Destination therapy – time for a paradigm change in heart failure therapy
DOI: https://doi.org/10.4414/smw.2013.13729
Markus
Wilhelm, Frank
Ruschitzka, Volkmar
Falk
Summary
Heart transplantation is only available for a limited number of patients with end-stage heart failure. Since the arrival of newer ventricular assist devices, mechanical circulatory support constitutes an alternative therapy for patients with advanced heart failure. The first-generation of pulsatile-flow devices were used only for bridging the sickest patients to transplantation. Frequent adverse events, limited durability and the patients’ discomfort made them unsuitable for lifetime support. The second-generation continuous-flow devices were smaller, quieter and more durable. Survival rates of patients improved significantly. This led to a marked growth of device implantations, largely caused by an increase of lifetime support. Survival of destination therapy patients is somewhat inferior to the survival of bridge-to-transplant patients, in part due to their co-morbid conditions which limit life expectancy. A subgroup of patients on destination therapy with advanced, but stable heart failure and a low-risk profile reach short-term survival rates equal or superior to the survival after heart transplantation. These patients may be offered the choice of destination therapy versus heart transplantation. However it remains unclear if long-term survival, quality of life and functional status on lifetime support can compete with the excellent results after transplantation. A trend to implanting devices at earlier stages of heart failure has begun. In a current trial, patients with advanced, but stable heart failure are randomised to destination therapy versus optimal medical therapy. The results of this trial will be expected to more precisely determine the place of mechanical circulatory support in the treatment of advanced heart failure.
Introduction
Heart transplantation is still being considered the gold standard for treatment of patients with end-stage heart failure. The actual 1-year survival following heart transplantation reaches 84% world-wide, according to the registry of the International Society for Heart and Lung Transplantation (ISHLT) [1]. While the number of patients listed for heart transplantation is steadily increasing, the quantity of heart transplantations has remained similar over the last years. This is a world-wide phenomenon which is well-known in Switzerland, in the Eurotransplant region as well as internationally [1–3]. From 2008 to 2011, the number of patients on the heart transplant waiting list in Switzerland increased by nearly 50%, but the total count of heart transplants only grew by 24% [3]. In the Eurotransplant countries, the amount of patients listed for heart transplantation doubled in the period from 2003 to 2011, whereas the number of heart transplants stayed the same [2]. In addition, there is a growing portion of patients with severely advanced heart failure who are not transplant candidates because of coexisting conditions such as advanced age or complications from renal disease and diabetes. This is the consequence of advancements in medical therapy which has helped to extend life expectancy without preventing progression of heart failure. In this context, mechanical circulatory support becomes an attractive alternative for the treatment of end-stage heart failure, particularly since technology has made rapid progress over the last few years.
Mechanical circulatory support in end-stage heart failure – from rescue therapy for transplant candidates to a transplant alternative
Heart transplantation can only be offered to a limited number of patients due to the shortage of donor organs, and its long-term complications such as chronic allograft vasculopathy and malignancy are still not controlled. In this light, mechanical circulatory support has gained increasing interest as an alternative to heart transplantation. Originally, ventricular assist devices were only used for temporary support to give the sickest patients on the waiting list the chance to survive until heart transplantation. In the landmark REMATCH trial, mechanical circulatory support devices were tested as lifetime therapy versus medical therapy, for the first time in history [4]. Patients with symptoms of NYHA class IV heart failure and ineligible for heart transplantation were randomised to receive a left ventricular assist device versus optical medical therapy (OMT) only. In the device group, there was a significant reduction in the risk of death from any cause, compared with the OMT group. Survival at one year was significantly higher in the device group (52%) than in the OMT group (25%). After two years, there was still a trend to better survival in the device (23%) as opposed to the OMT patients (8%). However, adverse events such as bleeding, neurologic dysfunction, device related infections and device failure continued to impair the survival benefit of the device patients.
