Hepatitis B and C in Switzerland – healthcare provider initiated testing for chronic hepatitis B and C infection
DOI: https://doi.org/10.4414/smw.2013.13793
Rainer
Fretz, Francesco
Negro, Philip
Bruggmann, Daniel
Lavanchy, Andrea
De Gottardi, Isabelle
Pache, Virginie
Masserey Spicher, Andreas
Cerny
Summary
Hepatitis B and hepatitis C are contagious liver diseases caused by the hepatitis B virus (HBV) and the hepatitis C virus (HCV), respectively. In particular, chronic infection with HBV or HCV is a major public health problem throughout Europe. The majority of persons chronically infected (65%–75%) are not aware of their infection status until symptoms of advanced liver disease appear. In addition, the peak in the number of patients suffering from advanced stages of the disease, such as cirrhosis and hepatocellular carcinoma, has not yet been reached. In order to reduce the current and future morbidity and mortality associated with chronic HBV or HCV infection, the timely detection of chronically infected persons, with follow-up and case management, is crucial. However, the current screening strategies in Europe and Switzerland have to be considered as inadequate to detect the majority of chronically infected persons. Hence, we emphasise the importance of an alternative approach: the healthcare provider initiated identification of HBV or HCV infection in defined risk groups. This entails determining whether a person is not only at risk of being chronically infected, but also at risk of becoming infected with HBV or HCV and, if necessary, testing for HBV or HCV infection.
Introduction
Hepatitis B and hepatitis C are contagious liver diseases caused by the hepatitis B virus (HBV) or the hepatitis C virus (HCV) [1–2]. HBV or HCV infection can be either acute or chronic. Chronic hepatitis virus infection is a major global public health problem, an important cause of morbidity and mortality from sequelae that include chronic liver disease and failure, cirrhosis, and hepatocellular carcinoma (HCC) [3–5].
Furthermore, chronic liver disease due to coinfection with HBV or HCV has become a major cause of death in persons infected with the human immunodeficiency virus (HIV) [3, 6]. Worldwide, about 1 in 12 persons (480–520 million people) are chronically infected with HBV or HCV [3]. In Europe, about 14 million people are chronically infected with HBV and 9 million people are chronically infected with HCV, compared with 1.5 million infected by HIV [7]. Chronic viral hepatitis is a silent killer because of the frequently asymptomatic nature of chronic HBV and HCV infection. The majority of persons chronically infected (i.e. 65%–75% of the infected) are not aware of their infection status until symptoms of advanced liver disease and failure, cirrhosis and HCC appear [3]. Consequently, case detection is difficult. Therefore, the timely detection of chronic hepatitis virus infection, with follow up and case management, has the potential to contribute significantly to secondary prevention of chronic hepatitis [8–9].
Although most countries in the European Union (EU) and Switzerland lack national action plans or national strategies for the prevention and control of viral hepatitis, different strategies for identification of chronic HBV and HCV infection are already in place. These include population screening and healthcare provider initiated testing. The objective of this review is, on the one hand, to raise awareness within the primary care community of the public health relevance of chronic hepatitis B and C infection in Switzerland and, on the other hand, to advocate the identification of the chronically infected by using healthcare provider initiated identification of HBV or HCV infection in defined risk groups.
Hepatitis B and C
Hepatitis B virus infection
HBV is a partially double-stranded deoxyribonucleic acid (DNA) virus belonging to the Hepadnaviridae family. The virus is mainly present in the blood, but is also present in saliva, semen, vaginal secretions, menstrual blood, any other body fluid containing blood, and in unfixed tissues and organs [1, 10].
Transmission of HBV
Transmission occurs by percutaneous and mucosal exposure to infective body fluids or by maternofoetal transmission occurring mainly at birth. The presence of the hepatitis B 'e' antigen (HBeAg) or HBV DNA above 104 copies/ml indicates high virus titre and higher infectivity of these fluids [11]. Since HBV is stable on environmental surfaces for at least seven days, indirect inoculation of HBV can occur via contaminated objects [1]. In areas of high endemicity (regions with an HBsAg prevalence of ≥8%), the most common route of transmission is perinatal, although the infection may be acquired also during the preschool years. In areas of intermediate endemicity (HBsAg prevalence 2%–7%), transmission is either perinatal or horizontal. In countries of low endemicity (HBsAg prevalence <2%), most HBV infections are acquired by horizontal transmission in adolescent or early adult life, that is, through unprotected sexual activities [12]. Blood transfusions were once a common route of transmission, but improved diagnostic tests and screening for HBV infection in blood donations have dramatically reduced the risk of acquiring HBV infection through transfusion [12].
Prevalence of HBV infection in Europe and in Switzerland
According to a review recently conducted by the European Centre for Disease Prevention and Control (ECDC), the prevalence data on the hepatitis B surface antigen (HBsAg) – an indicator of acute and chronic infection and thus for infectivity – in the general population ranged from 0.1% to 7% by country. Prevalence estimates for HBsAg that were considered representative were available for these countries only: Belgium (0.7%), Czech Republic (0.6%), Denmark (0.1%), Finland (0.2%), Germany (0.6%), Greece (2.1%), Ireland (0.1%), Italy (regional and national data ranged from 0.2%‒4.3%), the Netherlands (0.1%), Romania (5.6%), Slovakia (0.6%) and Turkey (regional and national data ranged from 2.5%‒9.0%) [9]. The prevalence of HBsAg carriers in the general Swiss population is estimated to be 0.3% [11]; this means that 23,500 persons in Switzerland are currently HBV-infected.
