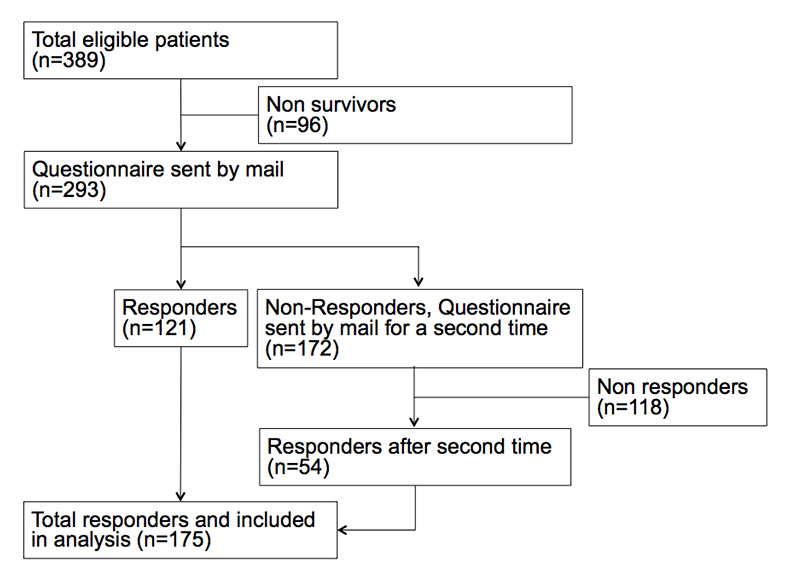
Figure 1
Patient inclusion flow chart AP-DRG Hospital.
DOI: https://doi.org/10.4414/smw.2013.13790
The increasing costs for medical treatment put an important burden on societies world-wide. In 2010, the total national health expenditures in the US amounted to $2.6 trillion, which related to 17.6% of the gross national product (GNP) [1]. In the same year, the annual cost in Switzerland amounted to $62.5 translating into 11.4% of GNP [2]. A large part of these costs are due to expensive inpatient treatment and are directly correlated to length of hospital stays (LOS) in fee-for-service (FFS) based systems. Shortening LOS is thus an important measure for cost control. In addition, studies have found that shorter LOS may result in improved patient outcomes, including lower risk for hospital acquired infections and hospital-acquired disabilities, and improved patient satisfaction [3, 4]. For these reasons diagnosis related groups (DRG) based reimbursement systems have been propagated, where hospitals are paid a lump sum based on the patient’s condition, mainly independent of the LOS [5]. However, the effects of such incentives on quality of care and patients’ outcomes remain largely unknown and an increase of so-called “bloody exits” is postulated, which would be preterm discharge of patients not diagnosed and treated properly. There is literature about this topic, mostly from the United States and dating back to the 1980’s when the country introduced a DRG system [6–8].
The beginning of the development of the DRG system started at Yale University in the 1970’s. The Swiss DRG is based on the German (G-) DRG, which is based on the Australian refined (AR-) DRG. The source of this Australian version lies in the all-patient (AP-) DRG [9].
In Switzerland, although hospital remuneration was based primarily on FFS before 2012, a smaller group of hospitals already used a reimbursement system similar to the AP-DRG as a precursor of the SwissDRG introduced on January 1st, 2012. The AP-DRG system in Switzerland consists of about 648 case groups. To put a patient into a case group the following information is needed: main diagnosis, secondary diagnoses, the surgical and diagnostic interventions, the age, sex, the kind of discharge and, for newborns, the birth weight. The aim of this information is to put patients into their appropriate case group. All patients in one group are supposed to have a similar course of disease and therapy [9] and are reimbursed by the same flat rate. The coding of the diseases is based on ICD-10 (International Classification of Diseases) and the CHOP (Swiss classification of surgical operations) [10].
A further advantage of the DRG is the possibility of benchmarking among hospitals. In that way it is possible to compare the severity of a patient’s disease by way of using the case-mix-index (= mean cost weight of a hospital).
