Figure 1
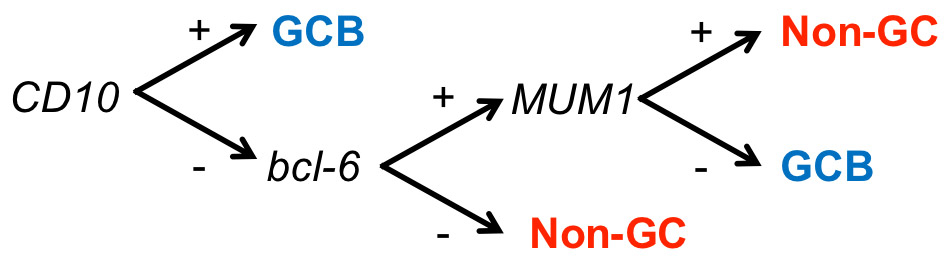
Hans algorithm for the determination of molecular DLBCL subtypes [15]. It is based on the immunohistochemical analysis of three markers (CD10, MUM1, and bcl-6).
DOI: https://doi.org/10.4414/smw.2013.13748
A case series from the Inselspital, Bern, and a critical appraisal of this determination in Switzerland
Glossary of diffuse large B-cell lymphoma molecular subtypes
DLBCL: diffuse large B-cell lymphoma, GEP: gene-expression profiling, IHC: immunohistochemistry
DLBCL-subtypes determined by means of GEP: GCB-DLBCL: germinal centre B-cell - like diffuse large B-cell lymphoma ABC-DLBCL: activated B-cell - like diffuse large B-cell lymphoma
DLBCL-subtypes determined by means of an algorithm based on IHC (e.g. the Hans classification): GCB-DLBCL: germinal centre diffuse large B-cell lymphoma Non-GC-DLBCL: nongerminal centre diffuse large B-cell lymphoma
Diffuse large B-cell lymphoma (DLBCL), the most common type of lymphoma in adults, accounts for 30–40% of new lymphoma diagnoses in the Western world. Its incidence is increasing among all age groups and both sexes [1]. It is a clinically, morphologically and genetically heterogeneous disease. A significant proportion (20–40%) of patients cannot be cured with current chemotherapeutic regimens [2–7].

Figure 1
Hans algorithm for the determination of molecular DLBCL subtypes [15]. It is based on the immunohistochemical analysis of three markers (CD10, MUM1, and bcl-6).
A considerable number of the features of lymphomas are thought to derive from their nontransformed progenitor cells. Each of the currently recognised forms of B-cell lymphoma has been associated with a distinct step in normal B-cell differentiation [8], and this is useful for a general classification of the various lymphoma entities. In 2000, Alizadeh and coworkers [9] were able to classify 85% of DLBCL cases, by means of gene expression profiles (GEP), into two groups. The first group, germinal centre B-cell - like (GCB) DLBCL, originates from a germinal centre B-cell. The second group, possibly deriving from B-cells at a further stage of differentiation, has been termed activated B-cell - like (ABC) DLBCL. Most importantly, these molecular subtypes differ in their genetic alterations and possibly also in their clinical outcome [10–13]. Later studies have identified a third and distinct subtype termed "primary mediastinal B-cell lymphoma" (PMBL) [14].
GEP is the gold standard for defining the molecular DLBCL subtypes, but is currently not applicable to the routine diagnostic work-up of lymphoma cases. Therefore, several groups have tried to develop a substitute involving immunohistochemistry (IHC). The most widely used algorithm was published by Hans et al. in 2004 [15]. Their IHC analysis is based on three antibodies (against CD10, Bcl-6 and MUM1) and classifies DLBCL into two subgroups, which they termed GCB and nongerminal centre (non-GC) DLBCL (fig. 1), to distinguish them from the classification based on GEP. Compared with GEP, the positive predictive value of the "Hans classification" is 74% for the non-GC and 84% for the GCB subtype [15]. Recently published alternative algorithms with additional antibodies and/or more stringent scoring criteria improved the PPV for both subtypes to 93% [16]. Most importantly, compared with GEP, the IHC-based DLBCL classification detects reasonably well the distinct molecular alterations in the DLBCL subtypes [17]. Whether the molecular DLBCL subtypes have independent prognostic significance is currently controversial. The association is undisputed when the subgroups are determined using GEP [10, 12, 18], but the reported data on IHC-based classifications are controversial [12, 15, 18–20]. Recent clinical data suggest that the inferior outcome of ABC-DLBCL with standard chemotherapy can be overcome by using the R-ACVBP regimen [7].
At present, determination of DLBCL subtypes by means of IHC is neither a routine diagnostic procedure, nor part of the current WHO classification, nor does it at this time influence the therapy of DLBCL patients. However, the distinct molecular features of the DLBCL subtypes imply the future possibility for therapies targeting particular subtypes. In fact, the “Schweizerische Arbeitsgruppe für Klinische Krebsforschung” (SAKK) is currently participating in an international clinical trial using this molecular information in the treatment of DLBCL. The DLBCL subtypes will be of indisputable relevance in the near future. We therefore aimed to a) critically examine the current assessment of the DLBCL subtypes using IHC (Hans classification [15]) at our University Hospital, and b) investigate standardisation of this assessment in other major pathology institutions in Switzerland.
DLBCL patients were identified in early 2011 in the database of the Department of Medical Oncology of the University Hospital of Bern (Inselspital). We limited our search to the period after 2005, when staining for MUM1, one of the three markers used in the "Hans classification" of DLBCL subtypes [15], was established at the Institute of Pathology of the University of Bern. The following cases were excluded: DLBCL known to have transformed from a low grade lymphoma; primary DLBCL of the central nervous system or primary mediastinal B-cell lymphomas (PMBL); and DLBCL arising during immunosuppression or in HIV-infected patients. As expected for a tertiary referral medical centre, the 172 de novo or bona fide DLBCL cases identified in this search were diagnosed in a total of nine different academic and nonacademic pathology institutions, and many patients received significant parts of their treatment outside our institution. Our retrospective, single-centre cohort included the DLBCL cases (109/172, 63.4%) for whom the routine diagnostic work-up was performed at the Institute of Pathology of the University of Bern. Clinical and immunohistochemical (CD10, bcl-6, MUM1) data were taken from the medical records. Full information on the classification of the DLBCL in accordance with the Hans algorithm (i.e., data on all three immunohistochemical markers, fig. 1) was available in 51 (46.8%) of these 109 cases. The DLBCL subtype in accordance with the Hans classification was also determined for our cohort using tissue microarray (TMA, see below). Clinical information on the 109 DLBCL patients is provided in table 1. All patients gave written consent to this retrospective analysis, based on a patient information leaflet approved by the local ethics committee.
