Figure 1
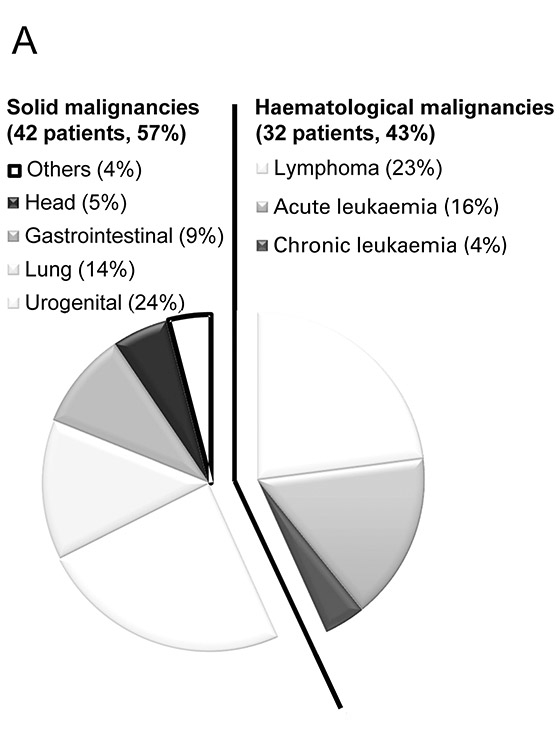
Relative prevalence of solid and haematological malignancies. Data is presented as the percentage of specific types of malignancies among the study population.
DOI: https://doi.org/10.4414/smw.2013.13741
Malignancy together with treatment related side effects cause substantial morbidity and mortality. According to the 2011 Swiss national cancer epidemiology registry, yearly 35,000 subjects out of the 8 million individuals living in Switzerland are newly diagnosed with cancer (incidence 0.44%/year) and 16,000 die yearly as a direct consequence of cancer (incidence 0.2% / year). In recent years intensified chemotherapy, transplantation of (autologous) stem cells as well as biologics such as certain antibodies, for example, have improved anti-cancer therapy but increased treatment related complications. Whether intensive care therapy should be given to patients with malignancies and the thus often unclear long term prognosis was and still is controversially discussed. Various studies have been locked into this question and have found a high in hospital mortality with only few long-time survivors [1–5]. Patients with haematological malignancy were identified to have especially poor outcome [6] and thus many studies have stratified patients with solid and haematological malignancies. Mechanical ventilation was shown to largely deteriorate outcome in haematological patients [7–10] and was found to almost always result in fatality in patients who received bone marrow- or stem cell transplantation [11–17]. This was particularly the case if the pulmonary complication occurred within fewer than 90 days after transplantation [18], or occurred after the engraftment period in the context of graft versus host disease [19]. On the other hand more recent studies have shown a noticeably better prognosis for oncology patients after intensive care therapy [1, 20]. Furthermore, several studies point out that tumour characteristics don’t necessarily have a prognostic value [6, 21, 22] and that selected patients with malignancy have ICU outcomes comparable to patients without malignancy [3].
To learn more about current practice and outcome of patients with malignancies we conducted a retrospective cohort study. In patients with solid and haematological malignancies at our institution we looked at the current base line characteristics, the therapy they received as well as their outcome. Data obtained was analysed to identify the most appropriate indicator to drive ICU treatment in regard to survival at one year after hospital admission.
The study was conducted at the medical intensive care unit (ICU) of the Zurich University Hospital, a Swiss 870 bed tertiary care referral hospital. All patients with solid or haematologic malignancies admitted within a one year window, starting January 2002 were eligible. Data on demographics, therapy applied and hospital outcomes was retrospectively extracted from data collected while treating the patient using the hospital’s electronic database (KISIMTM, Cistec®, Switzerland). Only one ICU admission per patient and time window entered analysis. Outcome data were obtained from the one year follow-up database of the ICU. For quality control and benchmark purposes, ICUs recognised by the Swiss Society of Intensive Care Medicine have to regularly deliver an anonymised minimal set of data, which includes ICU and hospital mortality. For our one year internal quality control we completed this set of data by assessing the one year mortality. This information is routinely obtained via a follow-up telephone call to the treating physician. The approval for the retrospective analyses of the data of this study was given by the ethical committee of the Department of Internal Medicine of the University Hospital Zurich and was compliant with the Declaration of Helsinki.