One of the challenges of the AP-DRG and the DRGs in general is that the hospital may have a financial incentive to discharge patients too early. For that reason good quality control is appropriate. Another challenge implementing the Swiss DRG is that the quality of care may decrease.
Before 2012, the reimbursement modalities for inpatient treatment in Switzerland differed between different regions and different states (cantons). Hospitals in some cantons used DRG based reimbursement systems and in other hospitals FFS based systems were used. This unique situation allowed the two systems to be compared, with a hospital for each one as proxy in a head-to-head comparison. We have previously compared the two systems in a secondary analysis of a former randomised-controlled trial (RCT) focusing thereby only on patients with pneumonia [11]. In that analysis, we found LOS in DRG hospitals to be significantly shorter compared to FFS hospitals by roughly 20% and no differences in outcomes. The current prospective study intended to expand these findings to other diagnoses.
Thus, within this survey study focusing on four well defined and important medical conditions, we aimed to compare LOS and different patient relevant outcomes between the two systems using a hospital for each system as proxy.
This was a prospective, two centre observational cohort study at the university departments of medicine from the University Hospital Basel and the Cantonal Hospital Aarau, Switzerland, between January and June 2011. The University Hospital Basel is a tertiary referral centre in North-western Switzerland with 670 beds which treats 31,600 hospitalised patients a year with a fee-for-service based reimbursement system. The Cantonal Hospital Aarau is a tertiary referral university-affiliated hospital with 630 beds treating 26,660 inpatients per year based on AP-DRG reimbursement.
The study protocol was approved by the two local ethical committees, and written informed consent was waived for this observational survey.
We included all patients with a final discharge diagnosis of (a) community-acquired pneumonia (ICD-10 codes J10.0, J12-18), (b) acute heart failure (ICD-10 code I50), (c) exacerbation of chronic obstructive pulmonary disease (COPD; ICD-10 code J44.1) and (d) total prosthesis of the hip (ICD-10 code S72.0-1). These diagnoses were selected because they represent a large number of inpatients within the medical and surgical spectrum. Due to the small sample size, we collapsed community-acquired pneumonia and COPD exacerbation into one category named “lower respiratory tract infection”. Patients were identified with the help of the electronic health records based on the final medical report in each of the two hospitals. Patients who did not survive their hospital stay were excluded, as well as patients who were hospitalised a second time during the study period.
Three months (range 2–4 months) after hospital discharge we sent patients a systematic, validated questionnaire described below asking about satisfaction with the hospital stay and quality of life (see Appendix http://www.smw.ch/fileadmin/smw/pdf/smw-13790-Appendix.pdf ). To improve the return rate of the questionnaires, we re-sent it a second time to non-responding patients.
The primary endpoint of this study was LOS defined as hospital admission until discharge. Secondary endpoints were hospital re-admission rates and patients’ satisfaction with the discharge procedure and quality of life. Re-admission was defined as a re-hospitalisation within three month of hospital discharge. To assess the patient’s overall satisfaction we used a percentage scale with a range of 0% (very unsatisfied) to 100% (very satisfied). Quality of life was measured with the EQ-5D instrument, which includes the 15-item tool EQ-5Dself-classifier and assesses the health related quality of life according to five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) [12, 13].
We used mean and standard deviation, or median and interquartile range to describe the population as appropriate. To investigate differences between LOS in the two hospitals, we calculated a generalised linear model (GLM) with a gamma distribution and a log link function as previously suggested for this type of continuous, non-normally distributed outcome data [14]. This approach was preferred over a Cox regression model due to violation of the proportional hazard assumption. We adjusted the model for the main predictors of LOS, namely age, gender, diagnosis and co-morbidities (anaemia, CHF, COPD, diabetes, hypertension, coronary heart disease, renal insufficiency, malignancy). For secondary binary endpoints, we calculated logistic regression models adjusted for the same covariates as described above. For other continuous outcome variables, we calculated linear regression models after ascertaining a roughly normal- distribution of data. Outcomes were compared within the different diagnoses groups to also check for consistency of effects.