The initial assessment of positivity/negativity of the three immunohistochemical markers in the diagnostic pathology reports was vague for 12 patients (e.g., terms like “questionably positive”, “weakly positive” were used). These cases were reviewed and scored again by E.G. using the published cut-off of 30% [15]. A TMA was constructed using tissue samples from the 109 DLBCL patients, in accordance with standard procedures, and analysed using IHC in order to determine the molecular subtype following the "Hans" algorithm and using the same cut-off score as for whole-tissue sections.
The three antigens were detected with the following primary antibodies: bcl-6 (clone GI191E/A8, dilution 1:400, Cell Marque, Rocklin CA; USA), CD10 (56C6; 1:50, Novocastra, Newcastle-upon-Tyne, UK), and MUM1 (MUM1p; 1:100, Dako, Glostrup, Denmark). Prior to incubation with the primary antibody, 2–3 μm paraffin-embedded sections were dewaxed, rehydrated, and pretreated by boiling in 10 mM Tris – 1 mM EDTA, pH 9.0, in a microwave oven. Slides were then incubated for 5 minutes in 3% hydrogen peroxide (H2O2) with 0.1% sodium azide (NaN3) to block endogenous peroxidase activity. Sections were then (and after all subsequent steps) washed in Tris-buffered saline (TBS) and incubated with the primary antibody diluted in TBS with 0.5 % casein and 5% normal goat serum, for 60 minutes at room temperature. In the negative controls, an irrelevant primary antibody was used. Next, a horseradish peroxidase-based polymer visualisation system was applied (Envision+; Dako), developed in 0.02% 3,3'-diaminobenzidine (Sigma, St. Louis MO, USA) with 0.01% H2O2, counterstained with haematoxylin, and mounted.
The clinical information (date of initial diagnosis and date of death or last contact to calculate overall survival, and the age-adjusted International Prognostic Index (aaIPI) score) was retrieved from medical records and/or by contacting the colleagues performing the follow-up assessments. Curves depicting overall survival were plotted using the Kaplan-Meier method, and survival analysis was performed using the log-rank test or Wilcoxon test. Cox proportional hazard regression was used to analyse the effect of various risk factors on survival. Results were considered significant if the p-value was below 0.05. Statistical analyses were performed using GraphPad Prism 5 and Statview 5.0.1.
| Table 1: Clinical information on the cohort of DLBCL patients. | |||||||||||
| DLBCL subtype by IHC on TMA (research setting), and, where available, from the diagnostic work-up, deviations in red. Age, clinical stage, international prognostic index (IPI), and age-adjusted IPI (aaIPI) at diagnosis. Survival in months from diagnosis to death or last follow-up. Patients used for the survival analysis (fig. 3) in last column. IT, intrathecal therapy; 30–40 Gy, involved field (IF) radiotherapy; R-CHOP-14/21, R-CHOP chemotherapy given every 14 or 21 days, preceded by the # of cycles; R, Rituximab (Mabthera®) | |||||||||||
| # | Subtype | Age | Stage | IPI | aaIPI | Regimens | Survival(month) | Alive | Remarks | Survivalanalysis | |
| GCB-DLBCL | 1 | GC | 58 | IIA | 1 | 6 x R-CHOP-14+2 x R, IF-RT 20 Gy | 26 | Yes | √ | ||
| 2 | 91 | IB | NA | NA | 3 x R-COP | 8 | No | ||||
| 3 | 64 | IIIA | 3 | 2 | 7 x R-CHOP+1xR, IF-RT 39.6 Gy | 54 | Yes | √ | |||
| 4 | GC | 68 | IVEB | 5 | 3 | 6 x R-CHOP-14+2xR; 2 x ESAP | 8 | No | SAKK 38/07; relapsed | √ | |
| 5 | Non-GC | 47 | IIIAE | 2 | 2 | 3 x R-CHOP-14; 2 x CODOX-M/IVAC | 51 | Yes | √ | ||
| 6 | Non-GC | 79 | IIA | 1 | 6 x R-CHOP-21 | 60 | Yes | √ | |||
| 7 | GC | 64 | IIIA | 2 | 1 | 6 x R-CHOP-14 + 2 x R | 32 | Yes | SAKK 38/07 | √ | |
| 8 | Non-GC | 77 | IAE | >1 | NA | 3 x R-C(H)OP-21, IF-RT 36 Gy | 18 | Yes | Epirubicin in cycle 1 only | √ | |
| 9 | 68 | IVB | 3 | 2 | 8 x R-CHOP | 15 | Yes | √ | |||
| 10 | GC | 77 | IAE | 2 | 1 | 3 x R-bendamustine + 3 x R | 40 | Yes | |||
| 11 | Non-GC | 82 | IIAE | 1 | 6 x R-CHOP-21 + 2 x R | 33 | Yes | √ | |||