Figure 1
Relative prevalence of solid and haematological malignancies. Data is presented as the percentage of specific types of malignancies among the study population.
Scores were calculated based on data extracted. For baseline characterisation the simplified acute physiology score (SAPS II) according to Le Gall et al. [23] was used. SAPS II is based on the first 24 hours after ICU admission and reflects the worst performance looking at 12 physiological variables (heart rate, blood pressure, body temperature, ratio paO2/FiO2, urinary output, creatinine, leucocytes, potassium, sodium, bicarbonate, bilirubin, Glasgow coma scale) as well as patients demographics (age, reason for ICU admission, metastatic tumour, haematological tumour or AIDS). These parameters allow the calculation of an integer ranging between 0 and 163 and results in a predicted mortality that is pure statistics. The higher the score, the higher the predicted mortality.
SAPS II is neither validated nor suited to describe how the patient’s disease state evolves over time. Therefore other scores such as the sequential organ failure assessment score (SOFA) [24, 25] have been developed. The SOFA score is composed of 6 items that individually score the respiratory, cardiovascular, hepatic, coagulation, renal and neurologic system function. Each item ranges from 1 (normal organ function) to 4 (severely impaired function); items are added up resulting in SOFA scores ranging from 6 (no organ failure) to 24 (most sick) points.
Patients were SOFA scored at admission, day 3, 5 and 10 as well as on the day of ICU discharge. Scores were calculated in retrospect by adding up the number of points assigned to each item based on the worst value recorded during the specific day [26]. From SOFA scores obtained, the number of organs in failure (failing organ defined as organ specific SOFA score ≥3 [24, 25]) was analysed and evaluated for predicting outcome.
Data was anonymously manually entered into Excel (Microsoft), rearranged and analysed by the SPSS (SPSS Statistics 17.0; Chicago, Illinois) and NCSS (NCSS 2007; Kaysville, Utah) software packages which were also used for data editing. There were no missing data for baseline characteristics as well as for the hospitalisation period. However in 5 patients data collection was not completed for the one year follow up. In these cases data was entered as censored at the time point of the last observation for survival analysis but data was excluded in endpoint analysis.
Survival at one year was the primary endpoint for analysis and was defined as being alive at day 365 after hospital admission. For this endpoint we stratified the cohort in patients having either haematological or solid malignancies. We also stratified the cohort into subgroups defined by the number of organs they were found to have in failure at the specified time points. In all subgroups outcome at one year was calculated. As statistical models, we used Kaplan-Meier plots and Log rank estimates.
Second endpoints included survival at time points other than one year after hospital admission, as well as descriptive analysis of patient characteristics, therapy delivered and complications or outcomes in general or concerning their malignancies (i.e. haematological as compared to solid malignancies).
To analyse these endpoints we used parametric (Wilcoxon) analysis for ordinal parameters split up in no more than two groups and parametric Kruskal-Wallis One-Way ANOVA on ranks followed by pairwise comparison when more than two groups of ordinal data were compared, chi-squared tests for categorical outcomes or proportions or Fisher’s exact test in case of n <5. Calculation of sensitivity and specificity together with plotting receiver operator curves and calculation of the area under the curve (AUCROC) was used to analyse test performance. Tests used are specified together with p-values obtained.