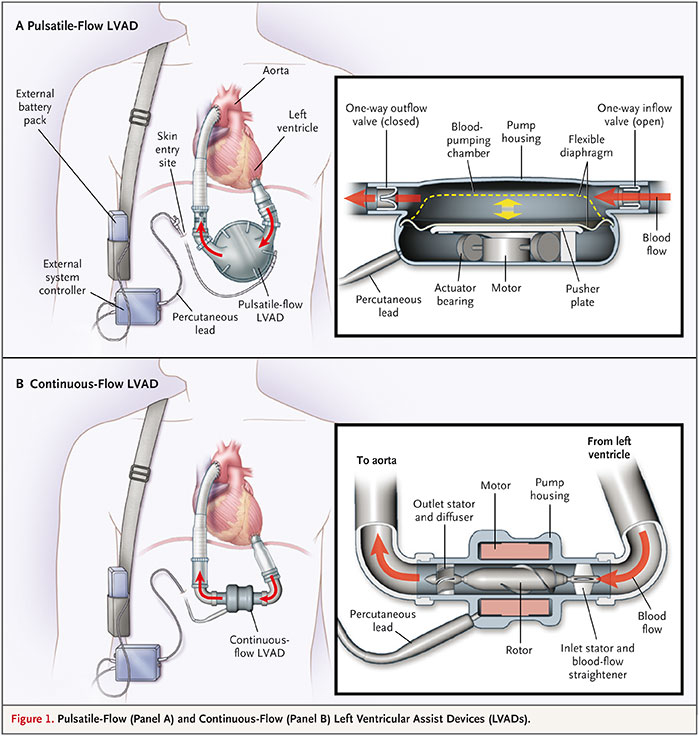
Figure 1
The first-generation pusatile-flow pump HeartMate I (A) and the second-generation continuous-flow pump HeartMate II (B). From: Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241-51. © 2009 Massachusetts Medical Society. Reprinted with kind permission.
In the REMATCH trial, the HeartMate I (originally Thermo Cardiosystems, Inc., Woburn, MA, USA; now: Thoratec Inc., Pleasanton, CA, USA) was used for mechanical support (fig. 1A). It represents the group of first-generation pulsatile-flow devices which also included the Novacor N100 (originally Baxter, later World Heart Inc., Ottawa, Canada). As they were displacement pumps consisting of a large housing weighing >1 kg, they did not fit into the pericardium and needed to be implanted inside the abdominal wall or intra-peritoneally. From the beginning of mechanical circulatory support in the mid 1980s, such devices were primarily used for temporary support to give patients with an imminent risk of death on the waiting list the chance to survive until heart transplantation. Although the pumps were successful in bridging 60–70% of patients to heart transplantation, they were associated with a multitude of adverse events, such as bleeding, thrombus formation causing stroke and peripheral embolism, infection, and mechanical wear requiring device exchange [5, 6].
In addition, the large size and noise affected the patients’ well-being significantly. These shortcomings prevented such devices from being used in a larger patient population. Not surprisingly, infection and failure of the HeartMate I device were prominent events which influenced the results of the device group in the REMATCH trial unfavourably. Thus, the most common causes of death in the device group were sepsis (41% of deaths), most frequently resulting from device-related infection, and device failure (17% of deaths). Within the first three months after implantation, the probability of device-related infection was as high as 28%. At 2 years after implantation, the probability of device failure was 35%, and the device had to be replaced in 15% of patients [4].
