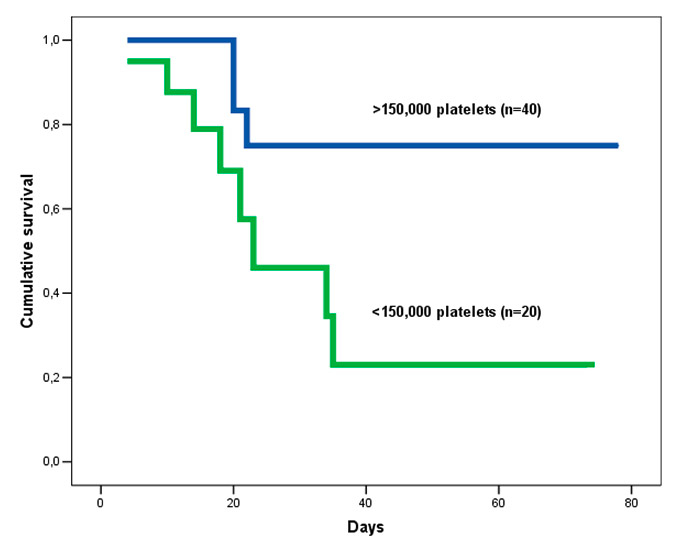
Figure 1
Cumulative survival rate of H1N1 influenza respiratory failure patients with thrombocytopenia.
DOI: https://doi.org/10.4414/smw.2013.13788
Since the outbreak of influenza A H1N1 of swine origin in 2009, clinicians have sought to identify mortality risk factors associated with this new pandemic. The number of patients requiring admission to an intensive care unit (ICU) for acute respiratory infection due to influenza A H1N1 is higher than those admitted for seasonal influenza [1]. Patients with H1N1 influenza can develop acute respiratory failure due to rapidly progressive lower respiratory tract disease together with abrupt onset and severe hypoxaemic illness. Approximately 9% to 31% of hospitalised patients have been admitted to ICU. A high person-to-person transmission rate was seen in young and previously healthy individuals, with ICU mortality rates which range from 14% to 46%. As a result, the World Health Organization declared a level 6 public health emergency in 2009 [1–6]. Clinical conditions such as pregnancy, obesity, chronic cardiovascular conditions, lung or metabolic disorders, chronic renal disease, liver cirrhosis, alcoholism, history of smoking, haemoglobinopathy, immunosuppression and older age have been associated with increased mortality [1–3]. However, only a few measurable biochemical/haematological markers, such as creatinine kinase and lactate dehydrogenase, able to predict poor outcome have been reported [2]. We hypothesised that measurable variables, which can report objective results could be helpful together with other clinical variables in assessing prognosis in those patients.
Further analysis of cases of acute respiratory failure due to influenza H1N1 are needed because of the great variety of clinical presentations in different geographical areas and population groups [1, 2]. ICU patients in Mexico mainly presented with primary viral pneumonia and mortality was extremely high [4]. However, Australia and the USA documented more frequently acute exacerbation of chronic obstructive pulmonary disease (COPD) patients or bacterial co-infection, which were associated with a better outcome [5, 6]. This report provides details of patients admitted to the ICU with acute respiratory failure due to H1N1 influenza in our population. In summary, the main aim of the present report was to show which variables on and during admission were associated with increased in-hospital mortality.
This prospective, observational study was carried out in two ICUs between August 2009 and March 2011. The period included two waves of pandemic H1N1 influenza during 2009–2010 and 2010–2011.
The Hospital Universitari de Bellvitge (HUB) is a tertiary level hospital with 850 general care beds and 44 ICU beds. The Hospital General de L’Hospitalet (HGH) is a secondary level hospital with 190 general care beds and 11 ICU beds. Together, the two hospitals have a reference population of approximately 1,200,000 people. At the time this study was performed, all patients from the healthcare area were referred to these two ICUs. During the study period, 114 patients were admitted in both hospitals owing to influenza H1N1. Of these, 60 (52%) needed ICU admission.
The primary goal of our report was to study which factors were associated with in-hospital mortality, such as an evaluation of body mass index (BMI) and its influence on mortality. We also describe mechanical ventilation needs and potential complications, and check how accurate ICU scores in our population were. The study was approved by the Institutional Ethics Committees at both hospitals. Informed consent was waived due to the observational nature of the study.
