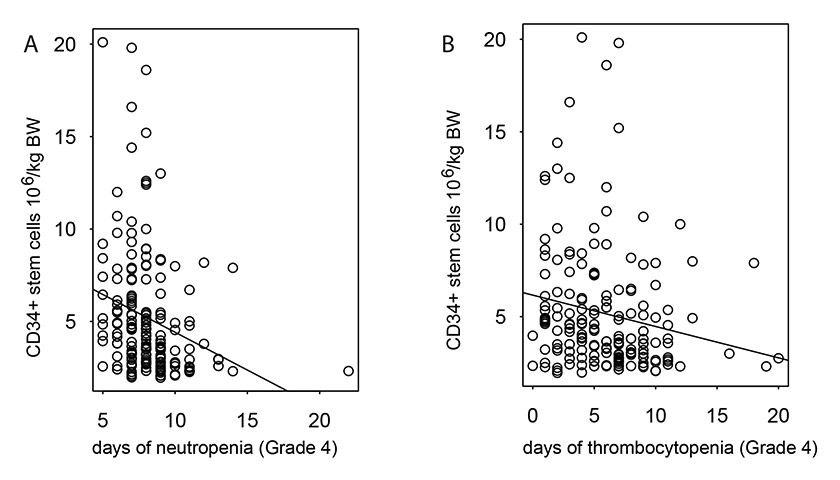
Figure 1
Correlation of the number of reinfused CD34+ stem cells with the length of neutropenias (r = –0.25, fig. A) and thrombocytopenias (r = –0.18, fig. B) after ASCT.
DOI: https://doi.org/10.4414/smw.2013.13791
List of abbreviations
ASCT autologous stem cell transplantation
BEAM conditioning regimen with carmustine, etoposide, cytarabine and melphalan
BMI body mass index
CBV conditioning regimen with cyclophosphamide, carmustine and etoposide
CD34 cluster of differentiation 34
CHOP first-line regimen with prednisone, cyclophosphamide, doxorubicin and vincristine
CI confidence interval
CLL chronic lymphocytic leukaemia
CNS central nervous system
CORAL Collaborative Trial in Relapsed Aggressive Lymphoma
CR complete remission
CRu complete remission unconfirmed
Dexa-BEAM salvage regimen with dexamethasone, carmustine, etoposide, cytarabine and melphalan
DHAP salvage regimen with dexamethasone, cytarabine and cisplatin
DLBCL diffuse large B-cell lymphoma
DMSO dimethyl sulfoxide
EFS event free survival
EPOCH salvage regimen with prednisone, doxorubicin, vincristine, etoposide and cyclophosphamide
FDG Fluorodeoxyglucose
FL follicular lymphoma
G-CSF granulocyte-colony stimulating factor
HDC high dose chemotherapy
HL Hodgkin’s lymphoma
HR hazard ratio
ICE salvage regimen with ifosfamide, carboplatin and etoposide
MCL mantle cell lymphoma
NHL Non-Hodgkin’s lymphoma
OS overall survival
PET/CT positron emission tomography/computed tomography
PD progressive disease
PR partial remission
RBC red blood cells
SD stable disease
TCL T cell lymphoma
TRM treatment related mortality
High-dose chemotherapy (HDC) followed by autologous stem cell transplantation (ASCT) is being widely used as salvage treatment in patients with non-Hodgkin’s lymphoma (NHL) or Hodgkin’s lymphoma (HL) [1–3]. This treatment strategy has the potential to improve significantly survival compared with standard dosed salvage chemotherapy alone. It offers a chance for a cure in up to 50% of patients with chemosensitive relapse, while the results are still unsatisfactory in patients with primary refractory disease, where a cure rate of 10%–25% may be achieved [4, 5]; an important prognostic factor for survival is the response to salvage chemotherapy. Patients with complete remission (CR) before ASCT have a better survival than patients with partial remission (PR) [6, 7]. In addition, many centres offer HDC and ASCT also as consolidation treatment after first line therapy to patients with poor prognostic features, i.e., in patients with T cell lymphoma or primary central nervous system lymphoma. The autologous stem cell transplantation programme Zurich includes two hospitals, the University Hospital and the Triemli City Hospital. It was initiated in 1988, and we reported first results 11 years later after having performed more than 250 ASCTs, predominantly in patients with NHL, HL, multiple myeloma and germ cell tumours [8]. Recently, we reported our current results in patients with multiple myeloma, who received ASCT as consolidation treatment. Beside survival data comparable to published data, we observed minimal toxicity and a low treatment related mortality (TRM) of 0.5% [9].
Treatment modalities for HL and NHL have changed during the last 10 years owing to various improvements in this field; the possibility of harvesting peripheral blood stem cells omits the invasive bone marrow harvest procedure. The use of granulocyte-colony stimulating factors (G-CSF) reduces the time to engraftment and, thus, the risk of infectious complications during neutropenia [10, 11]. Patient management has also improved through implementation and continuous adjustment of international standard operating procedures. We retrospectively analysed the clinical course and the outcome of all patients with NHL and HL undergoing ASCT from December 2000 until May 2011 to identify possible risk factors for survival and to control for quality in comparison to published data. Further, we placed a special emphasis on the prognostic impact of positron emission tomography / computed tomography (PET/CT) before and after ASCT on treatment outcome.