Technical advancement of devices improves results – destination therapy becomes an attractive option
At the time when the REMATCH trial was ongoing, axial-flow pumps were introduced into clinical use. These so-called “second-generation” devices used an impeller to convey the blood resulting in a continuous flow. Hereby, the pump size could be markedly reduced. Implantation inside the pericardium became possible. The first such pump which was used clinically was the MicroMed DeBakey VAD (MicroMed Cardiovascular, Inc., Houston, TX, USA). However, following a wave of enthusiasm, the expectations with the pump were not met. The formation of thrombus inside the pump was a frequent adverse event which largely contributed to the disappointing outcomes. Survival on the pump of <60% could not compete with that of first-generation devices [7]. With the availability of further refined axial-flow pumps such as the Jarvik 2000 (Jarvik Heart, Inc., New York, NY, USA), the Berlin Heart Incor (Berlin Heart GmbH, Berlin, Germany) and the HeartMate II (Thoratec Inc., Pleasanton, CA, USA) (fig. 1B), the results with second-generation pumps improved markedly [8–10]. Since they were smaller (fig. 1C), with less noise and more durable than the first-generation pulsatile pumps, the patient comfort increased considerably. In the light of these advantages, they were thought to be more suitable for long-term support than the first-generation pulsatile pumps. As a consequence, a trial was launched to compare both device groups in end-stage heart failure patients who were not transplant candidates. Subsequently, patients in heart failure NYHA functional class IIIb or IV who were ineligible for heart transplantation were randomised prospectively to receive either the first-generation pulsatile-flow pump HeartMate I or the continuous-flow pump HeartMate II [11]. Survival at one and two years was significantly better in patients who were on a continuous-flow device (68% and 58%, respectively) compared to those who had a pulsatile-flow pump (55% and 24%, respectively) (fig. 2). Equally important, there were significant reductions in the rates of major adverse events among patients with a continuous-flow left ventricular assist device. In particular, the incidence of device-related infection and sepsis as well as the frequency of pump replacement were significantly reduced in patients with a continuous-flow device as compared with those on a pulsatile-flow pump. While valve or bearing failures occurred frequently in the pulsatile-flow pumps, there were no primary pump or bearing failures in the continuous-flow devices. Replacement of continuous-flow pumps was mainly required because of damage to the percutaneous controller lead. In this so-called HeartMate II destination therapy trial, the survival rate of patients with the HeartMate I was similar to the outcome among patients with the HeartMate I in the REMATCH trial. This indicates that, over a period of eight years since the REMATCH trial, there was no progress in survival with pulsatile-pumps, despite advances in peri- and postoperative management. In contrast, the 2-year survival of patients with a continuous-flow device was more than double compared to patients with pulsatile-flow pumps (fig. 1) [12].
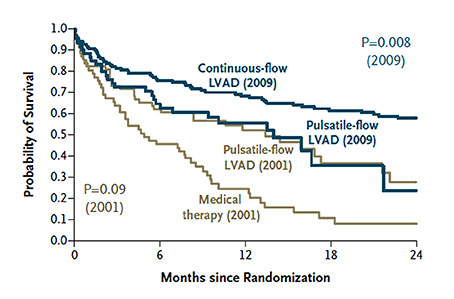
Figure 2
Survival rates in two trials of LVADs as destination therapy (REMATCH trial 2001, HeartMate II destination therapy trial 2009). From: Fang JC. Rise of the machines – left ventricular assist devices as permanent therapy for advanced heart failure. N Engl J Med 2009;361:2282-85. © 2009 Massachusetts Medical Society. Reprinted with kind permission.
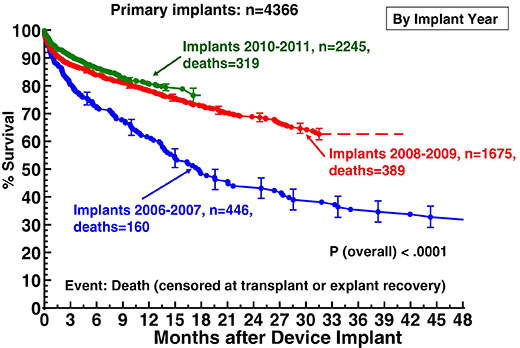
Figure 3
Actuarial survival following ventricular assist device implantation, stratified by era. From: Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant 2012;31:117–26. © 2012 Elsevier. Reprinted with kind permission.
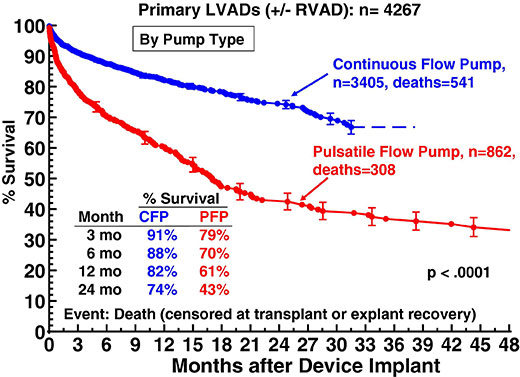
Figure 4
Actuarial survival following implantation of continuous-flow (CFP) versus pulsatileflow pumps (PFP). From: Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant 2012;31:117–26. © 2012 Elsevier. Reprinted with kind permission.