In Europe, the HBV prevalence in pregnant women is generally higher than in the general population and the antenatal HBsAg prevalence ranges from 0.1% to 4.4% by country [9]. In Switzerland, the frequency of HBsAg carriers among women of child-bearing age is estimated to be 0.7% [13]. A survey conducted in the year 1999 amongst patients with renal insufficiency undergoing chronic dialysis treatment in Switzerland revealed a frequency of HBsAg carriers of 1.4% [14]. The reported HBsAg prevalence in injection drug users (IDUs) in the EU varied widely, ranging from 0% in Belgium to 11.6% in Bulgaria in 2006. Generally, the HBsAg prevalence among IDUs is higher in countries in Central and Eastern Europe, when compared with those in Western Europe [9]. A prevalence study in Switzerland amongst patients with a history of long-term injection drug use in the years 1994 to 1996 documented a seroprevalence of the hepatitis B core antigen (HBcAg) as high as 73.2%, suggesting that the majority of individuals were, or had been, in contact with the virus [15]. This result is in line with the outcome of a review on anti-HBc seroprevalence in IDUs, which revealed a wide range of anti-HBc seroprevalence in Europe (4.2%–85.1%) [16]. In migrants and minority populations in Europe, a wide variation in HBsAg prevalence was found, ranging from 1.0% in resident first-generation migrants in the Netherlands up to 15.4% in Albanian refugees in Greece. However, the HBsAg prevalence among migrants was in general higher than in the general population [9].
HBV infection and disease
Only a small proportion of acute HBV infections may be clinically recognised; fewer than 10% of children and 30% to 50% of adults with acute HBV infection show icteric disease [1, 10]. After acute HBV infection, the risk of developing chronic infection varies inversely with age, being about 90% in infants infected at birth, 20% to 50% in children younger than 5 years, and 1% to 10% in persons infected at a later age [1, 10]. Furthermore, chronic HBV infection is common in persons with immunodeficiency [1]. About one-third of the chronically infected have elevated aminotransferases; liver biopsy findings range from normal to severe necroinflammatory hepatitis, with or without cirrhosis [1].
However, about 65% of these cases are unaware that they are chronically infected with HBV [3]. Cirrhosis, liver failure or HCC will develop in approximately 15% to 40% of individuals with chronic hepatitis B [12] and an estimated 15% to 25% of these will die prematurely either of cirrhosis or HCC [1, 3]. Patients with decompensated cirrhosis are candidates for liver transplantation [12].
The goal of antiviral therapy in chronic HBV infection is to achieve long-term viral suppression in order to prevent sequelae of chronic liver disease, and to reduce viraemia and, hence, the risk of contagion [5, 11]. Nucleoside and nucleotide analogues and pegylated interferon alfa are the most commonly used antiviral agents in the treatment of chronic HBV infection [5, 17]. However, current HBV treatments often do not eradicate HBV, although treatment for one year generally results in the reduction of serum HBV DNA to levels undetectable with polymerase chain reaction in up to 95% of patients. Because responses are not always durable, long-term and often life-long treatment is necessary to maintain viral suppression and continued clinical benefit [17].
For primary prevention, safe and effective vaccines against HBV infection have been available since 1982 [12].
Hepatitis C virus infection
HCV is an enveloped ribonucleic acid (RNA) virus belonging to a separate genus (Hepacivirus) in the Flaviviridae family and is classified into seven major genotypes with a broad variety of subtypes [18]. The distribution of genotypes varies geographically. In Europe, HCV genotypes 1 and 3 are most prevalent, and genotype 2 is commonly encountered in the Mediterranean area [5].
Transmission of HCV
HCV is efficiently transmitted via direct percutaneous exposure to infectious blood [5]. Depending on the viral load, HCV may be present in other body fluids such as saliva, sweat, tears or semen [19]. It was shown by using tissue culture assays that HCV is stable and may remain infective on environmental surfaces for several days. Hence, indirect inoculation of HCV can occur via contaminated objects [20]. Hepatitis C became a global epidemic in the 20th century as blood transfusions, haemodialysis and the use of injection needles to administer licit and illicit drugs increased throughout the world. The identification of the virus in 1989 led to measures to reduce healthcare-related exposure to HCV. With this reduction and the introduction of routine testing of donated blood, HCV infection through injection drug use (and intranasal drug use with the common use of utensils) has become the major source of exposure to HCV, and the primary mode of HCV transmission in Europe and in the US [3, 19]. Mother-to-child transmission has been documented, but appears rare [2]. Sexual transmission of HCV occurs infrequently. However, there seems to be a relevant and increasing risk of sexual transmission within certain populations, for example, among men who have sex with men (MSM), especially when coinfected with HIV, or among persons with high-risk sexual behaviour [19, 21–22].
Prevalence of HCV infection in Europe and in Switzerland
The anti-HCV prevalence (as an indicator of past or current HCV infection) in the general population in Europe ranges from 0.4% to 3.5% by country and from 0.2% to 10.4% by region within certain countries (especially Italy). Prevalence estimates that are considered representative were available for only three countries: the Czech Republic (0.2%), Germany (0.4%) and Romania (3.5%) [9]. Anti-HCV prevalence in the Swiss population, based on studies in subpopulations in the 1990s, is estimated to be between 0.7% and 1.0% [11]. Hence, between 55,000 and 78,000 persons in the general Swiss population are currently HCV-infected. However, the true anti-HCV prevalence in the general population of Switzerland might be up to 1.3% to 1.8%, as reported in a mathematical modelling study [23–24].
In Europe, the overall prevalence of anti-HCV is much higher than the HBsAg prevalence among IDUs and ranged from 30% to 98%, with a great variation within the different European countries [9]. Based on studies in the years 1994 to 1996, the anti-HCV prevalence rates in IDUs in Switzerland ranged from 56.4% to 82.2% [15, 25]. The anti-HCV prevalence in pregnant women in Europe ranges from 0% to 1.7% by country [9], and in Switzerland it was estimated to be 0.7% [26]. The prevalence of anti-HCV among migrants and minority populations of European countries ranges widely from 0% to 23.4% [9]. A survey amongst patients undergoing chronic dialysis treatment in Switzerland in 1999 found an anti-HCV prevalence of 5.1% [14].
HCV infection and disease
Only 25% of HCV-infected persons will develop acute hepatitis with jaundice or have elevated aminotransferases [19, 27]. The remaining 75% of HCV-infected persons exhibit no, or only nonspecific, clinical signs [19]. In contrast to HBV, 50% to 85% of infected adult persons will develop chronic HCV infection [5, 19]. Overall, 10% to 20% of patients with chronic HCV hepatitis will develop complications of chronic liver disease, such as cirrhosis, within two to three decades of onset, and 1% to 5% will develop HCC [28]. By 2007, HCV had surpassed HIV as a cause of death in the US [29]. Nevertheless, an estimated 75% of the infected people are unaware of their HCV infection [3].