Reported confidence intervals (CI) are two-sided 95% intervals and tests were performed at the two-sided 5% significance level. All analyses were performed with STATA 9.2 (Stata Corp, College Station, Texas).
A total of 1093 patients (FFS: N = 704, AP-DRG N = 389; s. fig. 1 and fig. 2) were identified in the database, a letter and questionnaire were sent to 897 (82.1%) living patients, and 450 (50.2%) returned a legible questionnaire after two send-outs (fig. 1). This total of 450 patients who responded was included in this study; 275 (61%) were from the FFS hospital and 175 from the DRG hospital. The questionnaires were fully completed in 79.8%; 12% had one question missing and 8.2% two or more. The most common diagnosis was lower respiratory tract infection (LRTI) with 48% (n = 217) which included the two diagnoses “community acquired pneumonia” and “exacerbation of chronic obstructive pulmonary disease”. Acute heart failure was present in 32.4% (AHF, n = 146) and total prosthesis of the hip was present in 19.3% (n = 87). Median age was 77 years and was significantly higher in the FFS hospital (p <0.001). A total of 48% of patients were male. Patients had a significant burden of co-morbidities, particularly hypertension (27%), coronary heart disease (27.6%) and congestive heart failure (22.2%) among others. The two hospitals differed significantly regarding the prevalence of coronary heart disease, diabetes and chronic renal failure with the FFS cohort showing more of these co-morbidities. Table 1 shows detailed baseline characteristics for the overall cohort, and separately for the FFS and the AP-DRG hospitals.

Figure 1
Patient inclusion flow chart AP-DRG Hospital.
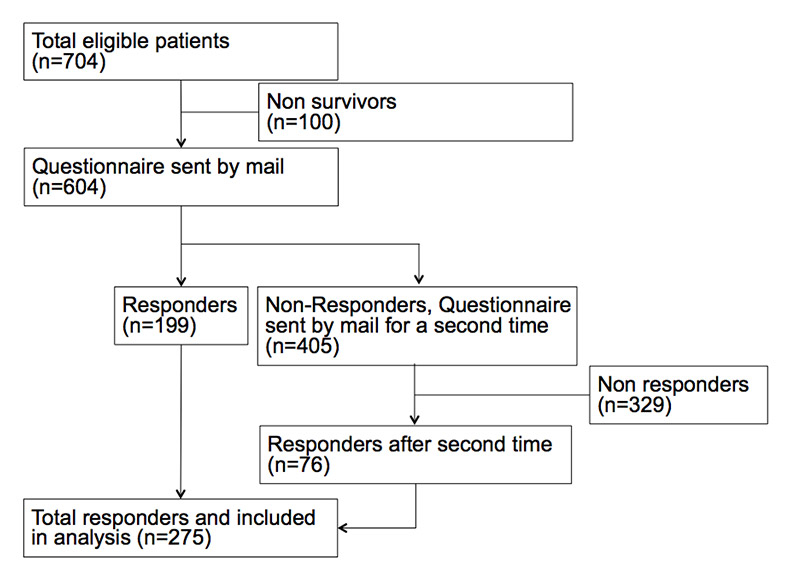
Figure 2
Patient inclusion flow chart FFS hospital.
Overall, the mean LOS was 9.0 days (95%CI 8.4, 9.6). LRTI patients had a LOS of 8.1 days (95%CI 7.2, 9.1), while it was longer in AHF patients (9.9 days, 95%CI 8.6, 11.5) and hip replacement patients (9.7 days, 95%CI 8.8, 10.8). Overall we found shorter LOS in the AP-DRG hospital as compared to the FFS hospital (unadjusted difference –0.15 days (–0.29, –0.01), p = 0.04). However, after adjusting for the above mentioned important confounding factors this difference did not reach statistical significance (adjusted difference –0.07 (95%CI 0.02, –0.08), p = 0.24). Similar results were found for LRTI and AHF patients; yet for total hip prosthesis patients, the association was opposite with longer LOS in the AP-DRG hospital, which again did not reach statistical significance in multivariate adjusted analysis. Detailed results for the overall cohort and for the three different diagnoses are displayed in table 2.