The success of continuous-flow pumps is also depicted in the most recent 4th report of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) [13]. This database analyses United States Food and Drug Administration (FDA)-approved durable mechanical circulatory support device implants in the United States. It now oversees five years of patient enrolment from 2006 to 2011. Overall survival on ventricular assist devices has progressively improved since 2006. In the most recent experience of the years 2010 and 2011, actuarial survival at one year amounts to 82% which is significantly higher than the 1-year survival of about 62% in the period of 2006 and 2007 (fig. 3). This is mainly caused by the excellent results in patients with continuous-flow pumps which experienced a dramatic increase of implants in recent years. In 2010, more than 95% of all implants were continuous-flow devices, and the number is still increasing. If stratified by pump type, it could be shown that the outcome of patients on continuous-flow pumps was significantly better than in patients who had a pulsatile-flow pump. Thus, survival at one year following implant was 82% for continuous-flow devices compared to only 61% in patients with pulsatile-flow pumps. After two years, 74% of patients on continuous-flow-pumps were still alive, as opposed to only 43% of patients with pulsatile-flow devices (fig. 4). As a consequence of the improved results with second-generation devices, the European Society of Cardiology upgraded the recommendation for destination therapy in the 2012 version of the Guidelines on the diagnosis and treatment of heart failure from a class IIb to a class IIa level B recommendation [14].
In the INTERMACS report no analysis was made with respect to survival of patients on continuous-flow pumps, stratified by year of implant. As already outlined above, having an implant, no matter if continuous-flow or pulsatile-flow, in the era 2010–2011 increased survival (82% at 1 year) as compared to previous years. In addition, being on a continuous-flow device between 2006 and 2011 also improved survival (at 1 year: 82%) compared to being on a pulsatile-flow device (at 1 year: 61%). Taken together, one might assume that 1-year survival of patients who received a continuous-flow device in 2010–2011 is even higher than 82% if one considers that the calculation by era included all pump types, not only the continuous-flow devices. Then, 1-year survival of patients who undergo a continuous-flow implant in the current era would reach values very much equal to the 1-year survival following heart transplantation which presently reaches 84% [1].
If one compares survival of primary continuous-flow implants according to the initial device strategy, data from INTERMACS show that survival of destination therapy patients comes close to that of bridge-to-transplant patients. One and two years after implant, survival of patients on destination therapy amounts to 78% and 72%, respectively, as compared to 89% and 86%in patients bridged to transplant, respectively [13]. The inferior survival rate of patients on destination therapy, compared to transplant candidates, is largely explained by the co-morbid conditions which make them ineligible for transplantation and have an unfavourable impact on life expectancy. It may also play a role that such patients are not considered for transplantation as rescue therapy in case of life-threatening device complications. In the light of the 2010–2011 INTERMACS data, it would be interesting to stratify the survival of patients on continuous-flow destination therapy by the implant year. It can be expected that patients on destination therapy would currently have a 1-year survival exceeding the 78% which reflect the analysis over the whole time period from 2006 to 2011.