Antiviral therapy with pegylated interferon alfa in combination with ribavirin was, until very recently, the gold standard for the treatment of hepatitis C [5]. However, with this regimen, the permanent eradication of HCV occurs in only one-half of the patients [5] and in fewer than 50% of patients infected with genotype 1 [30]. New antiviral agents specifically targeting the NS3/4a protease are potent inhibitors of HCV replication and can result in rapid declines in serum HCV RNA levels [30]. Clinical trials showed that in HCV genotype 1, treatment-naive HCV adult patients, the antiviral drug telaprevir in combination with pegylated interferon alfa and ribavirin can result in rates of sustained virological response (SVR; defined as undetectable HCV RNA in serum 24 weeks after the end of therapy) as high as 75% [31]. Treatment with the antiviral boceprevir in the same patient group resulted in SVR rates as high as 68% [32]. For the very first time, the newly achievable SVR rates of 68% to 75% in patients infected with HCV genotype 1 [31–32] reach the SVR rates of 65% to 82% in patients infected with HCV genotypes 2 and 3 after standard treatment with pegylated interferon alfa in combination with ribavirin [33]. Achievement of SVR potentially halts fibrosis progression, prolongs survival and decreases the risk of HCC or end-stage liver disease. In 2011, boceprevir and telaprevir have been approved in the EU and in Switzerland under special treatment conditions.
Although there is a clear need for a preventive vaccine and research in this field is ongoing, to date a safe and efficient vaccine against HCV does not exist [34].
Future challenges of HBV and HCV infection
It is impossible to predict with certainty the future epidemiological and clinical developments of hepatitis infection and disease. However, the worldwide peak in the number of patients suffering from cirrhosis and cancer, or of those waiting for a life-saving liver transplant, has not yet been reached, as many of those chronically infected with HBV or HCV (who acquired their infection two to three decades ago) have not yet reached the advanced stages of the disease. In the US, the extent of HBV and HCV infection have already reached epidemic proportions [29] and the World Health Organisation (WHO) compared HCV infection to a “viral time bomb” [35–36].
International assessments of the future situation of viral hepatitis may shed some light on the future development in Europe and in Switzerland. As an example, in the UK, the prevalence of chronic hepatitis B has increased dramatically from 150,000 to an estimated 325,000 persons, with a significant contribution from immigration from areas of high endemicity, such as Eastern Europe and Africa, within the period 2004 to 2008. Current migration figures suggest that the number of chronically HBV-infected cases in the UK will keep on rising in the near future, and that most of the existing and newly arrived cases remain undiagnosed [37]. In contrast, the prevalence of chronic hepatitis C in the UK remains quite stable at an estimated level of 0.5% to 0.7% (i.e. 250,000–350,000 persons). It is assumed that this silent pool of HCV infection, if left unidentified and untreated, will result in a significant burden of advanced liver disease cases over the next 20 to 30 years. Given that young adults are infected with HCV (laboratory reports between 1995 and 2010 in England showed that 49% were in individuals aged between 25 and 39 years) these time intervals are clinically relevant [37–38]. It is predicted that the number of individuals in England developing HCV-related cirrhosis will double between 2005 and 2015 with a parallel increase in the incidence of decompensated liver disease and HCC, if left untreated [37, 39]. Cirrhosis of any cause and HBV infection are already responsible for the overwhelming majority of cases of HCC in the UK. An increase in the incidence of cirrhosis will be paralleled by an increase in the incidence of HCC in a few years from now [37].
Regarding HCV infection, the main effort in global HCV care (besides developing highly efficient and well-tolerated HCV compounds) should focus on overcoming barriers to HCV testing, assessment and therapy, especially in marginalised, but highly affected, populations like IDUs and migrants.
|
Table 1: Populations at risk of hepatitis B virus (HBV) infection. |
|
A. Clinical signs or symptoms of hepatitis
|
| Persons with elevated liver enzymes (transaminases) |
| Persons with clinical signs or symptoms of hepatitis |
| Patients with liver cirrhosis, fibrosis or hepatocellular carcinoma |
|
B. Risk factors
|
|
Medical
|
| Persons who have chronic liver disease |
| Persons who are undergoing or have undergone haemodialysis |
| Persons with HIV or other sexually transmitted infections |
| Persons with HCV infection |
| Patients before or during immunosuppressive treatment or chemotherapy |
| Recipients of organ transplants and blood products |
|
Demographic
|
| Persons and migrants born or having lived during childhood in geographic regions with HBsAg prevalence ≥2% |
| Persons and migrants visiting their relatives in geographic regions with HBsAg prevalence ≥2% |
|
Behavioural
|
| Families and household members of persons infected with HBV |
| Sexual partners of persons infected with HBV |
| Heterosexual persons with multiple sex partners |
| Men who have sex with men |
| People who inject or have ever injected drugs (injection drug users) |
| Long-stay travellers with contact with the local population in geographic regions with HBsAg prevalence ≥2% |
|
Occupational
|
| Healthcare workers at risk for occupational exposure to blood or blood-contaminated body fluids |
| Healthcare workers after exposure to blood or blood-contaminated body fluids (e.g. needlestick injury) |
| Public-safety workers at risk of occupational exposure to blood or blood-contaminated body fluids |
| Staff in psychiatric institutions or residents of welfare institutions for mentally disabled persons |
|
Others
|
| Newborns of HBV-infected mothers |
| Patients in psychiatric institutions or residents of welfare institutions for mentally disabled persons |
| Persons with imprisonment history |
| Persons with body piercings or tattoos if performed in poor hygienic environments |
| Victims of rape |
| Persons with accidental needlestick injury in public places (e.g. park or street) |
| From references [3, 11, 50, 55, 58], modified.
HBsAg = hepatitis B virus surface antigen, HCV = hepatitis C virus; HIV = human immunodeficiency virus. |
Identification of persons with chronic HBV or HCV infection
The main goals of the identification of chronically infected persons are to reduce the morbidity and mortality associated with cirrhosis and HCC [3]. There are different strategies for the identification of persons with chronic HBV or HCV infection. Besides voluntary testing requested by the person concerned (which is tackled by education and empowerment measures), two different strategies exist for identifying persons with HBV or HCV infection: population screening and healthcare provider initiated testing based on identified risk-factors in patients [3, 40–41].