| Table 1: Baseline characteristics: | ||||
| Type of malignancy | All | Solid1 | Haematological2 | Pvalue3 |
| Demographics | ||||
| Nbr. of Patients (%) | 74 | 42 (57) | 32 (43) | |
| Age, median yrs (IQR) | 62 (50-71) | 66 (57-73) | 56 (44-65) | 0.008 4 |
| Male (%) | 38 (51) | 24 (57) | 14 (44) | 0.3645 |
| SAPS, median (IQR) | 43 (29-64) | 39 (28-53) | 45 (29-74) | 0.2024 |
| SOFA median (IQR) | 6 (4-11) | 5 (3-9) | 8 (5-11 ) | 0.039 4 |
| Disease status | ||||
| Newly diagnosed7 (%) | 33 (45) | 19 (45) | 14 (44) | 0.8995 |
| Progression / relapse (%) | 28 (38) | 19 (45) | 9 (28) | 0.2075 |
| Stable / remission8 (%) | 13 (18) | 4 (10) | 9 (28) | 0.0626 |
| Antineoplastic therapy | ||||
| Chemotherapy (%) | 56 (76) | 24 (57) | 32 (100) | 0.001 6 |
| Days before ICU (IQR) | 14 (2-101) | 33 (1-289) | 13 (3-28) | 0.045 4 |
| SCT, BMT 9 | 6 (19) | |||
| Month before ICU (IQR) | 5.7 (2.7-20) | |||
| 1 Urogenital (n=18; 43%), lung (n=10; 24%), gastrointestinal (n=7; 17%), head (n=4; 10%), others (n=3; 7%); see also Figure 1 2 Lymphoma (n=17; 53%), acute leukemia (n=12; 38%) and chronic leukemia (n=3; 9%); see also Figure 1 3 Comparing patient groups suffering from solid and hematologic malignancies 4 Pvalue calculated using Wilcoxon Rank-Sum Test 5 Pvalue calculated using CHI-squared test 6 Pvalue calculated using Fisher's exact test 7 Newly diagnosed within the last 3 months before ICU admission 8 Absence of progression of malignancy, partial or complete remission 9 SCT (Autologous stem cell transplant); BMT (Bone Marrow Transplantation) | ||||
| Table 2:Leading indication for ICU admission: | ||||
| Type of malignancy | All | Solid* | Haematological* | P value* |
| Nbr. of patients (%) | 74 | 42 (57) | 32 (43) | |
| Cardiovascular1 (%) | 29 (39) | 23 (55) | 6 (19) | 0.004 2 |
| ACS (%) | 11 (15) | 9 (21) | 2 (6) | 0.1003 |
| Cardiac arrest (%) | 5 (7) | 3 (7) | 2 (6) | 0.9993 |
| Congestion (%) | 13 (18) | 10 (24) | 3 (9) | 0.1323 |
| Acute respiratory failure4 (%) | 9 (12) | 4 (10) | 5 (16) | 0.4883 |
| lnfection5 (%) | 13 (18) | 1 (2) | 12 (38) | 0.001 3 |
| Pneumonia (%) | 7 (9) | 1 (2) | 6 (19) | 0.038 3 |
| SIRS / septic shock (%) | 6 (8) | 0 (0) | 6 (19) | 0.005 3 |
| Neurological deterioration (%) | 6 (8) | 4 (10) | 2 (6) | 0.6923 |
| Postoperative monitoring (%) | 8 (11) | 4 (10) | 4 (13) | 0.7253 |
| Other6 (%) | 9 (12) | 6 (14) | 3 (9) | 0.7203 |
| Organ dysfunction | ||||