Better outcome of destination therapy in patients with lower risk – destination therapy challenges heart transplantation
The INTERMACS survival analyses included patients with all risk profiles and did not differentiate for various risk classes such as INTERMACS levels 1–7. A risk factor analysis for the entire cohort identified cardiogenic shock, corresponding with INTERMACS level 1, as one of the most prominent risk factors for death within the first three months after implant. Other risk factors for early death were INTERMACS level 2, older age, history of cardiac surgery (CABG or valve) and deteriorating renal or hepatic function [13]. It is assumed that patients with less advanced heart failure and a lower risk profile at the time of implantation have better outcomes. An attempt was made by three US institutions to stratify clinical outcomes following continuous-flow device implants by the preoperative risk according to the INTERMACS classification [15]. Not surprisingly, survival was greater the less sick the patients were before implantation. Patients with ambulatory advanced heart failure (INTERMACS level 4–7) had a significant better 3-year survival as compared to patients in cardiogenic shock (INTERMACS 1) (95.8% vs 51.1%, p = 0.011). There was also a trend that 3-year survival of patients with INTERMACS level 4–7 was superior to that of inotrope-dependent patients (INTERMACS 2-3) (95.8% vs. 68.8%, p = 0.065). Survival of patients in INTERMACS level 1 was not different from survival of patients in INTERMACS levels 2–3 (51.1% vs. 68.8%, p = 0.18). The validity of these results is limited since it was a retrospective study across multiple sites, the size of the groups was relatively small, the distribution of patients across the groups was not even, and some baseline characteristics, such as age, were different between groups. Moreover, the study did not discriminate between bridge-to-transplant patients and patients on lifetime support.
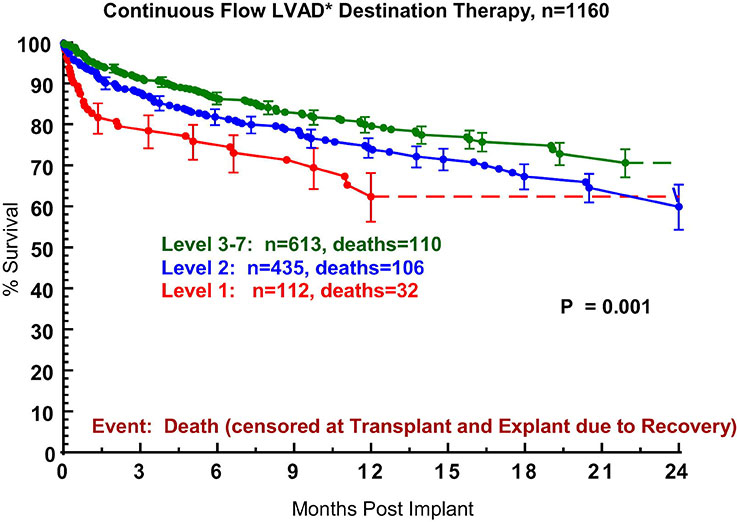
Figure 5
Actuarial survival after continuous-flow device destination therapy, stratified by INTERMACS level at the time of implant. INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support [20]. From: Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson L, et al. Long-term mechanical circulatory support (destination therapy): On track to compete with heart transplantation? J Thorac Cardiovasc Surg. 2012;144:584‒603. © 2012 Elsevier. Reprinted with kind permission.
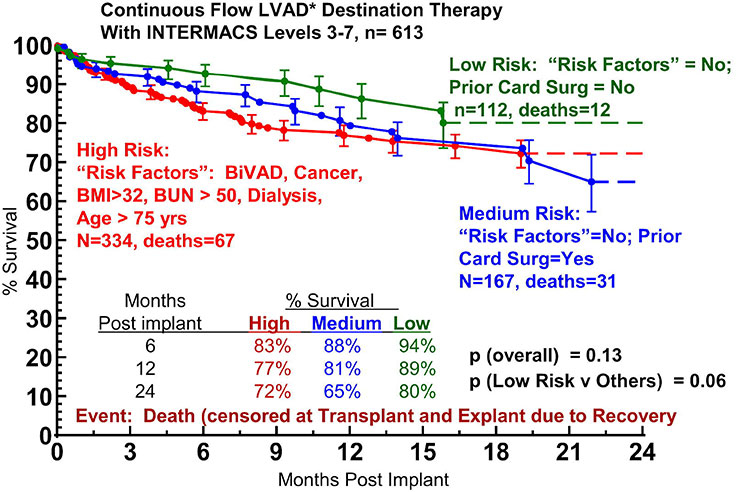
Figure 6
Actuarial survival after continuous-flow device destination therapy of patients with INTERMACS levels 3-7, stratified by high-, medium-, and low-risk patients. INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVAD, left ventricular assist device. From: Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson L, et al. Long-term mechanical circulatory support (destination therapy): On track to compete with heart transplantation? J Thorac Cardiovasc Surg. 2012;144:584‒603. © 2012 Elsevier. Reprinted with kind permission.