Population screening
Population screening is a strategy used to detect HBV or HCV infection in asymptomatic individuals, enabling earlier intervention and management to reduce morbidity and mortality. However, the costs of screening (including diagnosis, medical management and, if appropriate, treatment) should be economically balanced in relation to possible expenditure on medical care as a whole [42]. Screening the general population for HBV or HCV is very unlikely to be cost-effective, for one reason because of the low prevalence of infection in the general population [41, 43–45]. Therefore, targeted screening, that is, screening defined populations with a high risk of HBV or HCV exposure and pregnant women (to prevent perinatal transmission of HBV only), is an adequate strategy [3, 46–49]. Screening high-risk populations is already done throughout the European region [9].
In the EU and in Switzerland, there are no national policies for screening for HBV in migrants [9]. However, antenatal screening is widely conducted throughout the EU member states and in Switzerland [9, 50]. Screening for both HBV and HCV infection in blood donations is conducted throughout the EU and in Switzerland [9, 51]. On the other hand, HCV screening during pregnancy is not recommended, since the risk of vertical transmission of HCV is low and currently not reducible, and a treatment for infants is not available [43, 46]. Apart from a range of professional consensus statements which support screening for HCV in IDUs, there are currently no screening policies for HCV in IDUs implemented in the EU and in Switzerland [9].
In the US, HCV infection is most prevalent among adults born between 1945 and 1965 [52]. A large proportion of these persons probably acquired their infection from activities related to injection drug use in the 1960s and 1970s [53]. Consequently, the existing US strategies for the identification of HCV infection were amended in 2012 by implementation of HCV screening of all adults born during 1945 to 1965 (“birth-cohort screening”) [54].
Healthcare provider initiated testing
The current screening strategies in Europe and in Switzerland cover only antenatal screening for HBV infection in pregnant women and screening for HBV or HCV infection in blood donations. Hence these screening strategies have to be considered as insufficient for identifying other individuals at high risk of HBV or HCV infection, who belong to other subpopulations and who form the majority of chronically infected persons. Hence, the healthcare provider initiated identification of HBV or HCV infection among defined risk groups is a valuable instrument in secondary prevention [55]. This approach entails a (primary) healthcare provider determining whether a person is not only at risk for being chronically infected, but also at risk of becoming infected with HBV or HCV, by reviewing the patient’s history and, if necessary, testing for HBV or HCV infection. A greater variety of risk factors than those covered by the screening strategies in place, such as demographic, behavioural, occupational and medical risk factors, and clinical signs or symptoms of hepatitis, may serve as such indicators [3].
Consequently, persons with chronic HBV or HCV infection should be referred for medical care and case-management, and persons found to have risk factors for HBV or HCV infection should receive counselling about prevention (those at risk for becoming infected with HBV should be additionally offered vaccination) [3].
In the US, this approach has been presented as a key concept for the prevention and control of hepatitis B and C in the national strategy published in 2010 [3]. Outside the US, a similar identification process for chronic HBV and HCV infection was implemented in the national action plan of France (Plan national de lutte contre les Hépatites B et C, 2009–2012) [56]. In Ireland, the risk-factor-based approach for the identification of chronic infection was implemented in the national strategy to control HCV infection (National Hepatitis C Strategy 2011–2014) [57]. In 2009, an European expert group including, amongst others, members of the European Liver Patients Association (ELPA), the European Association for the Study of the Liver (EASL), the Institut de Veille Sanitaire (InVS), the French Ministry for Health and Sports, Health Protection Scotland, and the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), published a document with recommendations for the promotion of healthcare provider initiated testing for HBV and HCV infection [55].
In Switzerland, up to now there were limited recommendations regarding risk-factor based identification of HBV infection. In contrast, the risk-factor based identification approach for HCV infection has been recommended in Switzerland since 1993 [50, 58–59]. Hence, broadening the already existing risk-factor based identification approach for HCV and transferring this procedure to the identification of HBV infection in Switzerland is suggested. Furthermore, awareness of this identification procedure amongst the Swiss primary care community should be raised as it is they who are asked to implement the health-care provider initiated identification of HBV or HCV infection among defined risk groups in their day-to-day work with patients. This publication is intended to provide a base for further information for the healthcare community.
A consensus on the identified risk factors for chronic infection or for becoming infected with HBV or HCV, such as demographic, behavioural, occupational and medical risk factors, and clinical signs or symptoms of hepatitis are summarised in tables 1 and 2. These tables should serve as guidance for identifying persons belonging to these at-risk populations. These persons should have their history reviewed by a (primary) healthcare provider, should be considered for testing for HBV or HCV infection and, if warranted, referred for medical care and case management.
|
Table 2: At-risk populations for hepatitis C virus (HCV) infection. |
|
A. Clinical signs or symptoms of hepatitis
|
| Persons with elevated liver enzymes (transaminases) |
| Persons with clinical signs or symptoms of hepatitis |
| Patients with liver cirrhosis, fibrosis or hepatocellular carcinoma |
|
B. Risk factors
|
|
Medical
|
| Recipients of clotting-factor concentrates and blood products before 1987 |
| Recipients of blood transfusions before July 1992 |
| Recipients of solid-organ transplants before July 1992 |
| Patients who are undergoing or have undergone haemodialysis |
| Persons with HIV infection |
| Persons with HBV infection |
| Persons who have received repeated percutaneous injections |
| Persons who have had invasive medical and paramedical or dental work in settings with high prevalence or poor infection control practices |
|
Demographic
|
| Persons and migrants born or having lived in regions of high HCV endemicity |
|
Behavioural
|
| Persons who inject or have ever injected drugs (injection drug users) |
| Persons who use or have ever used intranasal drugs |
| Men who have sex with men |
| Household members or sexual partners of persons with HCV infection |
|
Occupational
|
| Healthcare workers at risk for occupational exposure to blood or blood-contaminated body fluids |
| Healthcare workers after exposure to blood or blood-contaminated body fluids (e.g. needlestick injury) |
| Public-safety workers at risk for occupational exposure to blood or blood-contaminated body fluids |
|
Others
|
| Persons with imprisonment history |
| Persons with body piercings or tattoos if being performed in poor hygienic environments |
| Children of HCV-infected mothers |
| Persons with accidental needlestick injury in public places (e.g. park or street) |
| From references [3, 11, 55, 58], modified.