The rate of re-hospitalisation for any reason was significantly higher in the FFS hospital (35% vs. 17.5%), which remained significant after multivariate adjustment (p = 0.01). Thereof, significantly more patients from the FFS hospital were re-admitted to acute-care institutions than from the AP-DRG hospital (11.7% vs. 5.2%, adjusted p = 0.014). This type of re-hospitalisation may lead to surplus cost for a hospital falling into the same budget as the primary hospitalisation.
Overall satisfaction with care was high and similar in both hospital types (86% vs. 83%). Yet, the proportion of patients not satisfied with the discharge process was significantly higher in the FFS hospital (15.3% vs. 9.7%, p = 0.02).
We investigated quality of life as assessed with a standardised questionnaire (EQ-5D) across five dimensions (mobility, self-care, usual activities, pain and depression). There was a significantly lower proportion of patients reporting problems with usual activities (37.9% vs. 42.4%, adjusted odds ratio (OR) 0.55 (95%CI 0.32, 0.91), p = 0.02) and with self-care (37.8% vs. 55.3%, OR 0.45 (95%CI 0.22, 0.93), p = 0.03) in the AP-DRG hospital compared to the FFS hospital. For the other quality of life items, no such difference was observed.
| Table 1: Baseline characteristics of included patients according to hospital reimbursement system. | ||||
| All patients (n = 450) | FFS hospital (n = 275) | AP-DRG hospital (n = 175) | p-value | |
| Demographics | ||||
| Mean age [years](±SD)* | 71.1 (±19.5) | 74.8 (±13.4) | 65.2 (±25.3) | <0.001 |
| Male sex | 214 (48%) | 134 (49%) | 80 (46%) | 0.53 |
| Main diagnosis | 0.27 | |||
| Lower respiratory Infection** | 217 (48.2%) | 128 (46.5%) | 89 (50.9%) | |
| Acute heart failure | 146 (32.4%) | 97 (35.3%) | 49 (28%) | |
| Total prosthesis of the hip | 87 (19.3%) | 50 (18.2%) | 37 (21.1%) | |
| Co-morbidities | ||||
| Anaemia | 34 (7.6%) | 24 (8.7%) | 10 (5.7%) | 0.24 |
| Congestive heart failure | 100 (22.2%) | 64 (23.3%) | 36 (20.6%) | 0.51 |
| COPD | 34 (7.6%) | 22 (8%) | 12 (6.9%) | 0.7 |
| Dementia | 12 (2.7%) | 9 (3.3%) | 3 (1.7%) | 0.30 |
| Diabetes | 58 (12.9%) | 45 (16.4%) | 13 (7.4%) | <0.01 |
| Hypertension | 122 (27.1%) | 83 (30.2%) | 39 (22.3%) | 0.07 |
| Coronary heart disease | 124 (27.6%) | 86 (31.3%) | 38 (21.7%) | 0.03 |
| Chronic renal failure | 53 (11.8%) | 44 (16%) | 9 (5.1%) | <0.01 |
| Malignancy | 27 (6%) | 18 (6.5%) | 9 (5.1%) | 0.54 |
| * Expressed as mean (±SD); p values refer to Mann-Whitney-U and chi-square tests ** Defined as having either community-acquired pneumonia or acute exacerbation of chronic obstructive pulmonary disease. | ||||
| Table 2:Primary and secondary endpoints with regard to hospital type. | |||||||
| Overall (n = 450) | FFS hospital (n = 275) | AP-DRG hospital (n = 175) | Unadjusted regression coefficient or odds ratio | p-value | Adjusted regression coefficient or odds ratio † | p-value | |
| Length of hospital stay, mean (IQR) | |||||||
| All patients | 9.0 (5, 12) | 9.5 (1, 30) | 8.2 (1, 26) | Coefficient – 0.15 (–0.29, –0.01) | 0.04 | Coefficient –0.07 (–0.02, 0.08) | 0.24 |
| Lower respiratory Infection | 8.1 (4, 11) | 8.8 (4, 12) | 7.1 (3, 9) | Coefficient – 0.22 (–0.44, 0.01) | 0.06 | Coefficient –0.09 (–0.35, 0.17) | 0.49 |