| Respiratory SOFA ≥ 2 (%) | 55 (74) | 31 (74) | 24 (75) | 0.9082 |
| Cardiovascular SOFA ≥ 1 (%) | 48 (65) | 28 (67) | 20 (63) | 0.6032 |
| Renal SOFA ≥ 1 (%) | 44 (59) | 26 (62) | 18 (56) | 0.8012 |
| Hepatic SOFA ≥ 1 (%) | 26 (35) | 10 (24) | 16 (50) | 0.036 2 |
| Cerebral SOFA ≥ 2 (%) | 17 (23) | 9 (21) | 8 (25) | 0.9342 |
| * Specifications cf table 1 1 all cardiovascular indications not related to SIRS / sepsis / septic shock 2 Pvalue calculated using CHI-squared test 3 Pvalue calculated using Fisher's exact test 4 excluding pneumonia und cardiac lung edema 5 including SIRS, sepsis, septic shock und pneumonia 6 renal and gastrointestinal indications, interventions and others | ||||
Within the predefined 12 month period, a total of 74 patients (median age 62 years) with underlying malignancies were admitted to the ICU and entered our data analysis (table 1). Of those, 42 (57%) suffered from solid and 32 (43%) form haematological malignancies. Urogenital origin was most common among solid malignancies (18 patients; 24%), whereas lymphoma (17 patients; 23%) was most prevalent among haematological malignancies (fig. 1). At ICU admission, patients with haematological malignancies were significantly younger (median age of 56 versus 66 years; p= 0.08), had a higher SOFA scores (8 versus 5; p= 0.039) and were more likely to be on chemotherapy or have had recent chemotherapy as compared to those with solid malignancies (100% of the patients versus 57%; p<0.001). In either type of malignancy around half of the patients had just been diagnosed with the malignancy (table 1).
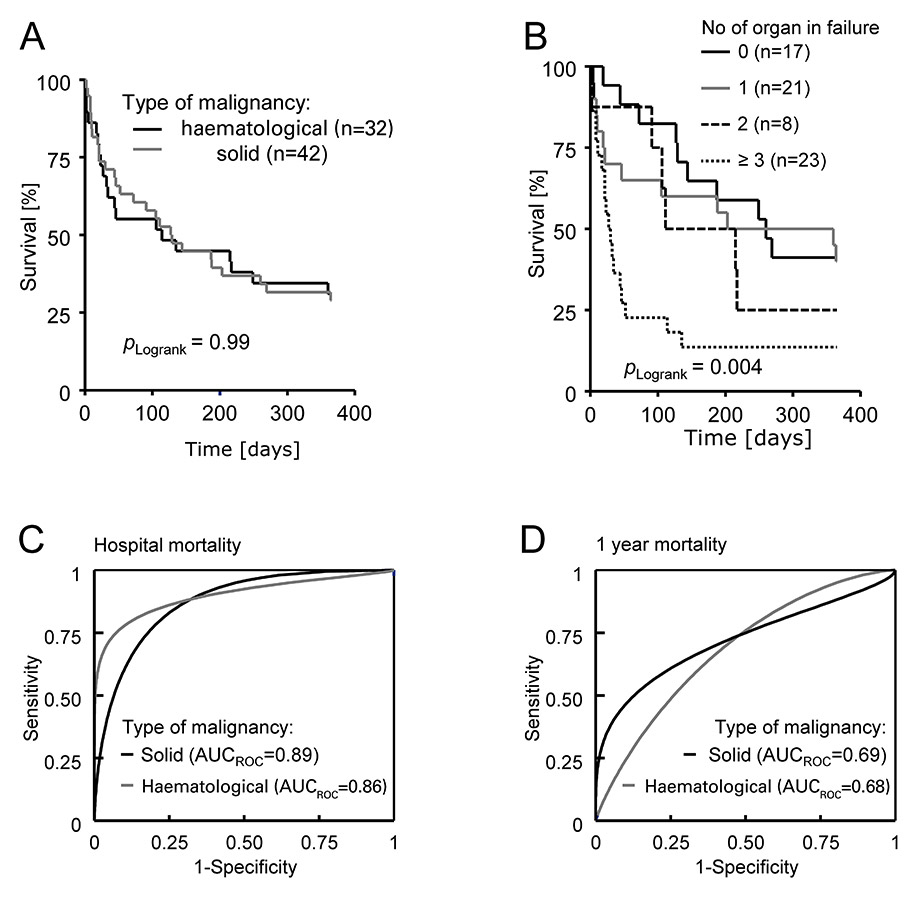
Figure 2
Survival analysis of patients with malignancies. (A) One year Kaplan-Meier survival curve stratified by the type of malignancy. Log rank comparison between groups yielded P = 0.99. (B) Survival plot stratified by the number of organs in failure. Log rank comparison between groups yielded P = 0.004 (C) Receiver operator curve stratified by the type of malignancy (solid, in black; haematological, in grey) analysing the number of organs in failure for predicting hospital fatality. AUCROC= 0.86 for haematological and 0.89 for solid malignancies. (D)Receiver operator curve stratified by the type of malignancy (solid, in black; haematological, in grey) analysing the number of organs in failure for predicting 1 year fatality. AUCROC = 0.68 for haematological and 0.69 for solid malignancies.