HBV = hepatitis B virus; HIV = human immunodeficiency virus. |
|
Table 3: Interpretation of hepatitis B serological diagnostic test results [3].
|
|
Antigen or antibody Test
|
Result
|
Interpretation
|
| HBsAg
Anti-HBc
Anti-HBs |
All negative |
Susceptible |
| HBsAg
Anti-HBc
Anti-HBs |
Negative
Positive
Positive |
Immune because of natural infection |
| HBsAg
Anti-HBc
Anti-HBs |
Negative
Negative
Positive |
Immune because of hepatitis B vaccination |
| HBsAg
Anti-HBc
IgM anti-HBc
Anti-HBs |
Positive
Positive
Positive
Negative |
Acutely infected
or
Reactivation (low titre of IgM anti-HBc) |
| HBsAg
Anti-HBc
IgM anti-HBc
Anti-HBs |
Positive
Positive
Negative
Negative |
Chronically infected |
| HBsAg
Anti-HBc
Anti-HBs |
Negative
Positive
Negative |
Interpretation unclear; could be due to
Resolved infection
False-positive anti-HBc test
Low-level chronic infection
Resolving acute infection |
| HBsAg = hepatitis B surface antigen; anti-HBc = total hepatitis B core antibody; anti-HBs = hepatitis B surface antibody; IgM anti-HBc = immunoglobulin M antibody to hepatitis B core antigen. |
Testing for HBV or HCV infection
Testing for HBV infection
Detection of specific HBV antigens or antibodies in serum confirms the diagnosis. Three clinically useful antigen-antibody systems have been identified for HBV: the HBsAg and antibody to HBsAg (anti-HBs); the HBcAg and antibody to HBcAg (anti-HBc); and the HBeAg and antibody to HBeAg (anti-HBe). Commercial kits are available for all markers except HBcAg. The serological markers can be used to identify the different phases of HBV infection. The preferred laboratory test for detecting current HBV infection is HBsAg, which can be detected in serum from several weeks before onset of symptoms to days, weeks, months or years after onset. The presence of HBsAg indicates that the person is infectious. The principal test for detecting recovery from HBV infection is anti-HBs as outlined in figure 1 and also shown in the annex. An alternative is to test initially for anti-HBc – which is present during acute, chronic, and resolved HBV infection – and, if the result is positive, to conduct follow-up testing for HBsAg and anti-HBs [3]. The IgM antibody to HBcAg (immunoglobulin (Ig) M anti-HBc) usually disappears within 6 months but may reappear at low titres if hepatitis reactivates with chronic hepatitis; nonetheless, this test may reliably diagnose acute HBV infection. The presence of HBeAg is associated with relatively high infectivity [1, 3]. Unfortunately, HBV markers can be misinterpreted, and this can lead to clinical errors in patient evaluation, counselling or treatment. Table 3 summarises the interpretation of HBV serological diagnostic test results. The detection of HBV DNA in blood serum with nucleic acid amplification techniques, such as the polymerase chain reaction (PCR), can be used as a marker for hepatitis B viraemia and hence may serve as a measure of infectivity [10].
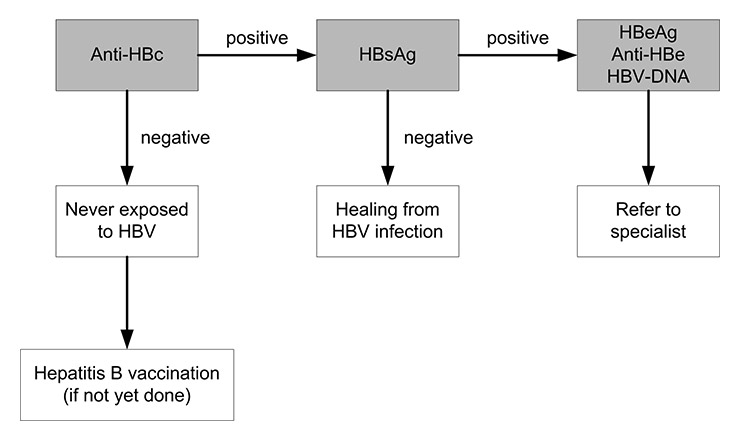
Figure 1
Testing for chronic hepatitis B virus (HBV) infection: absence of anti-HBc excludes HBV infection, while the presence of HBsAg indicates ongoing infection.
Anti-HBc = antibody to hepatitis B core antigen; Anti-HBe = antibody to hepatitis B ‘e’ antigen, HBeAg = hepatitis B ‘e’ antigen; HBsAg = hepatitis B surface antigen; HBV DNA = HBV deoxyribonucleic acid.
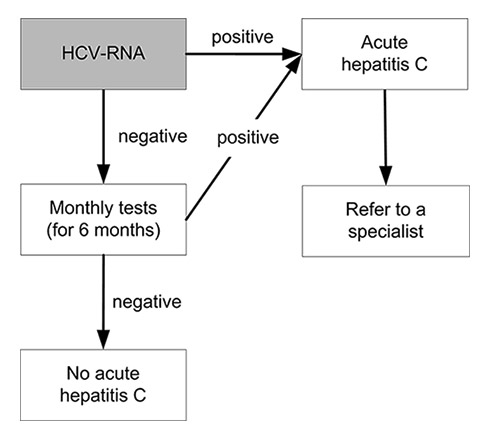
Figure 2
Testing for acute hepatitis C virus (HCV) infection after exposure: in the acute phase of infection HCV ribonucleic acid (RNA) becomes detectable before anti-HCV. A suspected acute HCV infection must be investigated by means of a HCV RNA test.