| 12 | GC | 78 | IVA | 3 | 2 | 6 x R-CHOP-14, IF-RT 40 Gy | 40 | Yes | √ | ||
| 13 | 83 | IVAE | >2 | >1 | 3 x R-COP | 58 | No | ||||
| 14 | 76 | IVAE | 5 | 3 | 6 x R-CHOP + 2 x R, IF-RT | 81 | Yes | √ | |||
| 15 | GC | 65 | IVA | 3 | 2 | 6 x R-CHOP-21 + 2 R + 4 x IT, IF-RT 30 Gy | 51 | Yes | √ | ||
| 16 | GC | 86 | IBE | NA | NA | R-COP | 1 | No | |||
| 17 | GC | 60 | IVAE | 3 | 2 | 6 x R-CHOP-21 + 4 x IT, IF-RT 40 Gy | 34 | Yes | √ | ||
| 18 | 60 | IAE | 1 | 6 x R-CHOP-21, IF-RT? | 15 | No | Testicular | √ | |||
| 19 | 52 | IA | 6 x R-CHOP, IF-RT | 87 | Yes | √ | |||||
| 20 | 64 | IIAE | 2 | 1 | 8 x R-CHOP-14 | 81 | Yes | √ | |||
| 21 | 82 | IVAE | 5 | 3 | 6 x R-CHOP | 9 | No | √ | |||
| 22 | 55 | IIA | 1 | 1 | 6 x R-CHOP, IF-RT 46 Gy | 68 | Yes | √ | |||
| 23 | GC | 42 | IIAE | 6 x R-CHOP-14 + 2 x R, IF-RT 36 Gy | 25 | Yes | √ | ||||
| 24 | GC | 65 | IVBE | 4 | 3 | 6 x R-CHOP-14 + R-maintenance; HD-MTX | 16 | No | Relapsed (CNS) | √ | |
| 25 | Non-GC | 58 | IIIBE | 3 | 2 | 6 x R-CHOP-14 | 36 | Yes | √ | ||
| 26 | GC | 69 | IAE | 1 | 6 x R-CHOP-14 + 4 x IT, IF-RT 46 Gy | 39 | Yes | √ | |||
| 27 | 67 | IIIAE | 3 | 2 | 6 x R-CHOP, IF-RT 36 Gy | 45 | Yes | √ | |||
| 28 | 58 | IIAE | 6 x R-CHOP | 81 | Yes | √ | |||||
| 29 | 42 | IIAE | 6 x R-CHOP + 2 x R | 72 | Yes | √ | |||||
| 30 | GC | 71 | IVEB | 3 | 2 | 5 x R-CHOP-21 + 3 x R | 49 | Yes | Lip. doxorubicin | √ | |
| 31 | 48 | IIAE | 1 | 6 x R-CHOP + 4 x IT, IF-RT 45 Gy | 52 | Yes | √ | ||||
| 32 | 76 | IAE | >1 | NA | 5 x R-CHOP + IF-RT | 35 | Yes | √ | |||
| 33 | GC | 71 | IIIB | 2 | 1 | 5 x R-CHOP-14 + 2 x R | 35 | Yes | √ | ||
| 34 | 19 | NA | NA | NA | 6 x CHOP (treated abroad) | 8 | Yes | Lost to follow-up | √ | ||
| 35 | Non-GC | 79 | IVEB | 5 | 3 | 1 x R-CHOP | 5 | No | √ | ||
| 36 | GC | 75 | IAE | >1 | NA | 6 x R-bendamustine | 34 | Yes | |||
| 37 | 78 | IIIBE | 2 | 1 | 6 x R-CHOP-14 + 2 x R | 35 | Yes | SAKK 38/07 | √ | ||
| 38 | 77 | IV | 4 | 2 | 4 x R-CHOP | 60 | No | √ | |||
| 39 | GC | 69 | IAE | 1 | 4 x R-CHOP-14 + 4 x R | 14 | No | Died of liver cirrhosis | √ | ||
| 40 | 41 | IAE | 8 x R-CHOP + 4 x IT, IF-RT 36 Gy | 68 | Yes | √ | |||||
| 41 | 64 | IAE | 2 | 1 | 6 x R-CHOP, IF-RT 46 Gy | 81 | Yes | √ | |||
| 42 | 33 | IAE | 6 x R-CHOP | 56 | Yes | √ | |||||
| 43 | GC | 80 | IVB | 3 | 2 | 6 x R-CHOP-14 | 21 | No | √ | ||
| 44 | 82 | IAE | >1 | NA | 4 x R-CHOP | 50 | No | √ | |||
| 45 | 56 | IIB | >1 | >1 | 8 x R-CHOP-14 | 56 | Yes | Lost to follow-up | √ | ||
| 46 | GC | 81 | IVA | 3 | 2 | 6 x R-CHOP-21 + 2 x R, IF-RT 36 Gy | 29 | Yes | √ | ||
| 47 | Non-GC | 81 | IAE | 3 | 2 | R-CHOP-21 | 7 | No | √ | ||
| 48 | GC | 71 | IAE | NA | NA | 6 x R-CHOP-21 + 2 x R | 68 | Yes | √ | ||
| 49 | 73 | IAE | 2 | 1 | 4 x R-CHOP + 3 x R + 4 x IT, IF-RT 40 Gy | 50 | Yes | √ | |||
| Non-GC-DLBCL | 50 | 74 | IIA | 1 | 6 x R-CHOP, IF-RT 36 Gy | 57 | Yes | √ | |||
| 51 | Non-GC | 61 | IVA | 4 | 3 | 8 x R-CHOP-21; 2 x R-ESAP + R-BEAM | 10 | No | Relapsed | √ | |
| 52 | Non-GC | 68 | IAE | 1 | 4 x R-CHOP-14 + 3 x HD-MTX, IF-RT 36 Gy | 35 | Yes | √ | |||
| 53 | 74 | IIAE | 1 | 6 x R-CHOP; R-gemcitabine | 21 | No | Relapsed | √ | |||
| 54 | 68 | IIIBE | 5 | 3 | 4 x R-CHOP; 2 x R-ESAP; 4 x VAPEC | 15 | No | Primary progression | √ | ||
| 55 | 58 | IV | 3 | 2 | R-CHOP + HD-MTX | 1 | No | Leukaemic | √ | ||
| 56 | Non-GC | 78 | IVBE | 3 | 2 | 6 x R-CHOP-21 + 2 x R | 19 | Yes | Lost for follow-up | √ | |
| 57 | Non-GC | 77 | IA | 1 | 3 x R-CHOP-14, IF 30 Gy | 32 | Yes | √ | |||
| 58 | Non-GC | 76 | IVB | 4 | 3 | 6 x R-CHOP-21 + 2 x R; 3 x ESAP + ibritumomab tiuexetan (Zevalin) + HD-melphalan (Alkeran) | 16 | No | SAKK 38/07; relapsed SAKK 37/05 | √ | |
| 59 | Non-GC | 85 | IVB | >3 | >2 | 2 x R-CVP | NA | No | Died; no additional information | ||
| 60 | Non-GC | 61 | IIA | 1 | 6 x R-CHOP-14 + 2 x R, IF-RT 46 Gy | 43 | Yes | SAKK 38/07 | √ | ||
| 61 | Non-GC | 80 | IIA | 2 | 1 | 6 x R-CHOP-21 | 20 | No | √ | ||
| 62 | 64 | IVE | NA | NA | R-CHOP | 2 | No | √ | |||