| Acute heart failure | 9.9 (5, 13) | 10.8 (7, 15) | 8.0 (4, 11) | Coefficient – 0.30 (–0.55, –0.04) | 0.023 | Coefficient –0.22 (-0.49, 0.06) | 0.126 |
| Total prothesis of the hip | 9.7 (6-12) | 8.8 (6-11) | 11.0 (8, 13) | Coefficient 0.23 (0.025, 0.43) | 0.027 | Coefficient 0.18 (–0.03, 0.39) | 0.1 |
| Re-hospitalisation for any reason | 28.4% (n = 122) | 35.0% (n = 94) ‡ | 17.5% (n = 28)§ | OR 0.39 (0.24, 0.64) | <0.01 | OR 0.39 (0.22, 0.67) | 0.01 |
| Unplanned re-hospitalisation in acute care institution | 9.3% (n = 39) | 11.7% (n = 31) | 5.2% (n = 8) | OR 0.42 (0.18, 0.92) | 0.03 | OR 0.31 (0.12, 0.79) | 0.014 |
| Overall satisfaction with care (0%–100%),mean (SD) | 84.7 (±24) | 86 (±21) | 83 (±27) | Coefficient –3.1 (–7.7, 1.4) | 0.2 | Coefficient –3.5 (–8.4, 1.3) | 0.15 |
| Satisfaction with discharge procedure | |||||||
| Not satisfied with discharge process | 13.2% (n = 53) | 15.3% (n = 38) | 9.7% (n = 15) | OR 0.60 (0.31, 1.13) | 0.11 | OR 0.41 (0.20, 0.85) | 0.02 |
| Date of discharge | |||||||
| Too early | 1.2% (n = 5) | 1.6% (n = 4) | 0.6% (n = 1) | OR 0.40 (0.04, 3.58) | 0.41 | OR 0.17 (0.06, 3.97) | 0.27 |
| Too late | 9.3% (n = 38) | 9.5% (n = 24) | 8.9% (n = 14) | OR 0.93 (0.47, 1.85) | 0.87 | OR 0.54 (0.24, 1.20) | 0.13 |
| Quality of life (QoL according to EQ-5D)on day 30 | |||||||
| Any problems with mobility | 45.3% (n = 204) | 44.7% (n = 123) | 46.3% (n = 81) | OR 1.06 (0.70, 1.56) | 0.75 | OR 1.33 (0.87, 2.01) | 0.20 |
| Any problems with self-care | 91.6% (n = 412) | 93.8% (n = 258) | 88.0% (n = 154) | OR 0.48 (0.24, 0.94) | 0.03 | OR 0.45 (0.22, 0.93) | 0.03 |
| Any problems with usual activities | 78.0% (n = 351) | 79.3% (n = 218) | 76.0% (n = 133) | OR 0.83 (0.53, 1.30) | 0.40 | OR 0.55 (0.32, 0.91) | 0.02 |
| Any problems with pain/discomfort | 54.7% (n = 246) | 54.6% (n = 150) | 54.9% (n = 96) | OR 1.01 (0.69, 1.48) | 0.95 | OR 0.99 (0.66, 1.40) | 0.94 |
| Any problems with anxiety/depression | 33.1% (n = 149) | 33.5% (n = 92) | 32.6% (n = 57) | OR 0.96 (0.64, 1.40) | 0.85 | OR 1.01 (0.70, 1.70) | 0.70 |
| * Results are derived from generalised linear model (GLM) with a gamma distribution for LOS, logistic regression for binary outcomes and linear models for continuous outcomes. † models adjusted for the main predictors of LOS, namely age, gender, diagnosis and co-morbidities (anaemia, CHF, COPD, diabetes, hypertension, coronary heart disease, renal insufficiency, malignancy).OR, odds ratio, Coefficient, regression coefficient ‡ In 96.7% (266/275) of questionnaires this information was provided. § In 88% (154/175) of questionnaires this information was provided. | |||||||
The aim of the current study was to investigate differences in patient and hospital relevant outcomes between two AP-DRG and FFS hospitals in Switzerland, as a baseline before a nation-wide adaption of the DRG system. The data from our survey suggest that AP-DRG hospitals may have an improved discharge process with higher patient satisfaction on discharge, lower re-hospitalisation rates and shorter LOS. This was only partly explained by a higher burden of co-morbidities and disease severity in FFS hospitals.