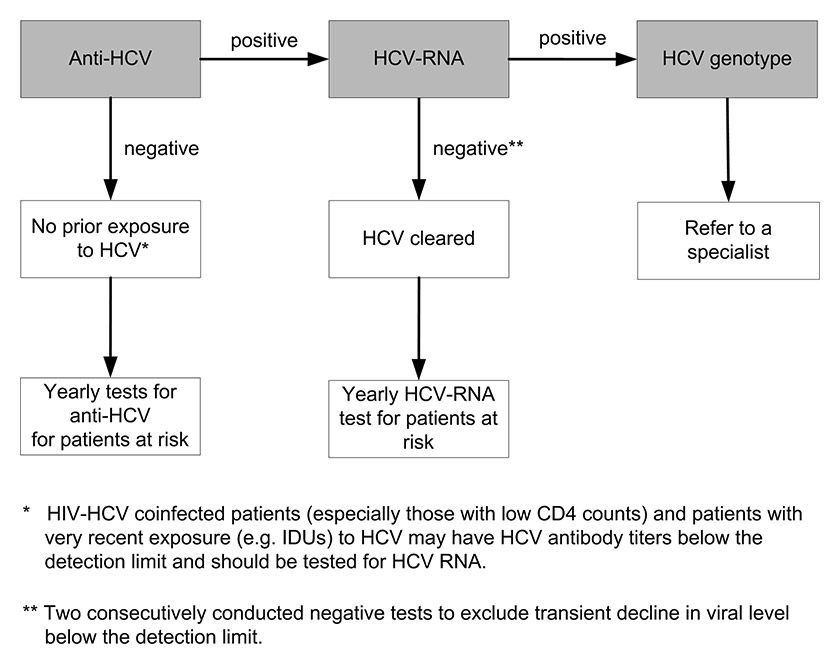
Figure 3
Testing for chronichepatitis C virus (HCV) infection: chronic HCV infection is defined as two consecutive positive HCV ribonucleic acid (RNA) tests at intervals of at least 6 months.
IDU = intravenous drug user.
Testing for HCV infection
Diagnosis depends on detecting HCV antibody (anti-HCV). Various tests are available for the diagnosis and monitoring of HCV infection. Tests that detect antibodies against the virus include the enzyme immunoassay (EIA) and the recombinant immunoblot assay. The same HCV antigens are used in both EIAs and the immunoblot assays. These tests do not distinguish between acute, chronic or resolved infection. EIA tests for the diagnosis of HCV are suitable for screening at-risk populations. A negative EIA test is enough to exclude a diagnosis of chronic HCV infection in immunocompetent patients. The high sensitivity and specificity of the current EIAs obviate the need for a confirmatory immunoblot assay in the diagnosis of individuals with clinical liver disease, particularly those with risk factors for HCV. Immunoblot assays may be useful as a supplemental assay in persons with a positive EIA who test negative for HCV RNA. Acute or chronic HCV infection in a patient with a positive EIA test should be confirmed by detection of the presence of HCV RNA in the serum. A single positive assay for HCV RNA confirms active HCV replication, but a single negative assay does not exclude viraemia and may reflect a transient decline in viral level below the level of detection of the assay. Therefore, a follow-up HCV RNA assay should be performed to confirm the absence of active HCV replication. Quantitative determination of HCV RNA levels and of HCV genotype provides information on the likelihood of response to treatment in patients undergoing antiviral therapy [2]. See figures 2 and 3 and also the annex for testing procedures for acute and chronic HCV infection.
What to do after a positive test for HBV or HCV infection
As indicated above, the presence of chronic hepatitis B or C infection may lead to cirrhosis, end-stage liver disease or HCC and, ultimately, to death. Therefore, persons found to be positive for either one or both of these infections need to be carefully investigated in order to determine whether they will benefit from medical care and case management, often comprising antiviral treatment or liver transplantation (rarely). Consequently, these persons should be referred to a liver specialist who will confirm the HBV or HCV infection, evaluate the liver status using appropriate procedures, often including a liver biopsy to assess the severity of the liver injury and to exclude the presence of HCC. Initiating life-saving anti-viral treatment for HBV or HCV infection needs to be evaluated carefully. The appropriate drug regimen is subject to constant improvements in the choice of drug combinations, treatment schedule and outcome determination, emphasising the need for a case management by an experienced liver specialist without delay.
Annex
Evolution of HBV infection and overview of testing for HBV or HCV infection
After the diagnosis of chronic HBV infection has been established, the infection is characterized by a first phase of immunotolerance (particularly in newborns and children) with high or very high viraemia (HBV DNA) and normal or quasi-normal transaminases. In this phase, HBeAg is detectable. As a consequence of an immunoreaction, the second phase of chronic hepatitis B infection, the immune clearance phase, develops, with elevated transaminases, detectable HBeAg and elevated HBV DNA levels. In this phase significant progression of fibrosis may occur and mutant forms of the virus may develop. During the third phase, the inactive HBsAg carrier phase, transaminases are normal and the viral replication is low. This phase coincides with seroconversion of HBeAg and emergence of anti-HBe. In some patients the only serological feature of an HBV infection is the presence of anti-HBc antibodies. Seroconversion of HBsAg to anti-HBs leads to resolution of HBV infection. However, some individuals may undergo reactivation into a fourth phase known as HBeAg-negative chronic HBV, where transaminases and HBV DNA may fluctuate over time.
|
Addresses of liver centres in Switzerland
|
| Addresses of liver centres. |
|
Basel
Abteilung für Gastroenterologie und Hepatologie
Universitätsspital Basel
Petersgraben 4
4031 Basel |
Bern
Universitätsklinik für Viszerale Chirurgie und Medizin
Inselspital
3010 Bern |
|
Genève
Service de Gastroentérologie et d’Hépatologie
Hôpitaux Universitaires
4 rue Gabrielle-Perret-Gentil
1211 Genève 14 |
Lausanne
Service de Gastroentérologie et Hépatologie CHUV
Rue du Bugnon 44
1011 Lausanne
Centre Saint-Martin
Rue Saint-Martin 7
1003 Lausanne |
|
Lugano
Centro di Epatologia
Clinica Luganese Moncucco
Via Moncucco 10
6900 Lugano |
Neuchâtel
Consultation de Maladies Inféctieuses
Département de Médecine
Hôpital Pourtalès
2002 Neuchâtel |
|
St. Gallen
Fachbereich Gastroenterologie/Hepatologie
Kantonsspital
9007 St. Gallen |
Zürich
Abteilung für Gastroenterologie und Hepatologie
Universitätsspital Zürich
Rämistrasse 100
8091 Zürich |
| Hepatitis centres for people who use drugs. |
|
Basel
Zentrum für Suchtmedizin ZfS
Haltingerstrasse 65
4057 Basel |
Genève
Unité des Dépendances
Département de Médecine Communautaire
Hôpital Cantonal
4 Rue Gabrielle-Perret-Gentil
1211 Genève |
|
Zürich
Arud, Zentren für Suchtmedizin
Konradstrasse 32
8005 Zürich |
|
References
1 Heymann DL, editor. Control of communicable diseases manual. 19th ed. American Public Health Association; 2008. Viral hepatitis B; p.284–93.