| Table 1: Patient characteristics. | |
| Parameter | PatientsN = 180 (100%) |
| Age <65 years – no. (%) ≥65 years – no. (%) Median – yr Range – yr | 163 (91) 17 (9) 53 18.7–67.9 |
| Gender Male – no. (%) Female – no. (%) | 117 (65) 63 (35) |
| BMI before ASCT Median – kg/m² Range – kg/m² | 24.9 16.2–45.7 |
| Lymphoma type and NHL subtypes Non-Hodgkin’s lymphoma – no. (%) – Follicular lymphoma – no. (%) – Chronic lymphocytic leukaemia – no.(%) – Marginal zone lymphoma – no. (%) – DLBCL – no. (%) – Mantle cell lymphoma – no. (%) – T-cell lymphoma – no. (%) – Other – no. (%) Hodgkin’s lymphoma – no. (%) | 134 (74) 18 (10) 1 (0.5) 1 (0.5) 59 (32) 25 (14) 25 (14) 5 (3) 46 (26) |
| Remission status before ASCT CR/CRu Less than CR | 78 (43) 94 (52) |
| ASCT indication Primary refractory disease Relapse (>3 months after 1st line treatment) Consolidation | 41 (23) 117 (65) 22 (12) |
| PET/CT for clinical monitoring Before ASCT – no. (%) After ASCT – no. (%) | 87 (48) 63 (35) |
| First line treatment – no. (%) CHOP +/– rituximab CHOEP +/– rituximab Hyper-CVAD +/– rituximab ABVD-like BEACOPP Other | 106 (59) 8 (4.5) 6 (3.5) 36 (20) 9 (5) 15 (8) |
| Number of previous treatment lines - median (range) Non-Hodgkin’s lymphoma – Low-grade lymphoma – DLBCL – Mantle cell lymphoma – T-cell lymphoma Hodgkin’s lymphoma | 2 (1–5) 2 (1–5) 2 (1–9) 2 (1–3) 2 (2–4) |
| BMI = body mass index; ASCT = autologous stem cell transplantation. NHL = non-Hodgkin’s lymphoma; DLBCL = diffuse large B-cell lymphoma; PET/CT = positron emission tomography / computed tomography. | |
Patients with NHL and HL who received an ASCT at our transplantation centre were analysed retrospectively, based on a prospective transplantation database. This analysis was approved by our local ethics committee.
Data regarding lymphoma stage, the salvage and conditioning regimens used, the response to treatment before and after ASCT, event free survival (EFS) and overall survival (OS) were collected. Additional variables like age, gender, body mass index (BMI), the amount of cluster of differentiation (CD) 34 positive cells mobilised, and PET/CT results were documented. We also collected data regarding the haematological toxicity and the supportive care of the patients during the post-transplant phase.
All patients received primary salvage chemotherapy as chosen by the attending oncologists. Salvage regimens used were ICE (ifosfamide, carboplatin, etoposide), DHAP (dexamethasone, cytarabine, cisplatin), EPOCH (prednisone, doxorubicine, vincristine, etoposide, cyclophosphamide), Dexa-BEAM (dexamethasone, carmustine, etoposide, cytarabine, melphalan) or CHOP-like (prednisone, cyclophosphamide, doxorubicin, vincristine) regimens. Stem cell mobilisation was usually performed after the second or third treatment cycle by administering daily filgrastim at a dose of 10 μg/kg body weight from day -3 on. Apheresis was performed at our transplantation centre until at least 2 x 106 CD34+ cells per kilogramme body weight were collected. Purging was not part of the procedure. The collected cell products were cryopreserved with 10% dimethyl sulfoxide (DMSO) and stored in liquid nitrogen until the day of ASCT. Cell thawing was performed immediately before retransfusion in a 37 °C water bath.
Conditioning chemotherapy consisted mainly of BEAM (carmustine 200–300 mg/m2, melphalan 140 mg/m2, etoposide 8 x 100–150 mg/m2, and cytarabine 8 x 200 mg/m2) or CBV (cyclophosphamide 4 x 1500 mg/m2, etoposide 6 x 100–150 mg/m2, and carmustine 200–300 mg/m2). Patients with poor performance status, organ dysfunctions like renal insufficiency, reduced pulmonary diffusion capacity or age over 65 years could receive a reduced dose of etoposide and/ or carmustine after discussion in our multidisciplinary transplantation board. As previously reported patients received daily filgrastim at a dose of 5 μg/kg body weight from day +5 on or pegfilgrastim 6 mg once on day +1 after ASCT to shorten time to engraftment [12].
Regarding the management of febrile neutropenia after ASCT, we comply with general European guidelines at our centre [13]. Briefly, patients received empiric broad spectrum antibiotic therapy with Pseudomonas-active β-lactam agents (like piperacillin-tazobactam or cefepime in first, carbapenems in second line). In case of obvious infections with gram-positive germs (i.e., catheter-infection) the patients also received vancomycin. According to international guidelines, the transfusion threshold after ASCT for patients with anaemia was haemoglobin of 7 g/dl (or higher in case of associated symptoms or cardiovascular disease), and for patients with thrombocytopenia thrombocyte levels of 10 x 109 /L (20 x 109 /L for patients with increased risk of bleeding) [14, 15].
Assessment of disease response was based on every available clinical or radiological evaluation, including PET/CT, as documented in the patient charts and the radiologic reports. Because of the low patient numbers, we only discriminated between patients with CR or unconfirmed CR (CRu), and patients with less than CR.
EFS was defined as time from ASCT to the time of first recurrence after achievement of CR or to the time of progression in patients with unconfirmed CR, as verified by clinical examination, imaging and biopsy, or to the time of death by any cause. OS was defined as time from ASCT to the time of death by any cause, as documented in the patient charts.
2-[fluorine-18]-fluoro-2-deoxy-D-glucose (FDG)-PET imaging data were acquired on a combined PET/CT in-line system (Discovery LS, RX or Discovery STE; GE Health Systems, Milwaukee, WI). This technique allowed the combination of CT-scans and PET images in a single session. PET/CT was available since 2001 at our institution, and has been increasingly used for staging and treatment monitoring of lymphoma patients during and after end of treatment. It was used individually at the treating physician’s discretion, and therefore PET data are not available for all patients. PET/CT scans performed during or after completion of salvage chemotherapy (pre-ASCT PET/CT) and within three months after ASCT (post-ASCT PET/CT) were evaluated. Image analysis was routinely carried out by two nuclear radiology physicians in consensus. PET/CT scans were assessed for residual pathologic FDG-uptake.
