Memories of emotional events have a persistence and vividness that other memories seem to lack. Abundant examples for this are found in the literature, for example in Marcel Proust’s "Remembrance of Things Past", where he describes how eating a madeleine evokes a strong memory of his childhood: "No sooner had the warm liquid mixed with the crumbs touched my palate than a shudder ran through me and I stopped, intent upon the extraordinary thing that was happening to me.…And suddenly the memory revealed itself. The taste was that of the little piece of madeleine which on Sunday mornings at Combray, when I went to say good morning to her in her bedroom, my aunt Léonie used to give me, dipping it first in her own cup of tea or tisane." Similarly, we all have intense and detailed memories of events such as the birth of a child or the death of a loved one. The psychologist William James wrote that “an impression may be so exciting emotionally as almost to leave a scar upon the cerebral tissues” [33].
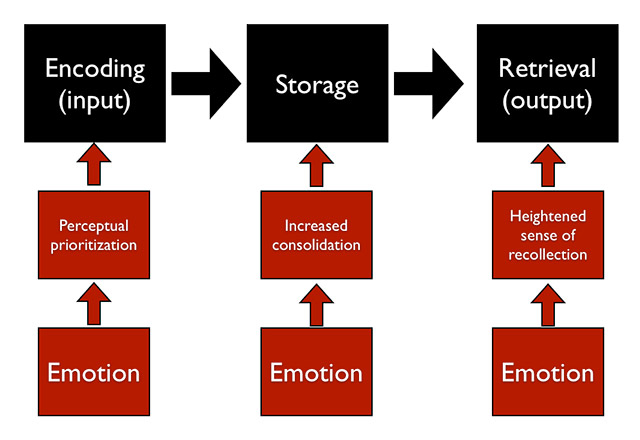
Figure 3
Schematic illustration of the impact of emotion on three different stages of memory processing: (1) By prioritising the perception of emotionally relevant information, encoding of this information may be strengthened. (2) By modulating the consolidation process of emotional information via increases in arousal, emotional information may form more robust memory traces. (3) By enhancing the subjective recollective experience of emotional information, emotional memories may become more central to the planning of current behaviour (see text for more details).
One of the most important neural regions underlying memory processes is the hippocampus, situated in the medial temporal lobe. Patients with lesions of the hippocampus suffer from amnesia, having difficulties remembering old memories or forming new ones [34]. The hippocampus is situated adjacent to the amygdala, which, similar to its role in attention and perception, can modulate the neural circuitry underlying memory processes during emotional situations. Thus, in addition to its important role in the acquisition and expression of implicit fear [35], the amygdala is central to the processing of explicit emotional memories via its interactions with hippocampal memory formation.
Memory processing can be divided into three stages: Encoding (the processing of information at the moment of perception), consolidation (the storage of information in the brain), and retrieval (the moment of remembering). Emotion can have a modulating effect at each of these stages [36–39]. As outlined in the previous section, perception and attention are focused on emotionally relevant information, which may result in preferential encoding of the emotional information [37]. As a further consequence, less attention is directed toward peripheral information, so that during encoding the emotional core aspects of a scene are well memorised, whereas the details of the surrounding context may be neglected. One example for this is the “weapon focus”; the presence of a weapon in a scene results in a good memory for the details of the weapon and other stimuli in close proximity, but a poor memory for the other details of the scene such as the face of the aggressor [40].
The storage of information in memory is not terminated immediately after encoding. It takes some time for memory traces to stabilise in a consolidation process which depends largely on the hippocampus [11]. During the consolidation phase, memories are fragile and prone to disruption and modification. The memory trace representing an event can be strengthened (in this case, the memory will be remembered later) or weakened (in this case, the memory is forgotten or distorted). Emotion may modulate this consolidation process: A strong emotional response elicits physiological arousal by which the amygdala can modulate hippocampal activation, leading to an augmentation of specific memory traces [36]. Via this mechanism, emotionally relevant events can profit from a stronger consolidation, which increases the possibility that the event is remembered later. This enhanced consolidation may occur some time after the encoded event, making a retrospective reinforcement of emotional memory content possible. Thus, events that contain important information for the well-being of the organism may be privileged in memory, and as a consequence, be retrieved and used to plan behaviour in the future [41].