To further improve the outcomes of destination therapy, careful patient selection will be of paramount importance. Patients for destination therapy should not be too sick and should be offered all other heart failure treatment options including OMT, AICD and cardiac synchronisation therapy as indicated by current guidelines. Various risk scores have been implemented to predict outcomes for patients with advanced heart failure, such as the Heart Failure Survival Score, and the Seattle Heart Failure Model. These models, however, have yet to be validated for patients who will undergo destination therapy using current continuous-flow LVADs [16]. The Destination Therapy Risk Score was the first risk prediction tool which was designed to evaluate patients for destination therapy. It was developed to predict the risk of 90-day in-hospital mortality in patients on pulsatile-flow LVADs as destination therapy [17]. Data were derived from a post-market surveillance study of the pulsatile-flow HeartMate I XVE LVAD, implanted for lifetime support. Due to the lack of other risk scores for destination therapy, the use of the Destination Therapy Risk Score was eventually extended to patients implanted with continuous-flow devices as lifetime support. However, when it was evaluated in a contemporary cohort of patients with a continuous-flow device as bridge-to-transplant or destination therapy, it became apparent that its utility in such populations was very limited [18]. Therefore, improved risk prediction models derived from the current era of continuous-flow LVAD implants are in urgent need and presently in development. In addition to the severity of advanced heart failure, as reflected by the INTERMACS profiles, co-morbidities have to be considered carefully when selecting patients for destination therapy. Irreversible end-organ failure, stroke, severe chronic obstructive lung disease and cancer limiting life expectancy to less than 2 years, psychiatric disorders and lack of social support may influence outcome of lifetime support negatively and should be considered as contraindication [16, 19].
In a recent study, an attempt was made for the first time to correlate different stages of heart failure and various preoperative risk profiles with the outcomes of destination therapy patients [20]. Nearly 1300 patients registered in the INTERMACS database from 2006 to 2011 were subject of this analysis, 1160 of which were implanted with continuous-flow devices for destination therapy. Use of a pulsatile-flow device, cardiogenic shock (INTERMACS level 1), use of a right ventricular assist device, history of cancer, history of cardiac surgery, older age, larger body mass index, dialysis and increased blood urea nitrogen were identified as risk factors for mortality. When patients on continuous-flow device destination therapy were stratified by severity of heart failure according to the INTERMACS profiles, it could be shown that 1-year survival of patients with advanced, but stable heart failure (INTERMACS levels 3–7) was superior (ca. 82%) to survival of patients in INTERMACS level 1 (cardiogenic shock, ca. 62%) and INTERMACS level 2 (depending on inotropes with progressive decline, ca. 75%) (fig. 5). Based on the risk factor analysis, patients with INTERMACS levels 3–7 were further classified into groups with low risk (no risk factor, no prior cardiac surgery), medium risk (no risk factor, but prior cardiac surgery) and high risk (risk factors such as BVAD, cancer, BMI >32, BUN >50, dialysis, age >75 yrs). Survival of the low risk group at 1 and 2 years amounted to 89% and 80%, respectively, which displayed a strong trend to be superior to survival of the medium risk (81% and 65%, respectively) and high risk (77% and 72%, respectively) group (fig. 6) [20]. Thus, the outcome of continuous-flow device destination therapy patients with advanced, but stable heart failure and a low risk profile competes favourably with survival at 1 and 2 years following heart transplantation in the current era (84.4% and 80.9%, respectively) [1].