| 63 | 78 | IA | 3 | 2 | 6 x R-CHOP-14 + 2 x R | 44 | Yes | √ | |||
| 64 | 84 | IV | >2 | >1 | 4 x R-CHOP-21 | 3 | No | √ | |||
| 65 | Non-GC | 80 | IAE | 2 | 1 | 5 x R-CHOP-21, IF-RT 40 Gy; R-bendamustine | 25 | No | Relapsed | √ | |
| 66 | 46 | IIIB | >3 | >3 | 6 x R-CHOP-14 | 72 | Yes | √ | |||
| 67 | 96 | IVBE | 5 | 3 | R-CHOP | 1 | No | √ | |||
| 68 | 27 | IAE | NA | NA | None | 92 | Yes | Hemicolectomy | |||
| 69 | 36 | II | 8 x R-CHOP, IF-RT 60 Gy; R-DHAB + R-BEAM | 85 | Yes | Relapsed | √ | ||||
| 70 | 80 | IAE | >1 | NA | 5 x R-COP | 14 | No | Died of cerebrovascular accident | |||
| 71 | 68 | IV | 4 | 3 | 1 x CHOP+1xR-CHOP-21 | 2 | No | √ | |||
| 72 | Non-GC | 72 | IVAE | 3 | 2 | 5 x R-CHOP-14+3xR | 39 | Yes | √ | ||
| 73 | GC | 21 | IAE | 1 | 6 x R-CHOP-21 | 67 | Yes | √ | |||
| 74 | 85 | IA | 1 | None | 73 | Yes | Surgery only | ||||
| 75 | 66 | IVB | 4 | 2 | 6 x R-CHOP-21; DHAP | 6 | No | Primary progression | √ | ||
| 76 | 72 | IVA | 3 | 1 | 6 x R-CHOP-21 + 4xIT + IF-RT | 9 | No | Testicular | √ | ||
| 77 | 68 | IIIB | >3 | >2 | 6 x R-CHOP + 2 x R; 6 x R-ICE+R-bendamustine | 18 | No | Relapsed | √ | ||
| 78 | Non-GC | 83 | IIIA | 3 | 2 | 6 x R-CHOP-21 + 2 x R | 35 | Yes | √ | ||
| 79 | Non-GC | 62 | IVB | 2 | 1 | 6 x R-CHOP-14 + 2 x R | 31 | Yes | √ | ||
| 80 | 79 | IAE | 1 | 6 x R-CHOP | 88 | Yes | √ | ||||
| 81 | 23 | IVBE | 3 | 2 | 8 x R-CHOEP | 65 | Yes | Haemophagocytosis | √ | ||
| 82 | 56 | IA | 4 x R-CHOP + IF-RT 36 Gy | 72 | Yes | √ | |||||
| 83 | 85 | IIBE | NA | NA | 4 x R-CHOP-21 | 7 | No | √ | |||
| 84 | Non-GC | 63 | IVAE | 3 | 1 | 6 x R-CHOP-14 + 2 x R | 53 | Yes | SAKK 38/07 | √ | |
| Unclear | 85 | GC | 73 | IIIA | 2 | 1 | 6 x R-CHOP-21 | 33 | Yes | ||
| 86 | GC | 47 | IVA | >1 | >1 | 6 x R-CHOP-14 | 33 | Yes | |||
| 87 | GC | 60 | IIIAE | 3 | 2 | 6 x R-CHOP-14 + 2 x R | 39 | Yes | |||
| 88 | 29 | IIB | 6 x R-CHOP 14, IF-RT 36 Gy | 62 | Yes | ||||||
| 89 | Non-GC | 76 | IV | >3 | >1 | 6 x R-CHOP-14 | 26 | No | |||
| 90 | 31 | IIB | 6 x R-CHOP-21 | 60 | Yes | ||||||
| Failure | 91 | 64 | IVB | 4 | 3 | 8 x R-CHOP + IT; HD-MTX + R-BEAM | 58 | Yes | Primary progression | ||
| 92 | Non-GC | 73 | IVA | 3 | 2 | 6 x R-CHOP-14 + 2 x R | 32 | Yes | SAKK 38/07 | ||
| 93 | 68 | IAE | 1 | 6 x R-CHOP, IF-RT 40 Gy | 86 | Yes | |||||
| 94 | 59 | IIA | 2 | 2 | 6 x R-CHOP + 2 x R, IF-RT 40 Gy | 39 | Yes | ||||
| 95 | 45 | IIIBE | 3 | 3 | 8 x R-CHOP-14 | 45 | Yes | ||||
| 96 | Non-GC | 68 | IVB | 3 | 2 | 6 x R-CHOP-21 | 19 | Yes | |||
| 97 | GC | 84 | IVBE | >4 | >2 | Refused | 4 | No | |||
| 98 | GC | 55 | IIA | 6 x R-CHOP-21, IF-RT 36 Gy | 51 | Yes | |||||
| 99 | 49 | IAE | 6 x R-CHOP-14 + 2 x R | 33 | Yes | SAKK 38/07 | |||||
| 100 | 51 | IIIB | 3 | 3 | 1 x etoposide, 6 x R-CHOP; 3 x ESAP+BEAM | 80 | Yes | Haemophagocytosis, primary progression | |||
| 101 | GC | 59 | IVAE | 2 | 1 | 6 x R-CHOP-14 + 2 x R | 27 | Yes | |||
| 102 | Non-GC | 86 | IIIAE | 3 | 2 | 5 x R-CHOP-21 (split) + 2 xR | 51 | Yes | |||
| 103 | Non-GC | 85 | IAE | 1 | Refused | 1 | Yes | Lost to follow-up | |||
| 104 | 72 | IE | >1 | NA | 6 x R-CHOP, IF-RT 36 Gy | 74 | Yes | ||||
| 105 | 90 | at least IIA | >2 | >1 | None | 1 | No | ||||
| 106 | GC | 48 | IA | 1 | 6 x R-CHOP-14 + 2 x R, IF-RT 46 Gy | 30 | Yes | SAKK 38/07 | |||
| 107 | 54 | IVBE | 2 | 1 | 8 x R-CHOP; 3 x R-ICE + R-BEAM | 65 | Yes | Relapsed (CORAL) | |||
| 108 | 92 | IVAE | 3 | 1 | IF-RT 30 Gy | 3 | No | ||||
| 109 | 54 | IA | 6 x R-CHOP, IF-RT 36 Gy; 3 x ESAP + BEAM + R-maintenance | 75 | Yes | Relapsed | |||||
The search in the database of the Department of Medical Oncology of the Inselspital revealed 172 newly diagnosed, bona fide, de novo DLBCL cases between 2005 and 2010. Forty-four percent of the patients in this retrospective cohort were male and 56% female. The age at diagnosis ranged from 17 to 96 years (median 68 years). The preponderance of female patients in our cohort is different from the reported distribution [21], but was not further investigated.