Inclusion criteria were: febrile (>38 ºC) acute illness; respiratory symptoms consistent with cough, sore throat, myalgia or influenza-like illness; acute respiratory failure requiring ICU admission; and microbiological confirmation of H1N1 influenza. Children and pregnant women were not included because they were not admitted to both hospitals during the study period.
The definition of community-acquired pneumonia was based on current American Thoracic Society and Infectious Disease Society of America guidelines [7]. Primary viral pneumonia was defined as acute respiratory distress in patients in the acute phase of influenza virus illness, unequivocal alveolar opacification involving a minimum of two lobes and negative respiratory and blood bacterial cultures. Bacterial co-infection was diagnosed in patients with confirmed influenza virus infection showing positive bacterial blood or respiratory cultures, based on the culture of tracheal secretions obtained with a bronchoscopy after intubation. Specimens from nasopharyngeal-swab were collected at admission and respiratory secretions were also obtained in intubated patients. Qualitative H1N1 RT-PCR was performed in accordance with local microbiology protocols and by the microbiologist in charge.
In all patients the decisions regarding ICU admission and treatment, including the need for intubation and type of antibiotic and antiviral therapy administered, were made by the attending physician.
Data on and during ICU admission were recorded from the medical registry of each patient in real time using a standardised questionnaire for the authors and collected in the database, in order to evaluate variables potentially associated with in-hospital mortality. The following information was recorded on admission: demographic data, comorbidities, time from illness onset to first antiviral dose and laboratory data. Microbiological findings, mechanical ventilation needs, treatment characteristics and presence of adverse events (e.g., need for vasopressor drugs, or renal replacement techniques) were obtained during hospital stay. To determine illness severity, the Acute Physiology And Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA) scoring systems were applied to all patients within 24 hours of ICU admission.
Usual laboratory and clinical thresholds were used for survival analysis when appropriate. The threshold platelet count used to define thrombocytopenia was 150 × 109/l or below, based on local laboratory protocols and the current literature [8]. BMI of the patients was classified using the WHO classification. BMI which ranged from 25 to 30 kg/m2 was classified as overweight and BMI >30 kg/m2 was classified as obesity. Patients with a BMI <25 kg/m2 were classified as normally weighted patients.
The study was approved by the Institutional Ethics Committees at both hospitals. Informed consent was waived because of the observational nature of the study.
Statistical analysis was applied using PASW statistics 13.0 (SPSS Inc., Chicago, Illinois, USA). Data are expressed as mean ± standard deviation. We analysed differences between survivors and non-survivors. For the comparisons between the two groups the Mann-Whitney U test was used or, when appropriate, the two-sample t-test. The χ²-test was used to evaluate categorical prognostic factors. The one-sample Kolmogorov-Smirnov test was used to report normal distribution of our population and to assess the goodness-of-fit of the final regression models. A multivariate analysis was carried out using stepwise logistic regression model to identify independent risk mortality factors for in-hospital mortality. We included variables in the multivariate analysis which had significant results in the univariate tests. Survival analysis was carried out with the Kaplan-Meier estimator. Finally, a receiver operating characteristic (ROC) curve analyses were applied to check optimal cut-off values of the different scores for in-hospital mortality and to further evaluate the predictive power between them, considering the differences of the areas under the empirical ROC curves (AUC). In all variables a two-tailed p value of 0.05 was considered statistically significant.
Sixty patients from our healthcare area with acute respiratory failure due to H1N1 influenza were admitted between August 2009 and March 2011, 42 to the HUB and 18 to the HGH.

Figure 1
Cumulative survival rate of H1N1 influenza respiratory failure patients with thrombocytopenia.
All patients were treated with oral oseltamivir 300 mg per day which was administered through a naso-gastric tube or orally. Mean oseltamivir treatment duration was 8.7 ± 4.2 days. All patients received what was considered to be early antiviral therapy if administered within 48 hours [2]; as a result, there were no differences between the groups regarding treatment.
All patients were also initially treated empirically with antibiotics because of the severity of the respiratory failure, for a mean duration of 9.2 ± 4.7 days. The most frequent regimens were beta-lactams plus fluoroquinolones (66.7%, n = 40) and beta-lactams plus macrolides (15%, n = 9). Two patients received ceftriaxone as monotherapy.