Descriptive statistics (median and range, or counts) were calculated for all variables. Event free and overall survival were calculated from the date of ASCT, and censored at the date of last follow-up. Survival curves were computed using the method of Kaplan and Meier, and compared using the log-rank test [16]. Risk factor analysis for survival was performed by univariate Cox proportional hazards models and multivariate Cox regression models. P-values <0.05 were considered statistically significant. All analyses were performed in the R programming language [17].
| Table 2: Conditioning and post-ASCT variables. | |
| Parameter (n = 180 patients) | Value |
| Conditioning regimen CBV – no. (%) BEAM – no. (%) Other – no. (%) | 72 (40) 103 (57) 5 (3) |
| CD 34+ cells reinfused Median – x106 cells /kg of body weight Range – x106 cells /kg of body weight | 4.1 1.96–20.1 |
| Duration grade 4 neutropenia, median (range) – days – after CBV conditioning, median (range) – days – after BEAM conditioning, median (range) – days | 8 (5–22) 9 (5–14) 8 (5–22) |
| Duration grade 4 thrombopenia, median (range) - days – after CBV conditioning, median (range) – days – after BEAM conditioning, median (range) – days | 6 (0–20) 6 (0–19) 6 (0–20) |
| Duration of filgrastim treatment: Median – days Range – days | 6 0–16 |
| Occurrence of neutropenic fever – no. (%) Use of antibiotics – no. (%) Use of fungistatics – no. (%) | 160 (89) 169 (94) 116 (64) |
| Platelet transfusions Median – no. of transfusions Range – no. of transfusions | 2 0–60 |
| Red blood cell transfusions Median – no. of transfusions Range – no. of transfusions | 2 0–43 |
| Hospital stay from day of ASCT Median – days Range – days | 15 9–72 |
| Treatment related mortality, overall – no. (%) | 3 (1.7) |
| CD = cluster of differentiation; ASCT = autologous stem cell transplantation; TRM = treatment related mortality; CBV = cyclophosphamide, carmustine and etoposide; BEAM = carmustine, etoposide, cytarabine and melphalan. | |
Between December 2000 and May 2011, a total of 180 consecutive ASCTs were performed in lymphoma patients at our centre. Of these patients, 59 (32%) were diagnosed with diffuse large B-cell lymphoma (DLBCL) and 46 (26%) with HL. About two-thirds were male (65%) and one-third female (35%). Eighty-seven (48%) received a PET/CT during or after salvage chemotherapy, and 63 (35%) also received PET/CT within 3 months after ASCT. In our patient cohort, 15% of DLBCL patients had received prior rituximab during previous treatment until 2006, while from 2007 on 93% of the DLBCL patients had already been pretreated with rituximab. Some of the patients had received first-line therapy at other hospitals or at oncological private practices before they were subsequently referred to our transplantation centre for salvage treatment and ASCT. The main patient characteristics are shown in table 1.

Figure 1
Correlation of the number of reinfused CD34+ stem cells with the length of neutropenias (r = –0.25, fig. A) and thrombocytopenias (r = –0.18, fig. B) after ASCT.
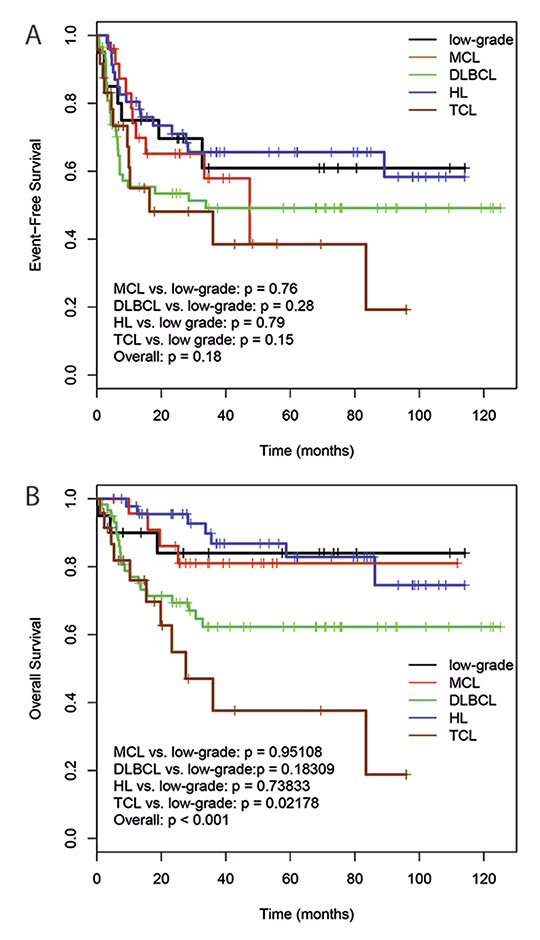
Figure 2
Event free survival (A) and overall survival (B) of the patients with respect to the different lymphoma subgroups.
MCL, mantle cell lymphoma; DLBCL, diffuse large B-cell lymphoma; HL, Hodgkin’s lymphoma; TCL, T cell lymphoma.
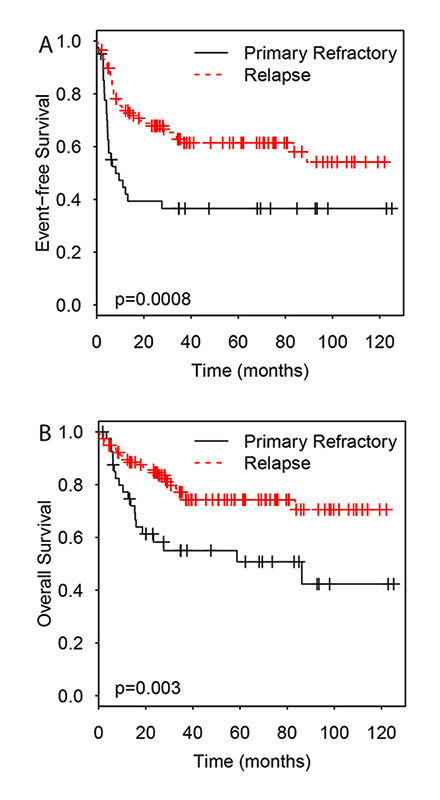
Figure 3
Impact of chemosensitivity to primary chemotherapy on event free survival (A) and overall survival (B).