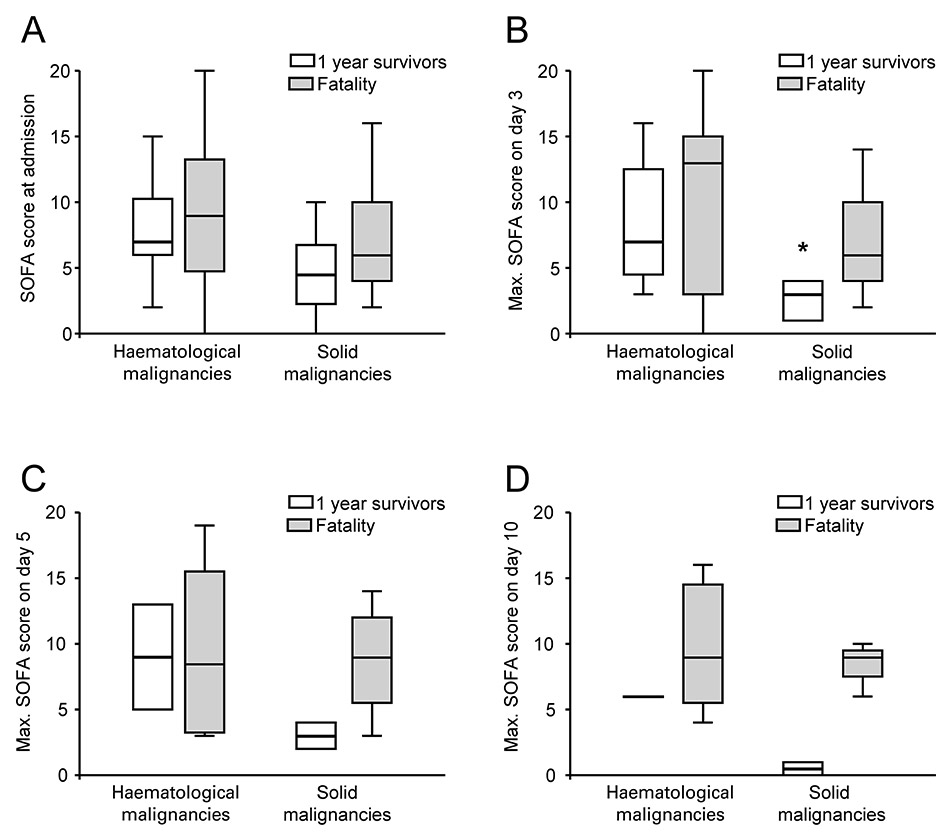
Figure 3
Admission SOFA scores and maximal SOFA scores obtained on day 3, 5 and 10 were stratified by the type of malignancy and survival status at one year. Data are given as box plots (showing median, line; central 50%, box and 2.5 and 97.5 percentile, whiskers) with individual panels for the day of admission (A), day 3 (B), 5 (C) and 10 (D) on the ICU. Parametric ANOVA on ranks revealed group differences of p= 0.008 (B) and p= 0.044 (C) with * significant post hoc group comparison (Bonferroni) at the p= 0.05 level.