Ten patients (16.6%) presented bacterial co-infection together with a positive RT-PCR (eight were Streptococcus pneumoniae, one was Staphyloccocus aureus and Klebsiella pneumoniae). Five patients (8.3%) developed secondary respiratory infection with Pseudomonas aeruginosa during ICU admission. All these patients survived without any influence on mortality. Eighteen patients (30%) received intravenous corticosteroids during ICU admission due to COPD exacerbation or bronchospasm.
In-hospital mortality was 20% (n = 12): 5% (n = 3) died from multiorgan failure and 15% (n = 9) patients from hypoxic respiratory failure. The variables recorded during ICU admission and univariate analysis are shown in table 1. The maximum time from illness onset to hospital admission, and time to first dose of antiviral delivery, was 48h. When data from survivors and non-survivors were compared, univariate analysis revealed significant differences in BMI, creatinine, haemoglobin, platelets, arterial pH, pCO2, and the rate of bacterial coinfection. There was no increase in mortality with greater BMI. In fact, patients with a BMI >25 kg/m2 had a better survival rate (90.6% vs 71.4%; Log Rank = 0.04); obese patients (BMI >30 kg/m2) had a 100% survival rate, compared with 80% among the overweight (25–30 kg/m2) and 70.4% in normal BMI (Log Rank = 0.019). Variables during follow-up were not included in the analysis. In the multivariate analysis, the presence of lower platelet count was statistically significant for in-hospital mortality (see table 2). Patients with thrombocytopenia at admission showed a lower survival rate (55% vs 92.5%; Log Rank = 0.008) (see fig. 1).
The need of support measures and adverse events were higher in non-survivors: they had a higher need of mechanical ventilation (MV), vasopressor drugs, renal replacement therapies and prone ventilation, and higher rate of non-invasive MV (NIV) failure. Despite the higher NIV failure rates in non-survivors, length of NIV in days was the same for both groups and there was no notable delay in tracheal intubation.
The mean APACHE II score was 13.3 ± 7 and the mean SOFA score was 5.4 ± 3.1; both were higher in non-survivors (APACHE II of 11.5 ± 5.7 vs 20.6 ± 7, p = 0.001 and SOFA of 4.5 ± 2.6 vs 9 ± 2.4; p = <0.001). The APACHE II showed a sensitivity and a specificity of 75% with a cut-off level of 14 (AUC 83.9 ± 6.7 (70.9–97, 95% CI);p = <0.001). The SOFA score had a better predictive value with a sensitivity of 75% and a specificity of 87.5% with a cut-off level of 6.5 (AUC 89.4 ± 4.1 (81.3–97.5, 95% CI); p = <0.001).
| Table 1:Characteristics of patients admitted with severe H1N1 influenza respiratory failure and univariate analysis. | ||||
| Total (n = 60) | Survivors (n = 48) | Non-survivors (n = 12) | p | |
| Population data | ||||
| Age (years) | 49.2 ± 14 | 48.8 ± 12.5 | 50.8 ± 12 | NS |
| Sex (male/ female) | 38 / 22 | 29 / 19 | 9 / 3 | NS |
| BMI (kg/m2 ) | 29 ± 6.3 | 29.8 ± 6.4 | 25 ± 3 | 0.03 |
| Days from symptoms onset to treatment | 1.2 ± 0.42 | 1 ± 0.8 | 1.5 ± 0.3 | NS |
| Mean ICU stay (days) | 15.5 ± 14.5 | 15 ± 8 | 17.8 ± 15 | NS |
| Mean hospital stay (days) | 18.1 ± 14.7 | 16.5 ± 13 | 24.5 ± 17.8 | NS |
| Medical history | ||||