All clinical data is shown in table 2. Patients with HL received predominantly EPOCH (n = 19, 41%) or DHAP (n = 19, 41%) as salvage chemotherapy, while DLBCL patients received predominantly ICE (n = 31, 53%) or EPOCH (n = 17, 29%) combined with rituximab.
The conditioning regimens mainly used were BEAM (n = 103; 57%) and CBV (n = 72; 40%). Five patients (3%) received other conditioning regimens. The use of conditioning regimen according to the lymphoma subtypes is presented in table 3. Patients received subsequently a minimum of 1.96 x 106 CD34+ stem cells per kilogramme of body weight (median 4.1 x 106 CD34+ cells; range, 1.96 x 106–20.1 x 106). The median duration of grade 4 neutropenia and the median time to engraftment were 8 days (range, 5–22 days) and 9 days (range, 7–23 days), respectively. The median duration of grade 4 thrombocytopenia was 6 days (range, 0–20 days). The number of CD34+ stem cells correlated inversely with the duration of grade 4 thrombocytopenia (correlation coefficient r = –0.1815) and the duration of grade 4 neutropenia (r = –0.2502) (fig. 1a, b). The median length of hospital stay from the day of ASCT was 15 days (range, 9–72 days). Patients received a median of 2 units (range, 0–60) platelet transfusions and a median of 2 units (range, 0–43) red blood cell (RBC) transfusions during hospital stay. Fever developed in 160 (89%) patients after ASCT, and the use of antibiotics for therapeutic purposes was necessary in 169 (94%) patients during the post-transplant period. Bacterial pathogens were isolated in 46 (26%) patients. Infectious agents most commonly isolated were Klebsiella pneumoniae (n = 6; 3%), Escherichia coli (n = 10; 6%) and coagulase-negative Staphylococcus sp. (n = 13; 7%).
Four (2%) patients died within the first 100 days after ASCT. One patient died due to disease progression, and the other three patients died due to infectious complications: one Pseudomonas aeruginosasepticaemia, one cytomegalovirus pneumonitis, and one Aspergillus fumigatuspneumonia. The TRM for the whole patient collective was 1.7%.
Sufficient follow-up data was available for all patients except for one female patient, who was lost 53 days after ASCT. She was from abroad and came to Switzerland only for the ASCT procedure. The median follow-up was 31 months (range, 0.29–136 months). The median EFS and OS were not reached until the end of follow-up for the whole patient collective. OS differed between patients with DLBCL, HL, mantle cell lymphoma (MCL), follicular lymphoma (FL) and T-cell lymphoma (TCL) significantly (p <0.001). TCL patients had a worse prognosis compared with the other lymphoma subtypes. No statistically significant differences were seen regarding EFS (fig. 2a, b). The 3-year EFS was 67% for HL patients treated with CBV predominantly from 2000 to 2005, and 65% for HL patients treated with BEAM as conditioning regimen from 2006 on. The corresponding 3-year OS were 88% and 82%, respectively. No differences were seen in HL patients treated in the late period with BEAM compared with the early period with CBV regarding initial lymphoma stage, length of CR before relapse or patient age. For DLBCL patients, 3-year EFS was 76% in the early period, and 27% in the late period. The corresponding 3-year OS were 76% and 54%, respectively. The patients with DLBCL treated during the early period with CBV tended to be younger than the DLBCL patients treated later with BEAM, although the difference did not reach statistical significance (p = 0.052). Patients with primary refractory disease had a statistically worse EFS and OS than patients with a relapse after response to initial treatment (3-year EFS 37% versus 63%, p = 0.0008; 3-year OS 55% versus 76%; p = 0.003) (fig. 3a, b). In contrast, no statistically significant survival differences were seen regarding the disease status before ASCT (CR/CRu versus less than CR: 3-year EFS 60% versus 51%, p = 0.12; 3-year OS 72% versus 69%, p = 0.58). Also no statistically significant differences were seen with regard to the duration of remission before relapse or the use of rituximab during salvage treatment.
Overall, 39 (45%) of 87 patients with pre-ASCT PET/CT had residual FDG-uptake considered pathological. Patients with partial remission continued with their scheduled procedure, while patients with no response or progressive disease received a new non-crossresistant salvage treatment. Twenty (32%) of 63 patients with post-ASCT PET/CT had residual FDG-uptake considered pathological. As a consequence, six patients received short-term follow-up with PET/CT within 3 months, four patients immediately received new chemotherapy, and three patients received radiotherapy. One additional patient was planned for radiotherapy but died as a result of lymphoma progression before treatment could be initiated. Two patients had a biopsy that was negative, and one patient underwent a splenectomy. In three patients the positive post-ASCT PET/CT had no impact on the follow-up schedule; two of these patients remained in complete remission, while one patient relapsed at a later point. PET/CT performed after ASCT could detect relapses with a positive predictive value of 0.7.
A positive PET/CT during or after completion of salvage chemotherapy but before ASCT was associated with inferior EFS (HR 2.72 (1.42–5.2), p <0.01) and OS (HR 2.65 (1.11–6.33), p <0.05) (fig. 4a, b). In addition, a positive PET/CT performed within 3 months after ASCT also impacted adversely on EFS (HR 4.74 (2.33–9.64), p <0.001) and OS (HR 7.11 (2.76–18.34), p <0.001) (fig. 4c, d).
In the patient subgroups with DLBCL and HL, only for the DLBCL patients a positive PET/CT before and after ASCT was associated with reduced EFS (both p <0.05), but not OS. No differences were observed for HL patients regarding PET/CT before ASCT. An analysis of the HL patient subgroup which had received PET/CT after ASCT was not possible due to the low number of events (figs 5 and 6).