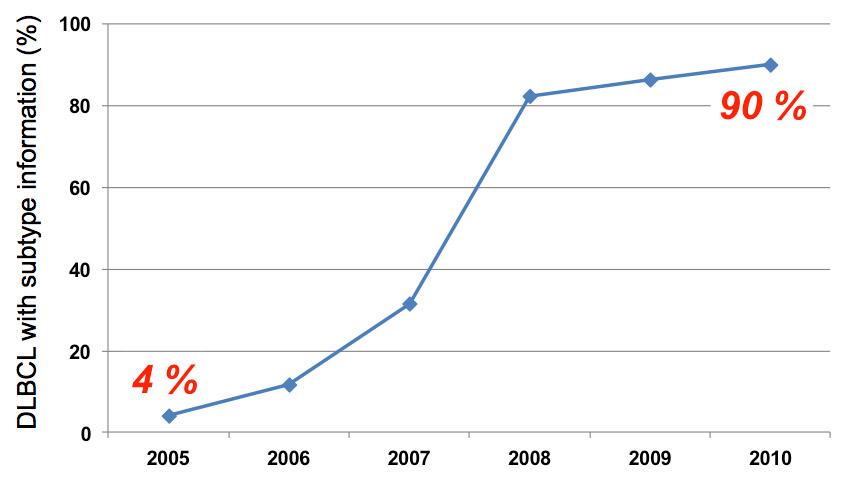
Figure 2
Increased availability over time of molecular DLBCL subtype information in accordance with to the Hans algorithm from routine diagnostics at the Inselspital Bern.
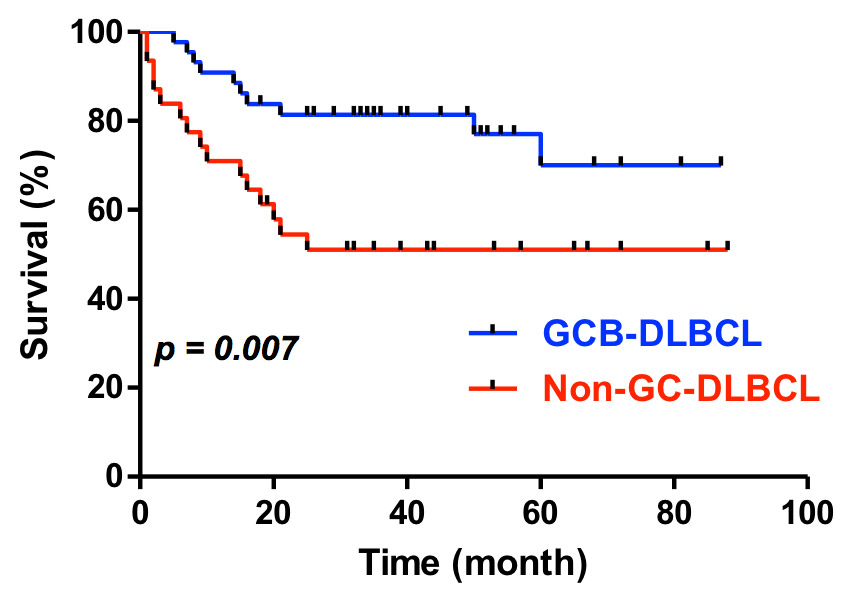
Figure 3
Survival of patients with DLBCL subtypes (determined using research techiques) treated with standard R-CHOP (GCB-DLBCL, n = 44, non-GC-DLBCL, n = 31, Wilcoxon test).
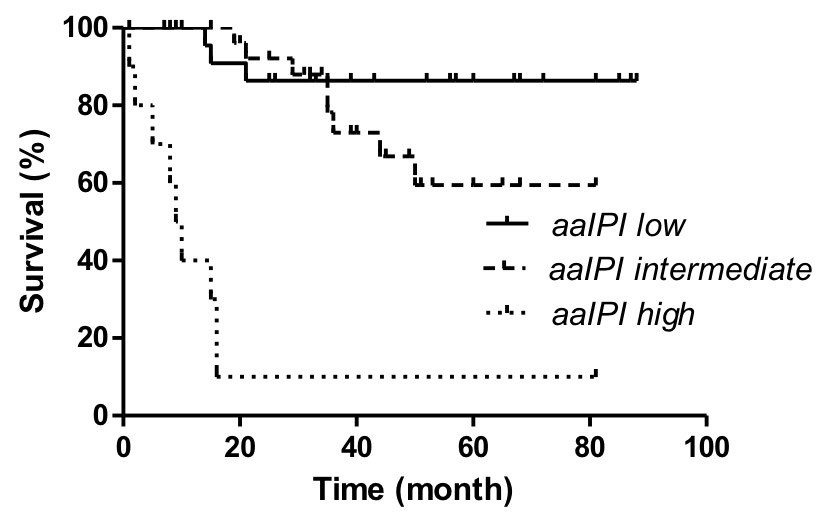
Figure 4
Survival of the DLBCL cohort by aaIPI score (low risk = aaIPI 0, intermediate risk = aaIPI 1 and 2, and high risk = aaIPI 3, p <0.0001 by log rank test).