On the basis of these analyses, the triage of selected patients from transplant to destination therapy may become debatable. The profile of such patients would include advanced, heart failure in stable condition, the implant of a continuous-flow device, absence of severe right ventricular failure, preserved renal function, body mass index and age suitable for transplant, no history of cardiac surgery, and no cancer [20]. The question remains whether the predicted 2-year survival is of sufficient duration for a paradigm shift from transplant to destination therapy. Maximal follow-up of patients on continuous-flow device destination therapy is currently only exceeding little more than 2 years and there is uncertainty about long-term survival [20]. In contrast, follow-up after heart transplantation now extends to almost 30 years, with excellent long-term survival [1]. In addition to survival, other outcome measures such as quality of life and functional capacity will have to be compared between patients on continuous-flow device destination therapy and transplant patients in order to further determine the role of lifetime mechanical circulatory support in heart failure therapy. Therefore, a prospective, controlled trial is suggested randomising selected patients to heart transplantation versus destination therapy. The results of continuous-flow implants have improved sufficiently that such a study can be ethically justified. In this discussion of destination therapy versus heart transplantation, one has to keep in mind that, since the REMATCH trial was performed, there has been no randomised comparison of survival and functional status with modern LVADs against contemporary medical therapy. Such a trial would also be of particular interest, because, parallel to the advancement of LVAD technology, outcomes with ambulatory cardiac failure have improved with targeted use of medical treatment, resynchronisation therapy, and cardiac failure management.
Destination therapy in “less sick” patients before they are typical transplant candidates
The success of continuous-flow devices has raised the question, regarding if the “less sick” heart failure patients should be a target for VAD therapy [21]. However, data from current trials would not justify implantation of LVADs into patients with class III symptoms. Traditionally, destination therapy has been limited to those advanced heart failure patients who are not eligible for transplantation, have less than 2 years of life expectancy, are on maximal heart failure medication and after implantation of AICD/CRT devices as indicated. Since the outcomes of destination therapy patients have improved markedly, there is already a clear trend for a shift to implant patients with a lesser grade of heart failure for lifetime support [13]. The “less sick” patients are considered to be advanced heart failure patients who are non-inotrope dependent and ambulatory, qualifying for INTERMACS levels 4–7. The natural course of such patients, if treated medically and with resynchronisation devices, is associated with a survival at 1 and 2 years of ca. 80% and 75%, respectively [21, 22]. Destination therapy in these patients must at least reach results equal or superior to the outcome of conservative management. A retrospective, single-centre study, compared patients in INTERMACS levels 4–6 receiving VAD therapy with their predicted survival without VAD, according to the Seattle Heart Failure Model [23]. The survival at one and two years following VAD implantation reached 85% and 80%, respectively, which was superior to the Seattle Heart Failure Model predicted survival, with an absolute reduction of 27% in mortality at 2 years. In this setting, however, one has to be aware that the net benefit for the patient with respect to survival is attenuated because the natural prognosis of the disease is also more favourable [21]. Destination therapy in such patients has to prove that it also enhances quality of life and functional status significantly, compared to optimal medical therapy. In the light of these uncertainties, a prospective, randomised, controlled trial was initiated to assess the use of chronic VAD therapy in patients who are less ill than those currently eligible for destination therapy. The trial became known as the REVIVE-IT pilot trial (Randomized Evaluation of VAD InterVEntion before Inotropic Therapy) and randomises VAD versus optimal medical therapy [24]. The hypothesis is that VAD therapy may improve survival, functional status and quality of life in those advanced heart failure patients who are neither inotrope-dependent nor exercise-intolerant and have not yet developed serious consequences from heart failure such as malnourishment, end-organ damage, and immobility. The results of the trial are expected with eagerness, the estimated primary completion date will be January 2015. The trial will further elucidate the role of current VAD therapy in the management of advanced heart failure. In the context of the evolving role of VADs, it is emphasised that this highly sophisticated treatment should be performed within an integrated program of care which offers all options including optimisation of medical and resynchronisation therapy as well as heart transplantation under the guidance of a dedicated team focusing on the patients’ preferences for heart failure management.