| Smokers | 46.7% (28) | 47.9% (23) | 41.6% (5) | NS |
| Hypertension | 38% (23) | 41.6% (20) | 25% (3) | NS |
| Diabetes mellitus | 18.3% (11) | 20.8% (10) | 8.3% (1) | NS |
| Dyslipidaemia | 31.6% (19) | 37.5% (18) | 8.3% (1) | 0.049 |
| BMI >30 kg/m2 | 30% (18) | 37.5% (18) | 0% | 0.021 |
| COPD | 30% (18) | 31.25% (15) | 25% (3) | NS |
| Liver cirrhosis | 15% (9) | 10.4% (5) | 33.3% (4) | NS |
| Cardiac Insufficiency | 26% (16) | 29.2% (16) | 16.6% (2) | NS |
| Laboratory findings on admission | ||||
| [Na+] (mmol/l) | 136 ± 4.5 | 137.2 ± 4.2 | 134.8 ± 5.5 | NS |
| [K+] (mmol/l) | 4 ± 0.9 | 3.9 ± 0.7 | 4.5 ± 1.3 | NS |
| [P+] (mmol/l) | 1.08 ± 0.53 | 1.06 ± 0.5 | 1.19 ± 0.7 | NS |
| ALT (μkat/l) | 0.93 ± 1.1 | 0.9 ± 1.05 | 0.9 ± 1.3 | NS |
| Albumin (g/l) | 28.9 ± 6 | 29.5 ± 5.7 | 26 ± 7.7 | NS |
| Creatinine (μmol/l) | 123 ± 119 | 108.2 ± 74 | 186.4 ± 220 | 0.043 |
| Urea (mmol/l) | 9.3 ± 7 | 8.4 ± 6.4 | 13.2 ± 8.7 | NS |
| Haemoglobin (g/dl) | 12.6 ± 2.3 | 13 ± 2 | 11.4 ± 3.2 | 0.033 |
| Leucocytes (×109/l) | 10.79 ± 7.3 | 11.24 ± 7.16 | 9.01 ± 8.14 | NS |
| Platelets (×109/l) | 195 ± 104 | 214 ± 101 | 113 ± 82 | 0.002 |
| INR | 1.23 ± 0.65 | 1.28 ± 0.8 | 1.22 ± 0.2 | NS |
| pH | 7.37 ± 0.1 | 7.4 ± 0.7 | 7.28 ± 0.15 | <0.001 |
| Arterial pO2 (mm Hg) | 109 ± 68 | 105 ± 63 | 118 ± 84 | NS |
| PaO2 /FiO2 | 160 ± 72 | 164 ± 70 | 136 ± 80 | NS |
| Arterial pCO2 (mm Hg) | 45 ± 22 | 41 ± 21 | 58 ± 24 | 0.04 |
| Arterial HCO3 (mmol/l) | 24.4 ± 4.2 | 24.1 ± 4.4 | 25.6 ± 3.8 | NS |
| Arterial lactate (mmol/l) | 1.9 ± 0.8 | 1.9 ± 0.8 | 2.1 ± 0.8 | NS |
| Bacterial coinfection rates | 35.7% (10) | 10.4% (5) | 41.6% (5) | 0.022 |
| Mechanical Ventilation needs | ||||
| NIV | 48.3% (29) | 45.8% (22) | 58.3% (7) | NS |
| Days on NIV | 2.75 ± 1.9 | 2.7 ± 1.8 | 2.9 ± 2 | NS |
| Invasive MV | 70% (42) | 62.5% (30) | 100% (12) | <0.001 |
| Days on invasive MV | 14.8 ± 12.6 | 11.2 ± 8.73 | 17.5 ± 14.86 | NS |
| Adverse event | ||||
| Vasopressor drugs | 76.7% (46) | 72.9% (35) | 91.6% (11) | <0.001 |
| Renal replacement therapy | 6 (10%) | 2.08% (1) | 41.6% (5) | <0.001 |
| Prone ventilation | 18.3% (11) | 8.3% (4) | 58.3% (7) | <0.001 |
| Non-invasive MV failure | 35.7% (10) | 19% (4) | 85.7% (6) | 0.003 |
| APACHE = Acute Physiology and Chronic Health Evaluation. SOFA = Sequential Organ Failure Assessment. BMI= Body Mass Index. INR = International Normalised Ratio. NIV = Non- invasive ventilation. MV = Mechanical Ventilation. Results are expressed as mean ± standard deviation or percentage. | ||||
| Table 2:Logistic regression model – dependent variable deceased during admission. | ||
| Odds ratio (95% CI) | p | |
| Age (years) | 1.020 (0.947–1.099) | 0.594 |
| Haemoglobin (g/dl) | 0.630 (0.339–1.169) | 0.143 |
| Platelets ×109/l | 33.520 (2.446–459.368) | 0.009 |
| Arterial pCO2 (mm Hg) | 1.018 (0.977–1.061) | 0.400 |
This report describes acute respiratory failure patients admitted to the ICUs for H1N1 influenza during two waves of the pandemic in Spain. We found that patients with thrombocytopenia at the time of admission, as well as other critically ill populations such as patients admitted with community-acquired pneumonia [8, 9], had an increased risk of death.