Of the bona fide 172 DLBCL patients, 109 had a pathological diagnostic work-up at the Institute of Pathology of the University of Bern and were thus included in our analysis. Staining for MUM1, the last immunohistochemical marker of the "Hans" algorithm to be established in Bern, was missing for 68 patients, 13 of whom also lacked information on CD10 and/or bcl-6. Given the current lack of clinical consequence, retroactive staining of these missing immunohistochemical markers was not done. We could assign 51/109 (46.8%) of the DLBCL cases a molecular subtype. This low number is not surprising because the three immunohistochemical markers were not systematically used in the routine diagnostic work-up of DLBCL. However, with increasing awareness among clinical pathologists at our institution, full information needed to assign the DLBCL subtype became increasingly available between 2005 and 2010 (fig. 2). Only three of the 172 DLBCL patients treated at the Department of Medical Oncology of the Inselspital in the period considered here, but with a diagnostic work-up outside of our Institute of Pathology of the University, had a complete "Hans" IHC data set. This negligible number clearly justifies our inclusion criteria that were a priori defined for practical reasons. 26/51 (51%) of the patients were assigned the GCB-subtype and 25/51 (49%) to the non GC-subtype of DLBCL (table 1). 11 cases (11/51, 21.6%) stained positive for both CD10 and MUM1. This marker constellation is not included in the "Hans classification" (fig. 1). We assigned these DLBCL cases to the GCB-subgroup after contacting the corresponding author of the original publication [15]. CD10 is considered an essential marker of GC differentiation [15, 22]. Notably, we found 3 (11.5%) of the 26 GCB-DLBCL cases to be CD10 negative.
The determination of the DLBCL subtype was expanded by constructing a tissue microarray (TMA) containing tissue samples from the 109 patients of our single-centre cohort, and using a concerted IHC analysis. Each DLBCL case was represented on the TMA by two punches. Material was not adequate for complete TMA work-up (e.g., no remaining tumour tissue available in small biopsy samples or missing blocks) in 19/109 (17.4%) of the cases. Determination of the molecular DLBCL subtype was inconsistent between the two punches in an additional six cases. Of the remaining 84 cases, 49 (49/84, 58.3%) could be assigned to the GCB subtype, and 35 (41.7%) to the non-GC subtype of DLBCL. Eight (16.3%) of the 49 GCB-DLBCL cases were CD10 negative, and 6 DLBCLs were positive for CD10 and MUM1. Notably, in the 39 cases with DLBCL subtype information from both the diagnostic and the research setting, the DLBCL subtype changed in seven cases from the non-GC to the GCB-subtype and in one case from GCB to non-GC after the analysis on the TMA (table 1).
The median follow-up of this retrospective single-centre cohort of 109 DLBCL cases was 35 months (range 1–92 months, table 1). Overall, 10/84 (11.9%) relapsed or had primary progression, and 30/84 (35.7%) have died, 13 in the GCB, and 17 in the non-GC group (table 1). Our survival analysis focused on the 75 patients with uniform given or planned first-line treatment with three to six cycles of standard R-CHOP (R-CHOP-14 or R-CHOP-21) for comparison with published data [4–6]. Because of the inconsistencies between the results of the routine diagnostic tests and the research technique outlined above, we also confined the survival analysis to the cases with an unequivocal subtype determination on the TMA (table 1). Overall survival of the patients with the GCB subtype of DLBCL was statistically longer than for the non-GC group (fig. 3). The subset of 43 uniformly treated patients with information obtained only within the diagnostic setting also showed a better survival of the GCB-subtype, however, this difference was not statistically significant (data not shown), most likely because of the small sample size. The aaIPI score clearly influenced the outcome in the uniformly treated patients of our retrospective cohort (fig. 4). In a multivariate analysis, the molecular subtype (from the TMA) turned out to be an independent prognostic factor for the overall survival in the uniformly treated patients (p = 0.04). This analysis also included the aaIPI score (0–1 versus 2–3) and the sex (data not shown).
To assess the current DLBCL subtype determination in Switzerland, we contacted six academic and other major pathology departments (Pathology Departments of the Universities of Basel and Zurich, Pathology Departments of Aarau, Chur, Lucerne and Bellinzona). We asked them to provide us with their IHC protocols for the three parameters in the "Hans classification" [15]. As shown in table 2, there was no concordance between the departments and, most importantly, with the published method [15]. Whereas most of the departments did not use predefined cut-offs for the definition of a positive result, one department applied cut-off scores calculated using analysis based on a receiver operating characteristic (ROC) curve [23]. We concluded that, compared to the original publication [15], there is no technical standard to define the DLBCL subtypes according to the Hans algorithm in Switzerland. This has to be taken into account when DLBCL subtype-specific treatment options emerge, and demonstrates the need for a central assessment in the context of possible future clinical protocols.