Conclusion
The technological advancement of current continuous-flow devices, as compared to the previous pulsatile-flow pumps, has led to a marked improvement in survival of patients on support. This has caused a dramatic increase of device implants over the last 2–3 years. The growth has largely been driven by the broader application of destination therapy. A subset of patients with less severe end-stage heart failure who have a low risk profile reaches a survival at 1 and 2 years at least equal to the survival following heart transplantation. It is time to offer such patients the chance to choose between transplant and lifetime support. A prerequisite for doing so is that the patient is informed about the uncertainty of long-term outcomes on destination therapy. To obtain solid data about the true value of both destination therapy and heart transplantation in end-stage heart failure, a randomised trial of contemporary VAD systems versus transplant is required. Since results of continuous-flow destination therapy implants have improved with selecting patients at lower risk, a shift to treat patients with lifetime support in earlier and less severe stages of heart failure has begun. A randomised trial allocating long-term device therapy to patients before they would probably be considered for heart transplantation is already underway. Since the survival benefit of destination therapy in “less sick” patients may be lower than in end-stage heart failure patients, the success of continuous-flow destination therapy in this patient population will be closely associated with its ability to significantly improve quality of life and functional status, as compared to optimal medical therapy.
References
1 Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report – 2011. J Heart Lung Transplant. 2011;30:1078–94.
2 Oosterlee A, Rahmel A. Eurotransplant International Foundation – Annual report 2011. http://www.eurotransplant.org
3 Swisstransplant Jahresbericht 2011. http://www.swisstransplant.org
4 Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–43.
5 El-Banayosy A, Deng M, Loisance DY, Vetter H, Gronda E, Loebe M, et al. The European experience of Novacor left ventricular assist (LVAS) therapy as a bridge to transplant: a retrospective multi-centre study. Eur J Cardiothorac Surg. 1999;15:835–41.
6 Frazier OH, Rose EA, Oz MC, Dembitsky W, McCarthy P, Radovancevic B, et al. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg. 2001;122:1186–95.
7 Goldstein DJ. Worldwide experience with the MicroMed DeBakey Ventricular Assist Device as a bridge to transplantation. Circulation. 2003;108(suppl1):II272–7.
8 Frazier OH, Myers TJ, Gregoric ID, Khan T, Delgado R, Croitoru M, et al. Initial clinical experience with the Jarvik 2000 implantable axial-flow left ventricular assist system. Circulation. 2002;105:2855–60.
9 Schmid C, Tjan TD, Etz C, Schmidt C, Wenzelburger F, Wilhelm M, et al. First clinical experience with the Incor left ventricular assist device. J Heart Lung Transplant. 2005;24:1188–94.
10 Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–96.
11 Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–51.
12 Fang JC. Rise of the machines – Left ventricular assist devices as permanent therapy for advanced heart failure. N Engl J Med. 2009;361:2282–5.
13 Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant. 2012;31:117–26.
14 McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J. 2012;33:1787–847.
15 Boyle AJ, Ascheim DD, Russo MJ, Kormos RL, John R, Naka Y, et al. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant. 2011;30:402–7.
16 Slaughter MS, Meyer AL, Birks EJ. Destination therapy with left ventricular assist devices: patient selection and outcomes. Curr Opin Cardiol. 2011;26:232–6.
17 Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, et al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007;116:497–505.
18 Teuteberg JJ, Ewald GA, Adamson RM, Lietz K, Miller LW, Tatooles AJ, et al. Risk assessment for continuous flow left ventricular assist devices: does the destination therapy risk score work? An analysis of over 1,000 patients. J Am Coll Cardiol. 2012;60:44–51.
19 Lietz K. Destination therapy: patient selection and current outcomes. J Card Surg. 2010;25:462–71.
20 Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson L, Miller M, et al. Long-term mechanical circulatory support (destination therapy): On track to compete with heart transplantation? J Thorac Cardiovasc Surg. 2012;144:584‒603.
21 Jeevanandam V. Are we ready to implant left ventricular assist devices in “less sick” patients? Semin Thoracic Surg. 2012;24:8–10.
22 Stewart GC, Stevenson LW. Keeping left ventricular assist device acceleration on track. J Am Heart Assoc. 2011;123:1559–68.
23 Eckman P, Rosenbaum A, Vongooru H, Basraon J, Kamdar F, John R, et al. Survival of INTERMACS profile 4–6 patients after left ventricular assist device implant is improved compared to Seattle Heart Failure Model estimated survival. J Card Fail. 2011;17:S38–9.
24 Baldwin JT, Mann DL. NHLBI’s program for VAD therapy for moderately advanced heart failure: the REVIVE-IT pilot trial. J Card Fail. 2010;16;855–8.