H1N1 influenza is an uncommon cause of acute respiratory failure in ICU, but is important because it can affect previously healthy and young individuals. The pulmonary problems showed a variety of clinical presentations: viral pneumonitis, exacerbations of asthma or COPD, exacerbation of an underlying disease (e.g. congestive heart failure) and pulmonary bacterial co-infection [1–6]. The rapidly progressive hypoxaemia means that most patients requiring ICU admission fulfil criteria for acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) [2].
Thrombocytopenia on admission appears to be a risk factor for mortality in critically ill patients and is related with higher illness severity, organ dysfunction, sepsis and renal failure [8]. A decrease in the platelet count among patients with ICU-acquired pneumonia has been reported [9] and the occurrence of thrombocytopenia after bloodstream infection is associated with increased mortality [10]. In fact, thrombocytopenia has been described as the main thrombohaemostatic disorder in other types of influenza infection, such as human bird influenza infection [11].
It is also used in organ failure scores, such as the SOFA score or Simplified Acute Physiology Score, which can explain the better predictive value of SOFA compared with APACHE that showed in our results. Thrombocytopenia has also been putatively associated with ALI and ARDS in patients whose underlying condition leads to endothelial inflammation [12]. Platelets may play a pathophysiologic role in mediating infection and/or inflammation [13], although the precise mechanism by which they do so has not been conclusively determined. Several mechanisms related to the biochemical features of platelet activation, release of platelet microparticles, platelet adherence to leucocytes and engulfment of pathogens by platelets in a process similar to phagocytosis have been hypothesised [14]. Platelets have also been described as a target of classic complement activation in autoimmune platelet destruction [15]. We therefore hypothesise that the platelet count is associated with the inflammatory response in the H1N1 setting, and that the presence of thrombocytopenia reflects an inadequate response of the immune system. Mortality does not appear to be due primarily to bleeding [8]; during the study period no bleeding episodes or transfusions were recorded. In addition, some authors have suggested that abnormal platelet count could be a better predictor of outcome than abnormal leucocyte count [14].
Obesity has been associated in the literature with higher prevalence of comorbid conditions, and previous studies have suggested that morbid obesity is an independent risk factor for complications requiring hospitalisation or ICU admission (and possibly death) in H1N1 infected patients [2, 3]. Paradoxically, recent findings present a fall in postoperative mortality in obese patients with ARDS [16]. ARDS involves an intense inflammatory response in the lungs, with accumulation of both pro- and anti-inflammatory cytokines [16]. Adipose tissue has been shown to produce soluble cytokine receptors, like tumour necrosis factor receptors, which are believed to neutralise the harmful effects of cytokines in the myocardium [17]. In fact, recent studies suggest that extreme obesity is not more closely associated with poor survival than normal weight [18]. This may explain the absence of any relationship between obesity and mortality in our study.
Finally, it is noted to say that the higher frequency of adverse events in non-survivors reflects their higher initial illness severity. In general, and also specifically in severe H1N1 influenza respiratory failure, non-invasive ventilation (NIV) is not recommended in hypoxaemic respiratory failure [19]. However, there are some reports of successful use of NIV and this is likely related to selection of less hypoxaemic patients for this mode of treatment [20].
The limitations of this study include those inherent in any single geographical area, its observational nature, the lower mortality which softens the value of predicting models, and the overall small number of patients. The selection bias of patients being already severely ill and therefore hospitalised on ICU makes our findings not transferable to other populations.
In summary, thrombocytopenia could be valuable marker of in-hospital mortality for patients with acute respiratory insufficiency due to influenza H1N1. However, due to the inherent limitations of our study these results should be taken cautiously. Future studies should be done in order to confirm our results
1 Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza, Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–19.