| Table 2: Immunohistochemical protocols currently used in Switzerland to assess molecular DLBCL subtypes in accordance with the Hans algorithm [15]. | ||||||
| Published protocol by Hans et al. [15]. Letters A to G denote the surveyed clinical pathology departments in Switzerland (C = Institute of Pathology, University of Bern) that provided their routine IHC protocols (as of May 2011) | ||||||
| Antigen | Clone | Source | Antigen retrieval | Dilution | Cut-off | |
| Published | CD10 | 56C6 | Ventana | Zitrat, 60', 95°C | 1 : 1 | 30% |
| bcl-6 | Polyclonal | Santa Cruz | EDTA, 60', 95°C | 1 : 75 | ||
| MUM1 | MUM1p | Falini et al. | EDTA, 30', 95°C | 1 : 10 | ||
| A | CD10 | 56C6 | Novocastra | 30', ER1 (pH6) | 1 : 20 | none |
| bcl-6 | PG-B6p | Dako | 30', ER2 (pH9) | 1 : 20 | ||
| MUM1 | MUM1p | BioSystems | 20', ER2 (pH9) | 1 : 100 | ||
| B | CD10 | 56C6 | Novocastra | Citrate, 15', 100°C | 1 : 10 | 10% |
| bcl-6 | PG-B6p | Dako | Citrate, 60', 80°C | 1 : 10 | 10% | |
| MUM1 | MUM1p | Dako | Citrate, 30', 100°C | 1 : 50 | 65% | |
| C | CD10 | 56C6 | Novocastra | Urea | 1 : 50 | 30% |
| bcl-6 | GI191E/A8 | Cell Marque | EDTA | 1 : 100 | ||
| MUM1 | MUM1p | Dako | Pressure cooker Citrate | 1 : 100 | ||
| D | CD10 | 56C6 | Novocastra | Bond ER2, 20', 95°C | 1 : 30 | None |
| bcl-6 | LN22 | Novocastra | Bond ER2, 20', 95°C | 1 : 20 | ||
| MUM1 | MUM1p | Dako | Bond ER2, 20', 95°C | 1 : 70 | ||
| E | CD10 | 56C6 | Leica | EDTA, 30' | 1 : 50 | None |
| bcl-6 | PG-B6p | Dako | EDTA, 30' | 1 : 40 | ||
| MUM1 | MUM1p | Dako | EDTA, 20' | 1 : 80 | ||
| F | CD10 | 56C6 | Ventana | Tris, 60', 100°C | Ready to use | None |
| bcl-6 | GI191E/A8 | Ventana | Tris, 90', 100°C | Ready to use | ||
| MUM1 | MUM1p | Dako | Tris, 30', 100°C | Ready to use | ||
| G | CD10 | 56C6 | Cell Marque | Citrate, 30', 95°C | Ready to use | None |
| bcl-6 | G/191E/A8 | Cell Marque | Citrate, mod., 90', 95°C | 1 : 50 | ||
| MUM1 | MUM1p | Dako | Citrate, 30', 95°C | 1 : 50 | ||
In the literature, there are conflicting reports on the use of IHC to determine the molecular subtypes of DLBCL [12, 15, 18–20]. We aimed to examine critically the current assessment of the DLBCL subtypes using IHC in a cohort of 109 patients. We also wanted to raise awareness of this technique, which we think will be important beyond a “simple” diagnosis of this lymphoma, perhaps becoming a default procedure to be used in addition to the current WHO classification.
We here report on our single-centre experience with this assessment, which gradually became available after 2005 (when immunohistochemical staining for MUM1 was established in Bern). We used the "Hans" algorithm [15], the first and most widely used of its kind. In summary, we could assign 58% of the DLBCLs to the GCB and 42% to the non-GC subtype, a distribution comparable to previously published data. In addition, after uniform treatment with the first-line standard, R-CHOP, patients with GCB-DLBCL were found to have a significantly longer survival than cases of the non-GC subtype. The study cohort included a relatively small number of patients, given the high number of cases with missing information on the molecular subtype (overall 76 of the 172 DLBCL patients (44.2%), 13/109 (11.9%) when combining the information from the diagnostic and the research setting, or 68/109 (62.4%) in the diagnostic setting). Furthermore, follow-up was limited to 35 months, and patients from a tertiary referral centre like ours may not be fully representative of the general patient population in Switzerland. Therefore, we acknowledge the limitations of our findings for a comprehensive comparison with published data. In addition, we do not have GEP data to assess the appropriateness of our assignments to the DLBCL subtypes using IHC. There is an an increasing body of evidence that DLBCL subtypes might be clinically and therapeutically relevant [7, 24, 25], and so having information on the molecular DLBCL subtype at hand (fig. 2) shows a welcome awareness of this potentially important biological feature.
We also report a disturbing lack of standardisation in DLBCL subtype determination by means of IHC in Switzerland. Furthermore, we saw inconsistent assignments of the DLBCL subtype between the routine diagnostic and research techniques in 8/39 (20.5%) patients (table 1). In the absence of GEP data, we cannot determine the correctness of these assignments. Given the possible implication of these results, we think that the controversial issue of DLBCL subtypes, not fully covered in the review recently published in this journal [26], cannot be ignored.
Firstly, the detected flaws included the antibodies, the antigen retrieval, and the cut-off scores. The latter ranged from no predefined scores, a uniform use of 30% as published by Hans et al. [15] to a rarely used but rational determination using a receiver operating characteristic (ROC) curve [23]. Although the technical shortcomings of IHC are known [27], this unappreciated issue might at least partially explain the reported conflicting results. Ideally, in parallel with an improvement of IHC algorithms, we would also attempt standardisation that may ensure the reliable and reproducible determination of DLBCL subtypes using an accepted algorithm. For practical reasons, these attempts should focus on the diagnostic, case-centred approach, rather than on high-throughput research techniques (such as IHC on TMAs) currently not suitable for individual DLBCL patients. This should be attempted through vigorous interlaboratory tests and comparisons, which are offered in Europe through, for example, the United Kingdom National External Quality Assessment Service (UK-NEQAS), the Swiss Society of Pathology (SGP) or the German Society of Pathology (DGP), and through a proactive and close collaboration between pathologists and clinicians. The controversial results presented here and in the literature might also be due to the retrospective nature of the IHC studies performed in the research setting on a heterogeneous set of patients, as opposed to prospective trials. In addition, an agreement is needed on how to handle cases that cannot be GEP-classified as GCB- or ABC-DLBCL. This hampers current IHC algorithms, including the Hans classification, as they include this heterogeneous group in the non-GC subtype [28].
Secondly, we spotted a disturbing misconception about the possible relevance of the DLBCL subtypes. The Hans algorithm was created as a surrogate for the molecular DLBCL subtypes determined using GEP and not primarily as another prognostic marker. Recent research has revealed a variety of DLBCL subtype-specific molecular alterations [17, 29–35] associated with different responses to chemotherapy [24, 25]. The NF-κB-pathway that is constitutively active in ABC-DLBCL and essential for the survival of this subtype [36] is one obvious subtype-specific therapeutic target. However, prospective trials testing this hypothesis have only recently been started. We think that a robust determination of the DLBCL subtypes has the potential to establish a predictive marker, one of the very few so far known in lymphoma. In the best-case scenario and analogous to, for example, HER2/neu positive breast cancer or the BRAFV600E mutation in melanoma, the prognostic significance then becomes a secondary issue and is moreover affected by variables unrelated to the accuracy of classification.