Finally, emotion can augment the subjective sense of recollection (independent of the correctness of the memory), which can increase the confidence we have in our memories. The vividness of the memory of emotionally relevant events is often taken as an indication that the memory is accurate. For example, many of us have very vivid memories of the circumstances under which we learned about the terrorist attacks on the World Trade Center in Manhattan in 2001. However, when a group of participants were asked to write down the exact circumstances on the day after the attack, and a few months later were asked to recall the situation, it was found that their memory had deteriorated to a similar extent as it did for neutral events. However, in contrast to neutral memories, participants were convinced that their emotional memories were correct, reflecting an increased sense of recollection [42]. At the neural level, an increased sense of recollection for neutral scenes is related to higher activation in the parahippocampus (which indeed reflects better memory performance for details of the situation), whereas for emotional scenes, the sense of greater recollection (but not necessarily better memory performance) is related to increases in amygdala activation [39]. Interestingly, the enhancement of the subjective recollective experience by emotion is more robust and consistent than its enhancement of accuracy for objective details. Whereas this seems not very adaptive at first glance, it may actually help us to react more efficiently in time-critical situations. In new or uncertain situations, we use memory information about similar previous situations to guide our thoughts and actions. In situations that require a rapid response, for example when confronted with an immediate threat, a hesitation to use a memory we are unsure about may be very costly. Enhanced confidence in memories from emotionally charged situations may lead to faster action in critical situations, even if the details of the memory are not absolutely accurate [38].
The impact of emotion on decision-making
According to the rational worldview, we base our decisions on logic: Whenever we are faced with a choice, we evaluate the options, weigh the possible consequences and their probabilities, and then choose the option with the highest utility. However, recent research has shown that emotion is central to the decision-making process, both as an input and an output [43]. Decisions and their consequences result in emotions (such as joy, relief, regret, or disappointment), and many of our choices are guided by the experience of these emotions or the anticipation of the emotions that may be elicited [interestingly, however, we are not very good at predicting which emotions we will feel in the future, see 44].
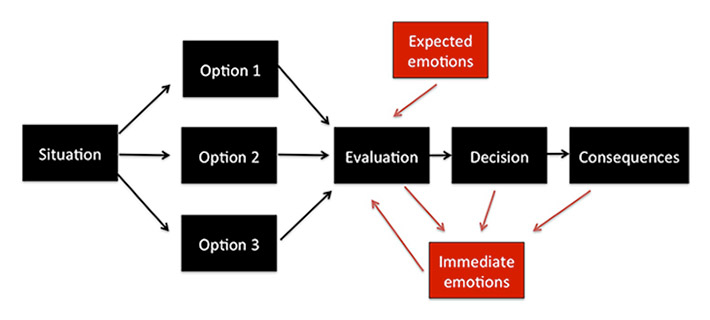
Figure 4
Schematic illustration of the stages of decision-making and the impact of emotion. When faced with a choice, we evaluate the options, weigh the possible consequences and their probabilities, and choose an option, and are confronted with the consequences. Expected emotions may influence our evaluation of behavioural options (for example, I may expect to be very happy once I buy the new luxury car, or I may have a gut feeling that it’s better to avoid a certain car dealer) and thus inform the choice at hand. Once a decision has been taken, the immediate consequences will also elicit emotions such as joy, relief, regret or disappointment, which may inform future choices or lead us to modify our current choice.