2 Rodríguez A, Alvarez-Rocha L, Sirvent JM, Zaragoza R, Nieto M, Arenzana A, et al; GETGAG; Infectious Diseases Work Group (GTEI) of Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC); Infections in Critically Ill Patients Study Group (GEIPC) of Spanish Society of Infectious Diseases and Clinical Microbiology. Recommendations of the Infectious Diseases Work Group (GTEI) of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC) and the Infections in Critically Ill Patients Study Group (GEIPC) of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) for the diagnosis and treatment of influenza A/H1N1 in seriously ill adults admitted to the Intensive Care Unit. Med Intensiva. 2012;36:103–37.
3 Rello J, Rodríguez A, Ibañez P, Socias L, Cebrian J, Marques A, et al. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care. 2009;13:R148.
4 Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E, et al; INER Working Group on Influenza. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–9.
5 Swerdlow DL, Finelli L, Bridges CB. 2009 H1N1 influenza pandemic: field and epidemiologic investigations in the United States at the start of the first pandemic of the 21st century. Clin Infect Dis. 2011;52:S1–3.
6 Kotsimbos T, Waterer G, Jenkins C, Kelly PM, Cheng A, Hancox RJ, et al. Influenza A/H1N1_09: Australia and New Zealand’s winter of discontent. Am J Respir Crit Care Med. 2010;181:300–6.
7 Brown SM, Jones BE, Jephson AR, Dean NC; Infectious Disease Society of America/American Thoracic Society 2007. Validation of the Infectious Disease Society of America/American Thoracic Society 2007 guidelines for severe community-acquired pneumonia. Crit Care Med. 2009;37:3010–6.
8 Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139:271–8.
9 Oguzulgen IK, Ozis T, Gursel G. Is the fall in platelet count associated with intensive care unit acquired pneumonia? Swiss Med Wkly. 2004;134(29-30):430–4.
10 Vandijck DM, Blot SI, De Waele JJ, Hoste EA, Vandewoude KH, Decruyenaere JM. Thrombocytopenia and outcome in critically ill patients with bloodstream infection. Heart Lung. 2010;39:21–6.
11 Wiwanitkit V. Haemostatic disorders in bird flu infection. Blood Coagul Fibrinolysis. 2008;19:5–6.
12 Gelderman MP, Chi X, Zhi L, Vostal JG. Ultraviolet B light-exposed human platelets mediate acute lung injury in a two-event mouse model of transfusion. Transfusion. 2011;51:2343–57.
13 Semple JW, Freedman J. Platelets and innate immunity. Cell Mol Life Sci. 2010;67:499–511.
14 François B, Trimoreau F, Vignon P, Fixe P, Praloran V, Gastinne H. Thrombocytopenia in the sepsis syndrome: role of hemophagocytosis and macrophage colony-stimulating factor. Am J Med. 1997;103:114–20.
15 Mirsaeidi M, Peyrani P, Aliberti S, Filardo G, Bordon J, Blasi F, et al. Thrombocytopenia and thrombocytosis at time of hospitalization predict mortality in patients with community-acquired pneumonia. Chest. 2010;137:416–20.
16 Memtsoudis SG, Bombardieri AM, Ma Y, Walz JM, Chiu YL, Mazumdar M. Mortality of Patients With Respiratory Insufficiency and Adult Respiratory Distress Syndrome After Surgery: The Obesity Paradox. J Intensive Care Med. 2012;27:306–11.
17 Suter PM, Suter S, Girardin E, Roux-Lombard P, Grau GE, Dayer JM. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am Rev Respir Dis. 1992;145:1016–22.
18 Martino JL, Stapleton RD, Wang M, Day AG, Cahill NE, Dixon AE, et al. Extreme obesity and outcomes in critically ill patients. Chest. 2011;140:1198–206.
19 Namendys-Silva SA, Hernández-Garay M, Rivero-Sigarroa E. Non-invasive ventilation for critically ill patients with pandemic H1N1 2009 influenza A virus infection. Crit Care. 2010;14:407.
20 Estenssoro E, Ríos FG, Apezteguía C, Reina R, Neira J, Ceraso DH, et al; Registry of the Argentinian Society of Intensive Care SATI. Pandemic 2009 influenza A in Argentina: a study of 337 patients on mechanical ventilation. Am J Respir Crit Care Med. 2010;182:41–8.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.