In a univariate Cox proportional hazards analysis in patients with DLBCL and HL, initial stage, the number of previous chemotherapy lines, PET results and patient age were adverse risk factors for EFS. Initial stage, number of previous treatment lines, PET results, patient age, grade 4 thrombocytopenia, and number of RBC and platelet units transfused were adverse prognostic factors for OS (table 4). Due to the generally favourable outcomes in our patients and the low number of events a multivariate analysis could only be performed for EFS, which identified initial lymphoma stage and number of previous treatment lines as independent risk factors (table 5).
| Table 3: Conditioning regimens used according to lymphoma subtype. | |||
| BEAM | CBV | Other | |
| Hodgkin’s lymphoma – n (%) | 22 (48) | 24 (52) | 0 (0) |
| Non-Hodgkin‘s lymphoma – n (%) – Low-grade lymphoma – DLBCL – Mantle cell lymphoma – T-cell lymphoma – Other | 11 (55) 30 (51) 21 (84) 17 (68) 2 (40) | 8 (40) 27 (46) 4 (16) 8 (32) 1 (20) | 1 (5) 2(3) 0 (0) 0 (0) 2 (40) |
| BEAM = carmustine, etoposide, cytarabine and melphalan; CBV = cyclophosphamide, carmustine and etoposide; DLBCL = diffuse large B cell lymphoma. | |||
| Table 4: Prognostic factors for event free and overall survival by univariate Cox proportional hazards analysis in diffuse large B cell lymphoma and Hodgkin’s lymphoma patients. | |||||||||
| Parameter | Event free survival | Overall survival | Observations Available (total = 105) | ||||||
| HR | Lower 95 CI | Upper 95 CI | p-value | HR | Lower 95 CI | Upper 95 CI | p-value | ||
| CD34+ cells mobilised (≥3 vs <3 x 106/kg) Previous irradiation (yes vs no) Patient age (per year) Response before ASCT (<CR vs CR(u)) CR less than 12 months (yes vs no) Initial lymphoma stage (III-IV vs I-II) Platelet transfusions (per unit) RBC transfusions (per unit) Duration of grade 4 neutropenia (per day) Duration of grade 4 thrombopenia (per day) Number of previous lines (≥3 vs <3) PET positive before ASCT (yes vs no) | 0.58 0.83 1.02 1.81 1.34 2.84 1.06 1.06 0.97 1.07 2.15 2.47 | 0.31 0.46 1.00 0.83 0.72 1.5 0.94 0.95 0.81 0.98 1.14 1.13 | 1.09 1.49 1.04 3.93 2.47 5.37 1.19 1.18 1.17 1.16 4.07 5.4 | 0.09 0.53 0.04 0.13 0.35 0.001 0.36 0.29 0.77 0.12 0.02 0.02 | 0.59 0.84 1.04 3.05 1.81 2.41 1.15 1.15 1.08 1.13 2.82 3.33 | 0.27 0.4 1.01 0.91 0.79 1.08 1.00 1.02 0.88 1.03 1.31 1.13 | 1.29 1.78 1.06 10.23 4.12 5.37 1.32 1.29 1.32 1.23 6.06 9.77 | 0.18 0.66 0.01 0.06 0.15 0.026 0.048 0.03 0.49 0.01 0.01 0.02 | 105 105 105 102 105 104 104 104 105 101 104 65 |
| HR = hazard ratio; CI = confidence interval; ASCT = autologous stem cell transplantation; CR = complete response; PET = positron emission tomography. | |||||||||
| Table 5: Multivariate Cox regression model for event free survival, including statistically significant parameters from the univariate analysis. | |||||
| Parameter | Coef. | HR = exp. (coef.) | 95% CI | p-value | Observations available (total = 105) |
| Initial lymphoma stage (III/IV vs I/II) PET+ before ASCT (yes vs no) Number of previous lines (≥3 vs <3) Patient age (per year) | 1.47 0.81 0.91 –0.009 | 4.34 2.26 2.48 0.99 | [1.47, 10.81] [1.00, 5.09] [1.10, 5.60] [0.96, 1.02] | 0.002 0.05 0.03 0.51 | 104 65 104 105 |
| HR = hazard; CI = confidence interval; PET = positron emission tomography; ASCT = autologous stem cell transplantation- | |||||
To identify risk factors for overall and event-free survival we evaluated the outcome data of our NHL and HL patients treated with ASCT. The number of previous chemotherapy lines, the responsiveness to first line chemotherapy, the initial lymphoma stage and the PET/CT-status before and after transplantation could be identified as important risk factors.
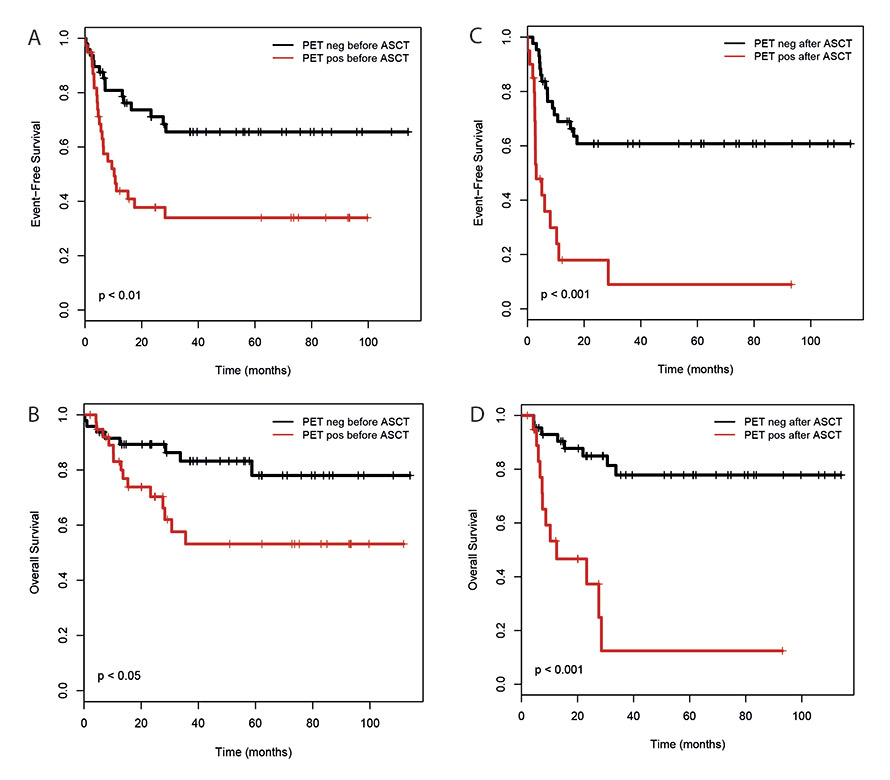
Figure 4
Impact of pre- and post-transplantation PET/CT on event free survival (A, C) and overall survival (B, D) in all patients. Pre-transplantation PET/CT was done during or after induction chemotherapy, post-transplantation PET/CT was done within 3 months after ASCT.