Only when all of these caveats are appropriately addressed we will be able to conduct prospective large clinical trials and ultimately to provide improved and possibly DLBCL-subtype specific therapies to our patients.
Acknowledgment:We thank our colleagues from the pathology departments in Switzerland to provide their current immunohistochemistry protocols, Dr. Andreas Kappeler and his team for the immunohistochemistry, Dr. Inti Zlobec and her team for construction of the TMA (both from the Instutite of Pathology, University of Bern) as well as Wing C Chan, University of Nebraska Medical Center, Omaha, USA, for useful discussions.
1 Fisher S, Fisher R. The epidemiology of non-Hodgkin’s lymphoma. Oncogene. 2004;23(38):6524–34.
2 Sehn L, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027–33.
3 Pfreundschuh M, Trümper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7(5):379–91.
4 Coiffier B. State-of-the-art therapeutics: diffuse large B-cell lymphoma. J Clin Oncol. 2005;23(26):6387–93.
5 Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9(2):105–16.
6 Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12(11):1013–22.
7 Recher C, Coiffier B, Haioun C, Molina TJ, Ferme C, Casasnovas O, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): an open-label randomised phase 3 trial. Lancet. 2011;378(9806):1858–67.
8 Lenz G, Staudt LM. Aggressive lymphomas. N Engl J Med. 2010;362(15):1417–29.
9 Alizadeh A, Eisen M, Davis R, Ma C, Lossos I, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–11.
10 Lenz G, Staudt LM. Aggressive lymphomas. N Engl J Med. 2010;362(15):1417–29.
11 Morin RD, Mendez-Lago M, Mungall AJ, Goya R, Mungall KL, Corbett RD, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476(7360):298–303.
12 Gutierrez-Garcia G, Cardesa-Salzmann T, Climent F, Gonzalez-Barca E, Mercadal S, Mate JL, et al. Gene-expression profiling and not immunophenotypic algorithms predicts prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Blood. 2011;117(18):4836–43.
13 Rui L, Schmitz R, Ceribelli M, Staudt LM. Malignant pirates of the immune system. Nat Immunol. 2011;12(10):933–40.
14 Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne R, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198(6):851–62.
15 Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–82.
16 Meyer PN, Fu K, Greiner TC, Smith LM, Delabie J, Gascoyne RD, et al. Immunohistochemical Methods for Predicting Cell of Origin and Survival in Patients With Diffuse Large B-Cell Lymphoma Treated With Rituximab. J Clin Oncol. 2011;29(2):200–7.
17 Pasqualucci L, Compagno M, Houldsworth J, Monti S, Grunn A, Nandula SV, et al. Inactivation of the PRDM1/BLIMP1 gene in diffuse large B cell lymphoma. J Exp Med. 2006;203(2):311–7.
18 Visco C, Li Y, Xu-Monette ZY, Miranda RN, Green TM, Tzankov A, et al. Comprehensive gene expression profiling and immunohistochemical studies support application of immunophenotypic algorithm for molecular subtype classification in diffuse large B-cell lymphoma: A report from the International DLBCL Rituximab-CHOP consortium program study. Leukemia. 2012:26:2103–13.
19 Ott MM, Horn H, Kaufmann M, Ott G. The Hans classificator does not predict outcome in diffuse large B cell lymphoma in a large multicenter retrospective analysis of R-CHOP treated patients. Leuk Res. 2012;36(5):544–5.
20 Castillo JJ, Beltran BE, Song MK, Ilic I, Leppa S, Nurmi H, et al. The Hans algorithm is not prognostic in patients with diffuse large B-cell lymphoma treated with R-CHOP. Leuk Res. 2012;36(4):413–7.
21 Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107(1):265–76.
22 Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503-11.
23 Tzankov A, Zlobec I, Went P, Robl H, Hoeller S, Dirnhofer S. Prognostic immunophenotypic biomarker studies in diffuse large B cell lymphoma with special emphasis on rational determination of cut-off scores. Leuk Lymphoma. 2010;51(2):199–212.
24 Dunleavy K, Pittaluga S, Czuczman MS, Dave SS, Wright G, Grant N, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009;113(24):6069–76.
25 Thieblemont C, Briere J, Mounier N, Voelker HU, Cuccuini W, Hirchaud E, et al. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: a bio-CORAL study. J Clin Oncol. 2011;29(31):4079–87.
26 Mey U, Hitz F, Lohri A, Pederiva S, Taverna C, Tzankov A, et al. Diagnosis and treatment of diffuse large B-cell lymphoma. Swiss Med Wkly. 2012;142:0.
27 Zu Y, Steinberg SM, Campo E, Hans CP, Weisenburger DD, Braziel RM, et al. Validation of tissue microarray immunohistochemistry staining and interpretation in diffuse large B-cell lymphoma. Leuk Lymphoma. 2005;46(5):693–701.
28 Choi WW, Weisenburger DD, Greiner TC, Piris MA, Banham AH, Delabie J, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009;15(17):5494–502.
29 Iqbal J, Sanger WG, Horsman DE, Rosenwald A, Pickering DL, Dave B, et al. BCL2 translocation defines a unique tumor subset within the germinal center B-cell-like diffuse large B-cell lymphoma. Am J Pathol. 2004;165(1):159–66.
30 Kato M, Sanada M, Kato I, Sato Y, Takita J, Takeuchi K, et al. Frequent inactivation of A20 in B-cell lymphomas. Nature. 2009;459(7247):712–6.
31 Morin RD, Johnson NA, Severson TM, Mungall AJ, An J, Goya R, et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet. 2010;42(2):181–5.
32 Pasqualucci L, Dominguez-Sola D, Chiarenza A, Fabbri G, Grunn A, Trifonov V, et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011;471(7337):189–95.
33 Lenz G, Davis RE, Ngo VN, Lam L, George TC, Wright GW, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319(5870):1676–9.
34 Compagno M, Lim WK, Grunn A, Nandula SV, Brahmachary M, Shen Q, et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459(7247):717–21.
35 Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463(7277):88–92.
36 Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194(12):1861–74.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.