We suppose that patients in University hospitals are usually sicker than other tertiary care centres due to higher frequencies of immunosuppressed and post-transplantation patients among others. In our study this was reflected by the higher proportion of patients with diabetes, coronary heart disease and chronic renal failure in the tertiary centre (FFS). Furthermore, these patients were older and had more problems with self-care and usual activities. The consequence may be that more of the patients from the FFS institution were re-hospitalised for any reason, probably because they were less independent and less mobile in everyday life. The relative frequency of re-hospitalisation to acute-care institutions was also significantly different. This is an interesting finding because usually DRG hospitals are incriminated for discharging patients too early causing too many re-admissions.
Prior to 2012, the majority of Swiss hospitals used the FFS system in order to account their inpatient services. The main idea of this system is that the hospital gets a flat tax for every day the patient is hospitalised. On top of this flat rate, the hospital receives additionally a fee for every performed service on the patient. The challenge of the FFS is that neither the severity of the disease nor the related treatment cost is mapped properly. Furthermore, it creates an incentive to hospitalise patients for too long [15].
As mentioned above, the FFS and the adapted AP-DRG was active at the same time in Switzerland until the end of 2011. This fact provided the unique chance to compare an AP-DRG like system and the FFS system in an otherwise relatively homogenous society and to detect the difficulties and differences between the said systems. With our study, we also built the basis for further studies in that domain. Pretto et al.(2010) conducted a study on elderly with fractures before the introduction of DRGs in Switzerland [10]. As important baseline data, these studies will help to recognise outcome changes after switching to DRG.
There are other studies comparing the AP-DRG and the FFS system in Switzerland. One of them focused on patients with community-acquired pneumonia from a randomised-controlled trial hospitalised in six centres within Switzerland [11]. Unlike the current study, it detected a significantly shorter LOS in AP-DRG hospitals compared to FFS hospitals, which was true even after adjustment for severity of illness. Mortality and patient satisfaction with the discharge process were similar in both systems. Also, quality of life was not different [11]. The current study extends these previous findings to other patient populations. Differences in this study regarding satisfaction with discharge and quality of life may be due to differences in discharge processes and a higher burden of co-morbidities, and needs further analysis.
The studies did not show a consolidated outcome in the field of re-admission; in some it remains constant [11] and in others there were more re-hospitalisations in the areas with AP-DRG [16]. Our study did show a significant difference between the FFS and AP-DRG institutions regarding acute care re-hospitalisations after discharge.
Busato et al. conducted a study comparing the FFS and the AP-DRG and found the following results in the AP-DRG areas: lower hospitalisation rates, shorter hospitalisation stays, but higher 90-day re-hospitalisation rates. Differences to this study were that the inpatient cost weight per 1000 population was almost equal and the outpatient cost weights lower [16]. The DRG system led to a shift from in-patient to ambulatory treatment of patients.
The current study has several limitations. First, the use of questionnaires after two to four months after hospital discharge may well not reflect patients’ opinion due to recalling difficulties. Furthermore, we recognised that some patients had several hospitalisations within a short period of time and didn’t know which hospitalisation to refer to. In these cases we excluded the patients to get high quality data; this procedure and the response rate of 50% may have introduced some selection bias. Third, not all questions were answered and in fact 12% of questionnaires did not feature any information on re-hospitalisation in the AP-DRG hospital (3.3% in the FFS institution). Fourth, reporting data from two centres in Switzerland may not be generalisable to other centres within the country or elsewhere. Furthermore, we cannot completely exclude some centre related confounding, as well as residual confounding effects on the patient level. Fifth, we were comparing the data from two centres with a different case mix; the patients in the FFS centre were sicker and showed more comorbidities and were less self-sufficient on discharge. The described differences between the two centres may have introduced some confounding regarding LOS and certainly regarding impediments in everyday life. For obvious reasons, we could not use cost-weights to adjust for these differences among the two institutions.