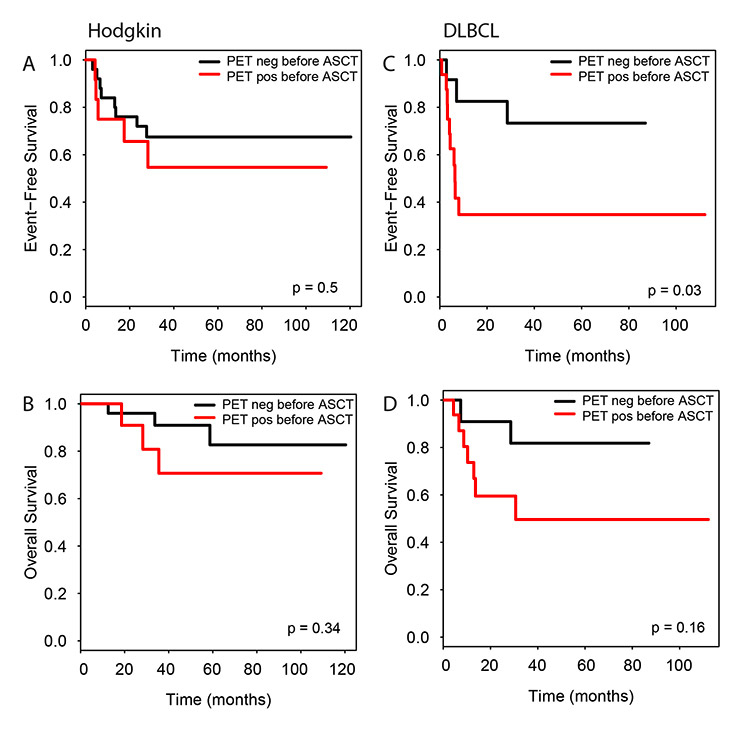
Figure 5
Impact of pre-transplantation PET/CT on event free and overall survival according to lymphoma entity. Hodgkin’s lymphoma (A, B) and DLBCL (C, D).
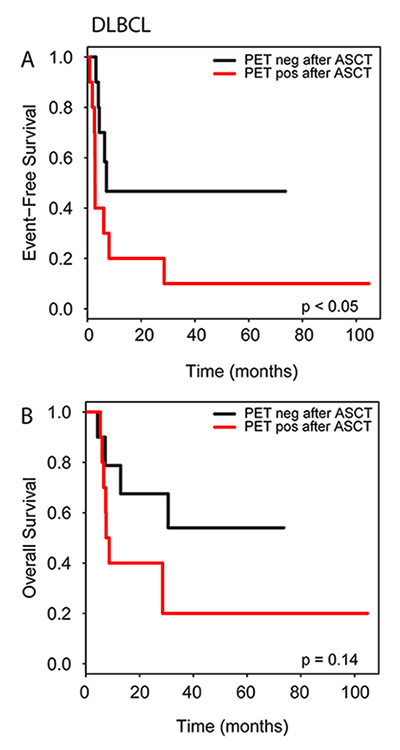
Figure 6
Impact of post-transplantation PET/CT on event free survival (A) and overall survival (B) in DLBCL patients.
Other factors with an impact on survival were an increased need for RBC and platelet unit transfusions, as well as the duration of grade 4 thrombocytopenia.
Patient selection criteria as well as the treatment underwent a practice change during the last ten years at our institution. Until 2005, patients received predominantly CBV as conditioning regimen, but from 2006 on BEAM was the preferred regimen due to its more favourable toxicity profile [18–20]. As a result of the optimisation of the transplant procedure and the supportive care measures, the indication for ASCT was continuously expanded, especially in patients suffering from DLBCL. As a consequence, older patients with more advanced DLBCL were increasingly selected for autotransplant during the observation period. In fact, the difference in patient age reached almost statistical significance and may explain the worse survival for the DLBCL patients who were autotransplanted from 2006 on. Another factor that may have impacted negatively on patient outcome in our study is the prior exposure to rituximab, since only a few patients had been pre-treated in the early period and most patients had received prior rituximab in the late period. Several studies have shown that rituximab during first line treatment was associated with worse survival in relapsing patients [21, 22]. In summary, the observed outcome of the patients receiving ASCT after BEAM is comparable to the results presented in the Collaborative Trial in Relapsed Aggressive Lymphoma (CORAL), confirming these data also in the daily practice outside of a clinical trial [21]. The low mortality rates after ASCT highlight that the impact on survival is not due to toxicities and subsequent differences in short-term outcome after transplantation, but rather due to patient heterogeneities [21, 23].
The most relevant risk factor identified in our study was the initial lymphoma stage. This finding confirms its established role as the main prognostic factor for patient outcome. Accordingly, lymphoma stage nowadays is the basis for a risk-adapted treatment decision, either directly in HL patients, or indirectly via the IPI score in DLBCL patients [24].
Increasing numbers of previous chemotherapy lines before ASCT were associated with worse survival. This may be explained by the lower chemosensitivity and more aggressive course of the underlying disease, which renders it prognostically disadvantageous [25, 26]. This is also supported by our observation that patients with primary refractory disease had a lower survival rate than patients with relapsed disease.