Recent neuroscientific studies show that emotion can help us make very “rational” choices, especially under complex conditions when the outcomes are uncertain. Brain-damaged patients with lesions of the ventromedial prefrontal cortex often show actions that are detrimental to their well-being, leading to financial losses, to losses in social standing, and to conflicts with family and friends. These patients show flat affect, i.e., reduced emotional reactivity to many types of events, reduced physiological reactivity and a very limited interoception of their bodily responses, whereas their intellect and problem-solving abilities remain largely intact [45]. Together, these observations have led to the hypothesis that emotions may play an important role in guiding our decisions. Congruent evidence has been obtained with the Iowa Gambling Task [46]. In this card game participants pick cards from four different decks (A, B, C, and D). With each card, they can win or lose certain amounts of money. Critically, and unknown to the participants, two card decks are more advantageous than the others: In decks A and B (the “bad decks”), there are some cards that lead to a substantial gain (100 USD), but there are also cards that lead to huge losses (minus 1,250 USD). Decks C and D (the “good decks”) contain cards that leads to smaller gains (50 USD), but also losses that are less severe (minus 250 USD). Thus, even if at first selecting decks A and B can lead to faster financial gains, in the long run, the good strategy is to select cards only from decks C and D. Healthy participants quickly develop a preference for the good decks, while showing stronger anticipatory skin conductance responses whenever choosing from the bad decks. In contrast, participants with lesions of the ventromedial prefrontal cortex usually do not manage to “read” the game: they keep choosing cards from the bad decks until the end of the game, and do not show increased skin conductance responses [47]. According to the somatic marker hypothesis [48], our choices and decisions are informed by bodily reactions that are triggered by emotion. These so-called somatic markers are body states that have been elicited by rewards or punishments in the past and been associated with certain situations or choices. When a person is deliberating several behavioural options, the physiological reactions associated to previous choices are re-enacted or anticipated in ventromedial prefrontal cortex, and may inform the current decision, e.g., by helping us reject less advantageous options. Whereas the somatic marker hypothesis is very influential and has inspired a large amount of research, it has nevertheless been criticised on the grounds that the causal role of peripheral feedback for decisions in the gambling task has not yet been convincingly demonstrated [49].
In a functional neuroimaging study investigating the brain bases of the impact of emotion on decision-making, we have recently asked our participants to distribute a monetary amount between themselves and a charitable organisation (they could choose between Greenpeace, Amnesty International or the Red Cross). In each of 100 trials, participants chose between an altruistic behaviour (giving money to charity) or an egoistic behaviour (keeping the money for themselves). Selfish participants, i.e., participants who donated little or no money to charity, showed increased BOLD signal in the amygdala and the ventral striatum, two neural regions that represent the reward value of a stimulus, when deciding to keep money for themselves. These affective responses may bias the choices of the participants toward more selfish behaviour at the cost of the charitable organisations [50, 51].
However, emotion may also help us ensure the long-term interests of the society by controlling individuals who behave too egoistically. In the Ultimatum Game, a game widely used in neuroeconomic experiments, one player (the proposer) receives an amount (e.g., 20 CHF) from the experimenter and has to propose a way of sharing the money between himself and the second player (the responder). The proposer can make any offer to the responder, from sharing equally (10:10) to keeping everything for him- or herself (20:0). However, in the next step the responder can either accept or reject the offer. If the responder rejects, neither the proposer nor the responder will get anything. A responder that acts on a strictly economic and rational basis would accept any offer larger than 0 (even a 19:1 split), because receiving 1 CHF is better than receiving nothing. However, this is not what is observed in studies all around the world [52]: Very frequently, responders reject offers of 5 CHF or less, as they perceive these offers as unfair. Thus, the personal financial gain is sacrificed to punish the unfair proposer. The perception of unfair offers is accompanied by increased activation in the anterior insula, a brain region involved in the representation of bodily changes and emotions such as disgust [53]. In fact, the more a responder’s insula is activated by an unfair offer, the more likely the responder will reject the offer. Economists have termed the foregoing of an individual gain in the interest of the group altruistic punishment. It has been shown that groups that apply altruistic punishment to keep free riders in check function much better in the long run [54]. Furthermore, it has been shown that altruistic punishment goes along with an activation of the striatum, which is involved in the representation of subjective reward in the brain [55]. Thus, even though altruistic punishment involves financial costs, it is nevertheless perceived as rewarding and thus can ensure the long-term collaboration at the group level. These “emotional” responses can be more “rational” than decisions purely based on (economic) reason.
