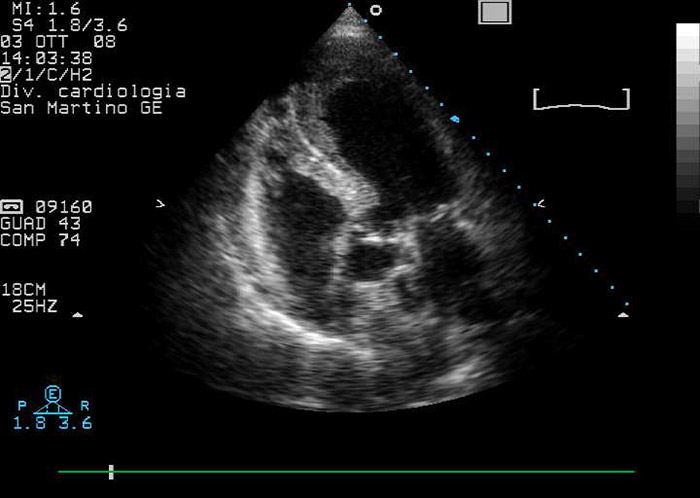
Figure 1
Echocardiographic modified four/five chambers view: note apical ballooning and apical mural stratified thrombus.
DOI: https://doi.org/10.4414/smw.2013.13797
Impaired left ventricle (LV) function, increased LV volume, apical aneurysm or endocardial injury might promote left ventricle thrombosis (LVT) in both anterior myocardial infarction (AMI) and apical ballooning syndrome (ABS). However, the clinical course of thrombosis in these settings is quite different.

Figure 1
Echocardiographic modified four/five chambers view: note apical ballooning and apical mural stratified thrombus.
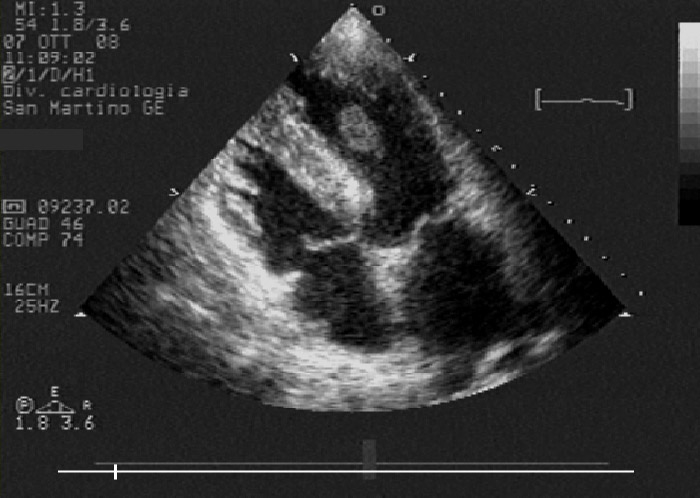
Figure 2
Echocardiographic modified four chambers view: note improvement of apical kinesis and floating, protruding left ventricle thrombus, whose morphology denotes a high risk of embolisation.
A 75-year-old woman was admitted to our Emergency Department complaining of leg pains and asthenia, following an intense episode of diarrhoea and vomiting. She was admitted to the Cardiology Division, San Martino Hospital and University of Genoa (Italy) within 24 hours of symptom onset. She was asymptomatic for chest pain. Physical examination revealed signs of leg ischaemia and hypoperfusion.
A routine electrocardiogram showed anterior-lead ST-segment elevation; transthoracic echocardiography (TTE) revealed apical ballooning, impaired LV ejection fraction (40%) and a stratified LVT (fig. 1). An ST elevation myocardial infarction (STEMI) complicated by acute leg ischaemia was then supposed. Recommended pharmacological treatment for STEMI was initiated [1]. Urgent coronary angiography and left ventriculography were performed, which showed normal epicardial coronary arteries and confirmed apical ballooning complicated by an apical LVT; STEMI was thus excluded and ABS was then postulated. Fogarty balloon thrombectomy was successfully executed to resolve the peripheral ischemia. Serum troponin I levels peaked at 1.09 µg/l.