Another important finding of our analysis was the impact of the PET/CT in the ASCT setting. A PET/CT with persistent pathologic FDG-uptake during or after salvage chemotherapy was associated with reduced EFS and OS. In addition, a positive PET/CT within 3 months after ASCT was also associated with a worse outcome. For the patient subgroup with DLBCL alone, a positive PET/CT before and after ASCT was associated with inferior EFS, but only a trend for inferior OS was seen. For the HL patient subgroup, no statistically meaningful differences were seen. The lack of statistically significant differences for survival in the subgroup analyses may be explained by the low number of events in our patient cohort, since, especially HL patients, had a very favourable long-term disease free time and overall survival rate. Only very few HL patients had persistent FDG-avid lesions in the PET/CT after ASCT, thus preventing a statistically meaningful analysis. On the other hand, one could argue, based on these data, that patients with HL benefit more from high dose therapy than DLBCL patients, even if the lymphoma cells show residual FDG-uptake before transplantation. A few other studies have reported on the impact of PET positivity before [27–32] and after [33, 34] ASCT in lymphoma patients, and are in line with our findings. We hypothesise, based on these results that pre- and post-transplantation PET/CT may be a useful prognostic marker for lymphoma patients.
In our risk factor analysis we found a correlation of increased requirement for RBC and platelet unit transfusions (and length of grade 4 thrombocytopenia, respectively) with survival. Transfusion of RBC or platelets was an adverse risk factor for OS, but not EFS. Interestingly, an increased need for RBC transfusions has also been linked to adverse outcome in patients undergoing cardiac surgery or surgery for hip fracture [35–37]. In the ASCT setting of lymphoma patients, the presence of anaemia has been identified as an independent risk factor for survival [38], but the influence of the number of blood product transfusions on the long-term outcome has not been broadly reported. It is not clear, however, whether the increased need for blood products correlates with an increased long-term risk of death due to immunomodulation or induction of chronic inflammatory events, or whether it is just reflecting a prognostically unfavourable, more severely ill patient subgroup with reduced bone marrow function in need for transfusions.
The main limitations of our study are its retrospective nature and the limited patient number. Retrospective evaluation of data is often hindered by missing or inadequate documentation. However, most of the individual data have been originally coded prospectively, and we are reporting on all consecutive patients transplanted during the evaluated time period, thus avoiding any form of selection bias. Another limitation may be that our patients came from one area of Switzerland and the results may therefore not be generalisable to the whole of Switzerland and other countries. Finally, since many patients had been initially pre-treated at other hospitals and the treatment had not been predefined, differences in first line chemotherapy regimens may have influenced the subsequent response to ASCT and consequently the survival rates.
In conclusion, the long-term survival data of our patients with HL and NHL are in agreement with the existing literature indicating treatment with international quality standards. Both CBV and BEAM are effective conditioning regimens for lymphoma patients. Most importantly, we present an out-of-study confirmation of the recently published CORAL data by demonstrating similar efficacy of BEAM as conditioning regimen followed by ASCT in patients with DLBCL. Responsiveness to primary chemotherapy, initial lymphoma stage, the amount of pre-treatment, and a PET/CT scan before and after ASCT are prognostic for survival. Interestingly, an increased need for blood product transfusions during the post-transplant period is associated with a higher risk for reduced survival in our patients. Our results may lay the foundation for further research in wider populations before specific treatment recommendations can be finally derived.
1 Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333(23):1540–5.
2 Chopra R, McMillan AK, Linch DC, Yuklea S, Taghipour G, Pearce R, et al. The place of high-dose BEAM therapy and autologous bone marrow transplantation in poor-risk Hodgkin’s disease. A single-center eight-year study of 155 patients. Blood. 1993;81(5):1137–45.
3 Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341(8852):1051–4.
4 Philip T, Armitage JO, Spitzer G, Chauvin F, Jagannath S, Cahn JY, et al. High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1987;316(24):1493–8.
5 Josting A, Sieniawski M, Glossmann JP, Staak O, Nogova L, Peters N, et al. High-dose sequential chemotherapy followed by autologous stem cell transplantation in relapsed and refractory aggressive non-Hodgkin’s lymphoma: results of a multicenter phase II study. Ann Oncol. 2005;16(8):1359–65.
6 Oyan B, Koc Y, Ozdemir E, Kars A, Turker A, Tekuzman G, et al. High dose sequential chemotherapy and autologous stem cell transplantation in patients with relapsed/refractory lymphoma. Leuk Lymphoma. 2006;47(8):1545–52.
7 Prince HM, Imrie K, Crump M, Stewart AK, Girouard C, Colwill R, et al. The role of intensive therapy and autologous blood and marrow transplantation for chemotherapy-sensitive relapsed and primary refractory non-Hodgkin’s lymphoma: identification of major prognostic groups. Br J Haematol. 1996;92(4):880–9.
8 Jost LM, Honegger HP, Stahel RA. High-dose chemotherapy with autologous bone marrow transplantation: 11 years’ experience in Zurich. Schweiz Med Wochenschr. 2000;130(3):60–9.
9 Samaras P, Blickenstorfer M, Haile SR, Siciliano RD, Petrausch U, Mischo A, et al. Validation of prognostic factors and survival of patients with multiple myeloma in a real-life autologous stem cell transplantation setting: a Swiss single centre experience. Swiss Med Wkly. 2011;141:w13203.
10 McQuaker IG, Hunter AE, Pacey S, Haynes AP, Iqbal A, Russell NH. Low-dose filgrastim significantly enhances neutrophil recovery following autologous peripheral-blood stem-cell transplantation in patients with lymphoproliferative disorders: evidence for clinical and economic benefit. J Clin Oncol. 1997;15(2):451–7.
11 Schmitz N, Ljungman P, Cordonnier C, Kempf C, Linkesch W, Alegre A, et al. Lenograstim after autologous peripheral blood progenitor cell transplantation: results of a double-blind, randomized trial. Bone Marrow Transplant. 2004;34(11):955–62.
12 Samaras P, Buset EM, Siciliano RD, Haile SR, Petrausch U, Mischo A, et al. Equivalence of pegfilgrastim and filgrastim in lymphoma patients treated with BEAM followed by autologous stem cell transplantation. Oncology. 2010;79(1–2):93–7.
13 de Naurois J, Novitzky-Basso I, Gill MJ, Marti FM, Cullen MH, Roila F. Management of febrile neutropenia: ESMO Clinical Practice Guidelines. Ann Oncol. 2010;21(Suppl 5):v252–6.
14 Guidelines for the use of platelet transfusions. Br J Haematol. 2003;122(1):10–23.
15 Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157(1):49–58.