2 Heymann DL, editor. Control of communicable diseases manual. 19th ed. American Public Health Association; 2008. Viral hepatitis C; p.293–95.
3 Colvin HM, Mitchell AE, editors; Committee on the prevention and control of viral hepatitis infections; Institute of Medicine. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Washington, DC: The National Academies Press; 2010. Available from: http://www.nap.edu/catalog/12793.html.
4 Lavanchy D. Chronic viral hepatitis as a public health issue in the world. Best Pract Res Clin Gastroenterol. 2008;22(6):991–1008.
5 Gonzalez SA, Keeffe EB. Chronic viral hepatitis: epidemiology, molecular biology, and antiviral therapy. Front Biosci. 2011;16:225–50.
6 Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol. 2008;48(2):353–67.
7 World Health Organization. Prevention & Control of Viral Hepatitis: Framework for Global Action. World Health Organization 2012. Available from: http://www.who.int/csr/disease/hepatitis/GHP_framework.pdf.
8 Veldhuijzen IK, Toy M, Hahné SJ, De Wit GA, Schalm SW, de Man RA et al. Screening and early treatment of migrants for chronic hepatitis b virus infection is cost-effective. Gastroenterology. 2010;(2):522–30.
9 European Centre for Disease Prevention and Control. Hepatitis B and C in the EU neighbourhood: prevalence, burden of disease and screening policies. Stockholm: ECDC; 2010. Available from: http://www.ecdc.europa.eu/en/publications/Publications/TER_100914_Hep_B_C%20_EU_neighbourhood.pdf http://www.ecdc.europa.eu/en/publications/Publications/TER_100914_Hep_B_C _EU_neighbourhood.pdf .
10 Robert Koch Institut. Ratgeber für Ärzte: Hepatitis B. Stand vom 01.08.2004 [cited 2011 Oct 29]. Available from: http://www.rki.de/DE/Content/Infekt/EpidBull/Merkblaetter/Ratgeber_HepatitisB.html?nn=2374512. German.
11 Bundesamt für Gesundheit, Referenzzentren für blutübertragbare Infektionen im Gesundheitsbereich. Prävention blutübertragbarer Krankheiten auf Patienten: Empfehlungen für Personal im Gesundheitswesen mit Hepatitis B-, Hepatitis C- oder HIV-Infektion. Richtlinien und Empfehlungen. Bern: Bundesamt für Gesundheit, 2011. German.
12 Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11(2):97–107.
13 Heininger U, Vaudaux B, Nidecker M, Pfister RE, Posfay-Barbe KM, Bachofner M et al. Evaluation of the compliance with recommended procedures in newborns exposed to HBsAg-positive mothers: a multicenter collaborative study. Pediatr Infect Dis J. 2010;29(3):248–50.
14 Ambühl PM, Binswanger U, Renner EL. Epidemiologie der chronischen Hepatitis B und C bei Dialysepatienten in der Schweiz. Schweiz Med Wochenschr. 2000;130:341–8. German.
15 Steffen T, Blättler R, Gutzwiller F, Zwahlen M. HIV and hepatitis virus infections among injecting drug users in a medically controlled heroin prescription programme. Eur J Public Health. 2001;11(4):425–30.
16 Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378(9791):571–83.
17 Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359(14):1486–500.
18 Gottwein JM, Scheel TK, Jensen TB, Lademann JB, Prentoe JC, Knudsen ML et al. Development and characterization of hepatitis C virus genotype 1-7 cell culture systems: role of CD81 and scavenger receptor class B type I and effect of antiviral drugs. Hepatology. 2009.
19 Robert Koch Institut. Ratgeber für Ärzte: Hepatitis C. Stand vom 01.04.2004 [cited 2011 Oct 29]. Available from: http://www.rki.de/DE/Content/Infekt/EpidBull/Merkblaetter/Ratgeber_HepatitisC.html?nn=2374512. German.
20 Ciesek S, Friesland M, Steinmann J, Becker B, Wedemeyer H, Manns MP et al. How stable is the hepatitis C virus (HCV)? Environmental stability of HCV and its susceptibility to chemical biocides. J Infect Dis. 2010;201(12):1859–66.
21 Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology. 2010;52(4):1497–505.
22 Wandeler G, Gsponer T, Bregenzer A, Günthard HF, Clerc O, Calmy A et al. Hepatitis C Virus Infections in the Swiss HIV Cohort Study: A Rapidly Evolving Epidemic. Clin Infect Dis. 2012 Sep 12. [Epub ahead of print]
23 Cornberg M, Razavi HA, Alberti A, Bernasconi E, Buti M, Cooper C et al. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int. 2011;31(Suppl 2):30–60.
24 Sagmeister M, Renner EL, Mullhaupt B, Wong JB. Simulation of hepatitis C based on a mandatory reporting system. Eur J Gastroenterol Hepatol. 2002;14(1):25–34.
25 Dubois-Arber F, Balthasar H, Huissoud T, Zobel F, Arnaud S, Samitca S et al. Trends in drug consumption and risk of transmission of HIV and hepatitis C virus among injecting drug users in Switzerland, 1993–2006. Euro Surveill. 2008;13(21). pii: 18881.
26 Prasad LR, Masserey Spicher V, Kammerlander R, Zwahlen M. Hepatitis C in a sample of pregnant women in Switzerland: seroprevalence and socio-demographic factors. Swiss Med Wkly. 2007;137(1-2):27–32.
27 Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81.
28 Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17(2):107–15.
29 Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271–8.
30 Thompson A, Patel K, Tillman H, McHutchison JG. Directly acting antivirals for the treatment of patients with hepatitis C infection: a clinical development update addressing key future challenges. J Hepatol. 2009;50(1):184–94.