Conclusions
Our review of recent research from the Affective Sciencesshows that the duality of reason versus emotion that has been propagated for a long time is not reflected in the architecture of the brain and the functioning of the mind. Emotion and cognition are closely intertwined, complex human behaviour emerges from dynamic interactions between multiple processes and brain networks. Emotion determines how we perceive our world, how we remember it, and which decisions we take. Like any other complex system, emotion may go awry, as illustrated for example by exaggerated attentional bias to threat in anxiety [23] or preferential memory for negative events in depression [56]. However, when functioning normally, emotion should be considered as useful guide, far from being irrational, that helps us navigate our complex environment.
References
1 Hebb D. The organization of behavior. 1949, New York: Wiley.
2 Scherer KR. Emotion serves to decouple stimulus and response, in The nature of emotion: Fundamental questions, P. Ekman and R.J. Davidson, Editors. 1994, Oxford University Press: New York/Oxford. p. 127–130.
3 Sander D. Models of emotion: The affective neuroscience approach, in Handbook of Human Affective Neuroscience, J.L. Armony and P. Vuilleumier, Editors. in press, Cambridge University Press: Cambridge.
4 Scherer KR. Appraisal considered as a process of multilevel sequential checking, in Appraisal processes in emotion: Theory, methods, research, K.R. Scherer, A. Schorr, and T. Johnstone, Editors. 2001, Oxford University Press: New York. p. 92–120.
5 Scherer KR. Toward a dynamic theory of emotion: The component process model of affective states. Geneva Studies in Emotion and Communication, 1987;1(1):1–98.
6 Scherer KR. The dynamic architecture of emotion: Evidence for the component process model. Cognition & Emotion. 2009;23(7):1307–51.
7 Scherer KR. On the nature and function of emotion: A component process approach, in Approaches to emotion, K.R. Scherer and P. Ekman, Editors. 1984, Erlbaum: Hillsdale. p. 293–317.
8 Siemer M, Mauss I, Gross JJ. Same situation – Different emotions: How appraisals shape our emotions. Emotion, 2007;7(3):592–600.
9 Scherer KR, Brosch T. Culture-specific appraisal biases contribute to emotion dispositions. Eur J Pers. 2009;23:265–88.
10 Brosch T, Sander D, Pourtois G, Scherer KR. Beyond fear: Rapid spatial orienting towards positive emotional stimuli. Psychological Science. 2008;19:362–70.
11 McGaugh JL. Memory – a century of consolidation. Science. 2000;287(5451):248–51.
12 Grandjean D, Sander D, Scherer KR. Conscious emotional experience emerges as a function of multilevel, appraisal-driven response synchronization. Conscious Cogn. 2008;17(2):484–95.
13 Brosch T, Sander D. The appraising brain: Towards a neuro-cognitive model of appraisal processes in emotion. Emotion Review. 2013;5:163–8.
14 Driver J. A selective review of selective attention research from the past century. Br J Psychol. 2001;92(1):53–78.
15 Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–15.
16 Brosch T, Pourtois G, Sander D, Vuilleumier P. Additive effects of emotional, endogenous, and exogenous attention: behavioral and electrophysiological evidence. Neuropsychologia. 2011;49(7):1779–87.
17 Ohman A, Lundqvist D, Esteves F. The face in the crowd revisited: A threat advantage with schematic stimuli. J Pers Soc Psychol. 2001;80(3):381–96.
18 Brosch T, Van Bavel JJ. The flexibility of emotional attention: Accessible social identities guide rapid attentional orienting. Cognition. 2012;125(2):309–16.
19 Phelps EA, Ling S, Carrasco M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychol Sci. 2006;17(4):292–9.
20 Brosch T, Pourtois G, Sander D. The perception and categorization of emotional stimuli: A review. Cogn Emot. 2010;24:377–400.
21 Grandjean D, Sander D, Pourtois G, Schwartz S, Seghier ML, Scherer KR, et al. The voices of wrath: Brain responses to angry prosody in meaningless speech. Nat Neurosci. 2005;8(2):145–6.