The ultimate reason for referral to the ICU was most frequently a cardiovascular event in patients with a solid malignancy (55% of the admissions) which was significantly less common in haematological patients (19%; p= 0.004). In contrast in haematological patients, infection was most frequently the reason for ICU admission (38% of the admissions) and was significantly less common in patients with solid malignancies (2%; p= 0.001). Pneumonia (19% versus 2%, p = 0.047) and sepsis (0% versus 19%, p = 0.009) were also significantly more prevalent among patients with haematological malignancies (table 2). The distribution of organ specific SOFA scores was comparable in either malignancy group except for the significantly more frequent hepatic disorders found in patients with a haematological malignancy (50% versus 24%, p = 0.036) when compared to solid malignancies (table 2).
There was a trend for patients with haematological malignancies to stay longer on the ICU which became significant (median 15 compared to 32 days; p= 0.002) for the total hospital stay as compared to patients with solid malignancies (table 3). Consistent with their longer hospital stays, they had significantly less common single organ failure (34% versus 71%; p= 0.002) but 3 or more organs in failure more frequently (53% versus 17%, p= 0.002; table 3). Patients with haematological malignancies were more frequently on a ventilator (75% as compared to 36% in patients with solid malignancy; p= 0.002), on vasopressors (66% and 33% respectively; p= 0.012) or received renal replacement therapy (34% and 10% respectively; p= 0.019) table 3). Nevertheless in either group similarly few patients had an uncomplicated ICU stay (26% in solid and 34% in haematological malignancy). Complications during the ICU stay occurred in patients with either type of malignancy at comparable levels except for significantly more cases of ventilator associated pneumonia in patients with haematological malignancy (38% versus 7%; p= 0.0034; table 3).
There were no significant differences in mortality over the observation period between patients with haematological and solid malignancies (fig. 2A). Overall ICU mortality was 26% (19/74) with 17% (7/42) in solid and 37% (12/32) in haematological malignancies. There were an additional 7 deaths on the ward after ICU demission resulting in a hospital mortality of 35% (26/74) for all patients; 29% (12/42) for patients with solid, and 44% (14/32) for haematological malignancy respectively. One year follow up was biased by the failure of follow up in 5 patients. From these remaining 69 patients 29% (20/69) were alive after one year, 31% (12/39) with solid and 27% (8/30) with haematological malignancies. Survival was significantly linked to the number of organs in failure (fig. 2B) while under intensive care therapy. The curves split according to the number of organs in failure within the first 100 days. Since the predictive value of a parameter tested at a specific time point can be estimated by calculation of the area under the curve (AUC) in receiver operator curves (ROC), we calculated AUCROC for the number of organs in failure for predicting hospital (fig. 2C) and 1 year (fig. 2D) fatality in patients with solid or haematological malignancies. The number of failing organs predicted hospital fatality with an AUCROC of 0.87 overall and of 0.86 for patients with solid and 0.89 for patients with haematological malignancies, whereas prediction was weaker for one year mortality (0.69 for patients with solid and 0.68 for patients with haematological malignancies). In order to corroborate this data we calculated sensitivity and specificity for specific cut off values. These are listed in table 4.
Serial evaluation of total SOFA scores was proposed to predict outcome in critically ill patients [26]. We calculated SOFA scores at admission and for the patients remaining on the ICU at day 3, 5 and 10 and analysed survival at one year (fig. 3A-D). Parametric analysis of variance yielded significantly different SOFA scores among the 4 groups on day 3 and 5. Post hoc group comparisons on single days showed a significantly lower SOFA score for one year survivors suffering from haematological malignancy, for analyses on days 5 and 10 the study was underpowered.