31 Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–16.
32 Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195–206.
33 European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55(2):245–64.
34 Garrone P, Fluckiger AC, Mangeot PE, Gauthier E, Dupeyrot-Lacas P, Mancip J et al. A prime-boost strategy using virus-like particles pseudotyped for HCV proteins triggers broadly neutralizing antibodies in macaques. Sci Transl Med. 2011;3(94):94ra71.
35 Piorkowsky NY. Europe’s hepatitis challenge: defusing the “viral time bomb”. J Hepatol. 2009;51(6):1068–73.
36 World Health Organization. State of the art of new vaccine research and development. 2006. Geneva; Switzerland. Available from: http://whqlibdoc.who.int/hq/2006/WHO_IVB_06.01_eng.pdf.
37 British Association for the Study of the Liver, British Society of Gastroenterology (Liver Section). A time to act: improving liver health and outcomes in liver disease. The national plan for liver services U.K 2009. Available from: http://www.bsg.org.uk/attachments/1004_National%20Liver%20Plan%202009.pdf http://www.bsg.org.uk/attachments/1004_National Liver Plan 2009.pdf .
38 Health Protection Agency. Hepatitis C in the UK; 2011. London, July 2011. Available from: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1309969906418.
39 Health Protection Agency. Hepatitis C in the UK; 2007. London, December 2007. Available from: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1204100441645.
40 Bundesamt für Gesundheit. Nationales Programm: HIV und andere sexuell übertragbare Infektionen (NPHS), 2011–2017. Dezember 2010. Available from: http://www.bag.admin.ch/hiv_aids/05464/05465/index.html?lang=de. German.
41 Andermann A. Screening for persons with underlying chronic disease. In: VHPB (Viral Hepatitis Prevention Board) meeting on identification and management of persons with chronic viral hepatitis in Europe; 2010 March 18–19; Budapest, Hungary: p. 2–5.
42 European Observatory on Health Systems and Policies. Policy brief: Screening in Europe. Geneva: WHO; 2006. Available from: http://www.euro.who.int/__data/assets/pdf_file/0007/108961/E88698.pdf.
43 The Writing Committee on behalf of the Consensus Panel. Expert Consensus Conference: The screening for hepatitis C virus infection in adults in Italy, May 5–6, 2005. Dig Liver Dis. 2006;38(7):445–51.
44 Goldberg D, Roudot-Thoraval F. In: VHPB (Viral Hepatitis Prevention Board) meeting on identification and management of persons with chronic viral hepatitis in Europe; 2010 March 18-19; Budapest, Hungary: p.1.
45 Sroczynski G, Esteban E, Conrads-Frank A, Schwarzer R, Mühlberger N, Wright D et al. Long-term effectiveness and cost-effectiveness of screening for hepatitis C virus infection. Eur J Public Health. 2009;19(3):245–53.
46 Thompson Coon J, Castelnuovo E, Pitt M, Cramp M, Siebert U, Stein K. Case finding for hepatitis C in primary care: a cost utility analysis. Fam Pract. 2006;23(4):393–406.
47 Castelnuovo E, Thompson-Coon J, Pitt M, Cramp M, Siebert U, Price A et al. The cost-effectiveness of testing for hepatitis C in former injecting drug users. Health Technol Assess. 2006;10(32):iii-iv, ix-xii, 1–93.
48 Stein K, Dalziel K, Walker A, Jenkins B, Round A, Royle P. Screening for Hepatitis C in injecting drug users: a cost utility analysis. J Public Health. 2004;26(1):61–71.
49 Sutton AJ, Edmunds WJ, Sweeting MJ, Gill ON. The cost-effectiveness of screening and treatment for hepatitis C in prisons in England and Wales: a cost-utility analysis. J Viral Hepat. 2008;(11):797–808.
50 Bundesamt für Gesundheit, Eidgenössische Kommission für Impffragen, Arbeitsgruppe «Prävention der Mutter-Kind-Übertragung von Hepatitis B». Empfehlungen zur Prävention der Mutter-Kind-Übertragung von Hepatitis-B. Ergänzung zu den Richtlinien und Empfehlungen Nr. 2 (ehemals Supplementum II). Bern: Bundesamt für Gesundheit, 2007. German.
51 Niederhauser C. 11 Jahre nationales Referenzzentrum für Infektionen durch Blut und Blutprodukte. Bull BAG. 2010;10:336–44.
52 Rein DB, Smith BD, Wittenborn JS, Lesesne SB, Wagner LD, Roblin DW et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012;156(4):263–70.
53 Murphy EL. The increasing burden of mortality from viral hepatitis in the United States. Ann Intern Med. 2012;157(2):149–50.
54 Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR 2012;61(No.RR-4). Available from: http://www.cdc.gov/mmwr/pdf/rr/rr6104.pdf.
55 European Liver Patients Association, European Association for the study of the Liver. Recommendations for the promotion of case-finding for viral hepatitis B and C, including targeted screening measures for risk groups, February 2009. Available from: http://www.elpa-info.org/tl_files/elpa_downloads/recommendations-consolidated-version-final.pdf http://www.elpa-info.org/tl_files/elpa_downloads/recommendations-consolidated-version-final.pd .
56 Ministère de la santé et des sports. Direction générale de la santé. Plan national de lutte contre les Hépatites B et C, 2009-2012. 2010, Paris. Available from: http://www.sante.gouv.fr/IMG/pdf/Plan_national_Hepatites.pdf. French.
57 Health Service Executive. HSE National Social Inclusion. National Hepatitis C Strategy 2011–2014. 2012, Dublin. Available from: http://www.hse.ie/eng/services/Publications/HealthProtection/HepCstrategy.pdf.
58 Bundesamt für Gesundheit. Hepatitis C in der Schweiz: Für eine individuelle Information und Beratung. Bull BAG. 2001;46:877–81. German.
59 Bundesamt für Gesundheit, Schweizerische Kommission für Impffragen, Schweizerische Expertengruppe für virale Hepatitis. Empfehlungen zur Hepatitis-B-Impfung. Richtlinien und Empfehlungen (ehemals Supplementum II). Bern: Bundesamt für Gesundheit, 1997. German.