16 Kaplan EL, Meier, P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457–81.
17 R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org. 2009.
18 Wang EH, Chen YA, Corringham S, Bashey A, Holman P, Ball ED, et al. High-dose CEB vs BEAM with autologous stem cell transplant in lymphoma. Bone Marrow Transplant. 2004;34(7):581–7.
19 Brice P, Bouabdallah R, Moreau P, Divine M, Andre M, Aoudjane M, et al. Prognostic factors for survival after high-dose therapy and autologous stem cell transplantation for patients with relapsing Hodgkin’s disease: analysis of 280 patients from the French registry. Societe Francaise de Greffe de Moelle. Bone Marrow Transplant. 1997;20(1):21–6.
20 Puig N, de la Rubia J, Remigia MJ, Jarque I, Martin G, Cupelli L, et al. Morbidity and transplant-related mortality of CBV and BEAM preparative regimens for patients with lymphoid malignancies undergoing autologous stem-cell transplantation. Leuk Lymphoma. 2006;47(8):1488–94.
21 Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–90.
22 Martin A, Conde E, Arnan M, Canales MA, Deben G, Sancho JM, et al. R-ESHAP as salvage therapy for patients with relapsed or refractory diffuse large B-cell lymphoma: the influence of prior exposure to rituximab on outcome. A GEL/TAMO study. Haematologica. 2008;93(12):1829–36.
23 Bolwell B, Kalaycio M, Sobecks R, Andresen S, McBee M, Kuczkowski L, et al. Autologous hematopoietic cell transplantation non-Hodgkin’s lymphoma: 100 month follow-up. Bone Marrow Transplant. 2002;29(8):673–9.
24 Ziepert M, Hasenclever D, Kuhnt E, Glass B, Schmitz N, Pfreundschuh M, et al. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(14):2373–80.
25 Nademanee A, O’Donnell MR, Snyder DS, Schmidt GM, Parker PM, Stein AS, et al. High-dose chemotherapy with or without total body irradiation followed by autologous bone marrow and/or peripheral blood stem cell transplantation for patients with relapsed and refractory Hodgkin’s disease: results in 85 patients with analysis of prognostic factors. Blood. 1995;85(5):1381–90.
26 Caballero MD, Perez-Simon JA, Iriondo A, Lahuerta JJ, Sierra J, Marin J, et al. High-dose therapy in diffuse large cell lymphoma: results and prognostic factors in 452 patients from the GEL-TAMO Spanish Cooperative Group. Ann Oncol. 2003;14(1):140–51.
27 Derenzini E, Musuraca G, Fanti S, Stefoni V, Tani M, Alinari L, et al. Pretransplantation positron emission tomography scan is the main predictor of autologous stem cell transplantation outcome in aggressive B-cell non-Hodgkin lymphoma. Cancer. 2008;113(9):2496–503.
28 Becherer A, Mitterbauer M, Jaeger U, Kalhs P, Greinix HT, Karanikas G, et al. Positron emission tomography with [18F]2-fluoro-D-2-deoxyglucose (FDG-PET) predicts relapse of malignant lymphoma after high-dose therapy with stem cell transplantation. Leukemia. 2002;16(2):260–7.
29 Spaepen K, Stroobants S, Dupont P, Vandenberghe P, Maertens J, Bormans G, et al. Prognostic value of pretransplantation positron emission tomography using fluorine 18-fluorodeoxyglucose in patients with aggressive lymphoma treated with high-dose chemotherapy and stem cell transplantation. Blood. 2003;102(1):53–9.
30 Svoboda J, Andreadis C, Elstrom R, Chong EA, Downs LH, Berkowitz A, et al. Prognostic value of FDG-PET scan imaging in lymphoma patients undergoing autologous stem cell transplantation. Bone Marrow Transplant. 2006;38(3):211–6.
31 Jabbour E, Hosing C, Ayers G, Nunez R, Anderlini P, Pro B, et al. Pretransplant positive positron emission tomography/gallium scans predict poor outcome in patients with recurrent/refractory Hodgkin lymphoma. Cancer. 2007;109(12):2481–9.
32 Moskowitz AJ, Yahalom J, Kewalramani T, Maragulia JC, Vanak JM, Zelenetz AD, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. 2010;116(23):4934–7.
33 Filmont JE, Czernin J, Yap C, Silverman DH, Quon A, Phelps ME, et al. Value of F-18 fluorodeoxyglucose positron emission tomography for predicting the clinical outcome of patients with aggressive lymphoma prior to and after autologous stem-cell transplantation. Chest. 2003;124(2):608–13.
34 Qiao W, Zhao J, Wang C, Wang T, Xing Y. Predictive value of (18)F-FDG hybrid PET/CT for the clinical outcome in patients with non-Hodgkin's lymphoma prior to and after autologous stem cell transplantation. Hematology. 2010;15(1):21–7.
35 Surgenor SD, Kramer RS, Olmstead EM, Ross CS, Sellke FW, Likosky DS, et al. The association of perioperative red blood cell transfusions and decreased long-term survival after cardiac surgery. Anesth Analg. 2009;108(6):1741–6.
36 Kuduvalli M, Oo AY, Newall N, Grayson AD, Jackson M, Desmond MJ, et al. Effect of peri-operative red blood cell transfusion on 30-day and 1-year mortality following coronary artery bypass surgery. Eur J Cardiothorac Surg. 2005;27(4):592–8.
37 Engoren M, Mitchell E, Perring P, Sferra J. The effect of erythrocyte blood transfusions on survival after surgery for hip fracture. J Trauma. 2008;65(6):1411–5.
38 Bierman PJ, Lynch JC, Bociek RG, Whalen VL, Kessinger A, Vose JM, et al. The International Prognostic Factors Project score for advanced Hodgkin’s disease is useful for predicting outcome of autologous hematopoietic stem cell transplantation. Ann Oncol. 2002;13(9):1370–7.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.
Authors’ contribution: Panagiotis Samaras and Dimitrios Zardavas contributed equally to this work. Frank Stenner-Liewen and Christoph Renner share senior-authorship