22 Brosch T, Grandjean D, Sander D, Scherer KR. Cross-modal emotional attention: Emotional voices modulate early stages of visual processing. J Cogn Neurosci. 2009;21:1670–9.
23 Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133(1):1–24.
24 Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24(4):1265–70.
25 Peelen M, Atkinson A, Andersson F, Vuilleumier P. Emotional modulation of body-selective visual areas. Soc Cogn Affect Neurosci. 2007;2:274–83.
26 Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron. 2001;30(3):829–41.
27 Vuilleumier P. How brains beware: Neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9(12):585–94.
28 Ohman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108(3):483–522.
29 Cunningham WA, Brosch T. Motivational salience: Amygdala tuning from traits, needs, values, and goals. Curr Dir Psychol Sci. 2012;21:54–9.
30 Sander D, Grafman J, Zalla T. The human amygdala: An evolved system for relevance detection. Rev Neurosci. 2003;14(4):303–16.
31 Vuilleumier P, Brosch T. Interactions of emotion and attention, in The Cognitive Neurosciences IV, M.S. Gazzaniga, Editor. 2009, MIT Press: Cambridge. p. 925–34.
32 Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7(11):1271–8.
33 James W. The principles of psychology. 1890, New York: Dover.
34 Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986;6(10):2950–67.
35 Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53.
36 Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42(5):855–63.
37 Phelps EA. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14(2):198–202.
38 Phelps EA, Sharot T. How (and why) emotion enhances the subjective sense of recollection. Curr Dir Psychol Sci. 2008;17(2):147–52.
39 Sharot T, Delgado MR, Phelps EA. How emotion enhances the feeling of remembering. Nat Neurosci. 2004;7(12):1376–80.
40 Loftus EF, Loftus GR, Messo J. Some facts about weapon focus. Law Hum Behav. 1987;11:55–62.
41 Montagrin A, Brosch T, Sander D. Goal-conduciveness as a key determinant of memory facilitation. Emotion, in press.
42 Talarico JM, Rubin DC. Confidence, not consistency, characterizes flashbulb memories. Psychol Sci. 2003;14(5):455–61.
43 Han S, Lerner JS. Decision making, in The Oxford Companion to the Affective Sciences, D. Sander and K.R. Scherer, Editors. 2009, Oxford University Press: New York.
44 Wilson TD, Gilbert DT. Affective forecasting – Knowing what to want. Curr Dir Psychol Sci. 2005;14(3):131–4.
45 Damasio AR. Descartes’ error: Emotion, reason, and the human brain. 1994, New York: Putnam Publishing.
46 Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends Cogn Sci. 2005;9(4):159–62.
47 Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275(5304):1293–5.
48 Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 1996;351(1346):1413–20.
49 Dunn BD, Dalgleish T, Lawrence AD. The somatic marker hypothesis: a critical evaluation. Neurosci Biobehav Rev. 2006;30(2):239–71.
50 Brosch T, Coppin G, Scherer KR, Schwartz S, Sander D. Generating value(s): Psychological value hierarchies reflect context-dependent sensitivity of the reward system. Soc Neurosci. 2011;6:198–208.
51 Brosch T, Coppin G, Schwartz S, Sander D. The importance of actions and the worth of an object: Dissociable neural systems representing core value and economic value. Soc Cogn Affect Neurosci. 2012;7:497–505.
52 Henrich J, Boyd R, Bowles S, Camerer C, Fehr E, Gintis H, et al. “Economic man” in cross-cultural perspective: behavioral experiments in 15 small-scale societies. Behav Brain Sci. 2005;28(6):795–815.
53 Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300(5626):1755–8.
54 Fehr E, Gachter S. Altruistic punishment in humans. Nature. 2002;415(6868):137–40.
55 de Quervain DJ, Fischbacher U, Treyer V, Schellhammer M, Schnyder U, Buck A, et al. The neural basis of altruistic punishment. Science. 2004;305(5688):1254–8.
56 Dalgleish T, Watts FN. Biases of Attention and Memory in Disorders of Anxiety and Depression. Clin Psychol Rev. 1990;10(5):589–604.