TTE after angiography revealed a protruding apical LVT. Heparin was continued and warfarin was added in the following days; target INR values were reached. TTE 96 hours after hospital admission showed a quite complete recovery in LV function and in regional wall motion; this situation probably promoted detachment of the apical LVT from the apical wall, so that the apical LVT assumed a floating morphology (fig. 2). On day six, the patient developed acute left arm ischaemia that fortunately required only medical therapy. At this time, TTE showed complete resolution of the apical LVT and complete recovery of LV ejection fraction.
Although evidence on apical LVT in ABS is mainly based on single reports and series of clinical cases, it is known that LVT has peculiar characteristics in ABS or AMI.
The incidence of LVT in AMI in the era of reperfusion therapy ranges from 8% to 20%, according to different reports [2, 3].
Though ABS is often associated with severe acute LV dysfunction, LVT was documented in 5.3% of cases and cardioembolic complications occurred in less than 1% in different studies [4, 5]. We report a case of a 75-year-old woman with ABS, moderately reduced ejection fraction and apical LVT, who had two embolic events during the same hospitalisation.
LV apical aneurysm is often more extensive in ABS than in AMI, but the incidence of LVT seems to be lower, probably because LV apical ballooning is usually transient and wall motion abnormalities often disappear within a few weeks. Therefore, the clinical course of thrombosis in ABS and AMI is quite different, but once LVT appears, embolic events may occur more frequently in ABS than in AMI. In addition, the improvement of contractility might promote the discharge of emboli [4, 6–8]. We believe that the improvement in LV function and recovery of apical wall motion abnormalities induced the complete detachment of the LVT from the apical wall, transforming a mural thrombus in a protruding, floating one. The dynamic, fast recovery in apical wall motion abnormalities promoted LVT detachment with consequent embolic complications, despite optimal anticoagulation therapy, as demonstrated in our case. This evolution makes LVT extremely dangerous when present in ABS.
We have documented what happened to an apical LVT in a patient affected by ABS, during the recovery of acute LV dysfunction and the progressive normalisation of an apical aneurysm. Indeed, we depicted through TTE follow up the dynamic evolution and the correlated risk of embolisation of the apical LVT in the hours following the acute event. Clinicians must be aware of this possibility and, therefore, TTE should be recommended to all ABS patients. Whether strict TTE follow up should be recommended in all patients with ABS remains unclear.
Funding / potential competing interests: All authors declare that they have no competing interests in relation to this article.
1 Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32(23):2999–3054.
2 Anzai T, Yoshikawa T, Kaneko H, Maekawa Y, Iwanaga S, Asakura Y, Ogawa S. Association between serum C-reactive protein elevation and left ventricular thrombus formation after first anterior myocardial infarction. Chest. 2004;125(2):384–9.
3 Seo Y, Maeda H, Ishizu T, Ishimitsu T, Watanabe S, Aonuma K, Yamaguchi I. Peak C-reactive protein concentration correlates with left ventricular thrombus formation diagnosed by contrast echocardiographic left ventricular opacification in patients with a first anterior acute myocardial infarction. Circ J. 2006;70(10):1290–6.
4 Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Nakama Y, et al. Incidence and treatment of left ventricular apical thrombosis in Tako-tsubo cardiomyopathy. Int J Cardiol. 2011;146(3):e58–60.
5 de Gregorio C, Grimaldi P, Lentini C. Left ventricular thrombus formation and cardioembolic complications in patients with Takotsubo-like syndrome: a systematic review. Int J Cardiol. 2008;131(1):18–24.
6 Robles P, Jimenez JJ, Alonso M. Left ventricular thrombus associated with left ventricular apical ballooning. Heart. 2007;93(7):861.
7 Y-Hassan S, Shahgaldi K. Thrombo-embolic renal infarction in a case of mid-ventricular takotsubo syndrome. Intern Med. 2011;50(19):2175–8.
8 Yoshida S, Miwa K, Matsubara T, Yasuda T, Inoue M, Teramoto R, et al. Stress-induced takotsubo cardiomyopathy complicated with wall rupture and thrombus formation. Int J Cardiol. 2012;161(1):e18–20.