In conclusion the current study suggests an improved discharge process at the AP-DRG hospital with higher patient satisfaction on discharge, lower re-hospitalisation rates and shorter LOS. However, this may be partly explained by a higher burden of co-morbidities and disease severity in the FFE hospital. It will be interesting to monitor these outcomes after the nation-wide DRG implementation in Switzerland; for this purpose the presented data provide a solid baseline.
Appendix 1: Questionnaire http://www.smw.ch/fileadmin/smw/pdf/smw-13790-Appendix.pdf
Acknowledgement:First of all, we would like to thank Professor Meredith Rosenthal, whose excellent class in health economics at Harvard School of Public Health PS and BLH were privileged to attend. The idea for this study emerged from within those classes and was expanded at Beehive’s in Boston. We are very grateful for Ursula Althaus’ work retrieving the patient data at Basel University Hospital. Nadine Binda and Sabina Roth were extremely helpful in labelling and posting the questionnaires.
1 Services CfMaM. National Health Expenditures 2010 Highlights. 2012.
2 Office SFS. Health care costs and financing in 2010. 2012.
3 Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782–93.
4 Carratala J, Fernandez-Sabe N, Ortega L, Castellsague X, Roson B, Dorca J, et al. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients. Ann Intern Med. 2005;142(3):165–72.
5 Donaldson C, Magnussen J. DRGs: the road to hospital efficiency. Health Policy. 1992;21(1):47–64.
6 Davis C, Rhodes DJ. The impact of DRGs on the cost and quality of health care in the United States. Health Policy. 1988;9(2):117–31.
7 Rosenthal MB. Beyond pay for performance – emerging models of provider-payment reform. N Engl J Med. 2008;359(12):1197–200.
8 Rosenthal MB. What works in market-oriented health policy? N Engl J Med. 2009;360(21):2157–60.
9 Fischer, Wolfram. Die DRG Familie. 2008. Available at: http://www.fischer-zim.ch .
10 Pretto M, Spirig R, Kaelin R, Muri-John V, Kressig RW, Suhm N. Outcomes of elderly hip fracture patients in the Swiss healthcare system: A survey prior to the implementation of DRGs and prior to the implementation ofa Geriatric Fracture Centre. Swiss Med Wkly. 2010;140:w13086.
11 Schuetz P, Albrich WC, Suter I, Hug BL, Christ-Crain M, Holler T, et al. Quality of care delivered by fee-for-service and DRG hospitals in Switzerland in patients with community-acquired pneumonia. Swiss Med Wkly. 2011;141:w13228.
12 Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–20.
13 Schrag A, Selai C, Jahanshahi M, Quinn NP. The EQ-5D – a generic quality of life measure-is a useful instrument to measure quality of life in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2000;69(1):67–73.
14 Dodd S, Bassi A, Bodger K, Williamson P. A comparison of multivariable regression models to analyse cost data. J Eval Clin Pract. 2006;12(1):76–86.
15 Grundlageninformationen zur Vergütung mit APDRG-Fallpauschalen. Available at: http://www.zmt.ch/stationaere_tarife/stationaere_tarife_apdrg/stationaere_tarife_apdrg_grundlageninformationen.htm.
16 Busato A, von Below G. The implementation of DRG-based hospital reimbursement in Switzerland: A population-based perspective. Health Res Policy Syst. 2010;8:31.
Funding / potential competing interests: The University Hospital Basel, Switzerland, provided a research grant for this study. All authors declare that they have no competing interests.
Authors’ contribution: NM, DT, PS contributed equally.