New therapeutic options in oncology significantly prolong survival and quality of life for many patients with malignancies but can also cause complications requiring intensive medical care treatment [27]. As a result, increasing numbers of patients have to make a decision for or against intensive care treatment with physicians supporting and advising them. However intensivists have been shown to often inaccurately predict outcome, especially in patients suffering from haematological malignancies [28] and the reliability of admission scores is also discussed controversially [3, 29–31] making the decision on whom to refer to critical care therapy difficult. The more and more stringent economic environment together with findings that patients with malignancies consume more critical care resources than non-oncological patients [31] further complicate such decisions. To review our local policy and to test for reliability of scores in our specific setting, we retrospectively analysed patients with malignancies in our single institution. We assessed baseline characteristics, indications for ICU admission, therapy provided together with complications occurring on the ICU as well as survival over a one year period.
As in earlier studies [11–17], the main reason for ICU admission in patients with haematological malignancies was infection. Infections were mainly pulmonary and most frequent in patients requiring mechanical ventilation (75% of the patients). Since infections frequently result in sepsis they also explain the frequent need for vasopressors, renal replacement therapy and occurrence of liver dysfunction. In contrast to earlier studies [3], we report higher SOFA scores on average in haematological compared to solid cancer patients. Nevertheless we found that the two groups had comparable outcomes despite haematological patients having higher SOFA scores. These were in part caused by significantly more frequent liver dysfunction in haematological malignancies, a condition linked to especially poor prognosis [6]. We did not observe worse outcomes in haematoligical as compared to solid malignancy patients. One possible explanation is the significant age difference (median age of haematological patients was 10 years younger than patients with solid malignancies). Age has been shown to heavily impact outcome in critically ill patients with malignancies [32]. Whether specific referral policies by haematologists further introduced a selection bias in our cohort cannot be clarified in our retrospective single institution study.
Our study confirms earlier findings [5, 6, 22, 33–36] showing a highly predictive value of the numbers of organs in failure in predicting outcome. Especially patients with 3 or more organs in failure were found to have a poor outcome in terms of hospital and one year survival. Assessment of the organs in failure is thus a helpful predictor for survival in patients already admitted to the ICU. We found that the number of organs in failure was better at predicting survival at hospital discharge than at one year. Loss of accuracy over time could be explained by fatalities caused by the underlying malignancy and not by the acute condition that resulted in ICU admission. The constant and high mortality observed after day 100 of hospitalisation and even in patients with no or one organ in failure supports this hypothesis. In contrast to scoring the maximal organs in failure, SOFA scores made on admission and subsequent days was not suited to predict outcome in our cohort, as reported earlier and for admissions scores [3, 29, 31].
The relatively small patient population is a major limitation of our study as well as the retrospective character that cannot control for selection bias. Similar to previous studies [1–6, 31] we cannot estimate in how many critically sick patients referral to the ICU was not desired, not evaluated or referral was ultimately denied. Thus our observations and conclusion might only be applied to patients already referred to an ICU but cannot help to decide who should be referred to a critical care unit. Further research will be required to better guide such decisions.
In conclusion we show that overall mortality was high in patients with malignancies but that one quarter of the patients survived for more than one year. Whereas admission SOFA score did not allow identification of patients with poor outcome, the number of organs in failure allowed the prediction of hospital fatality and one year survival in both solid and haematological malignancies. Accordingly we suggest that in addition to the diagnosis of malignancy and the potentially curative therapy applied, the severity of illness quantified as number of organs in failure should also tailor therapy and guide ICU management in the future.
| Table 3: Course during ICU stay: | |||
| Type of malignancy | Solid* | Hematologic* | P value* |
| Patients (%) | 42 (57) | 32 (43) | |
| LOS ICU in days, (IQR) | 3 (2-4) | 4 (2-12) | 0.0691 |
| LOS hospital (IQR) | 15 (8-23) | 32 (18-80) | 0.002 1 |
| Number of organ(s) in failure | |||
| 0-1 (open bar) | 0.002 2 | ||
| 2 (gray bar) | 0.9993 | ||
| ≥ 3 (black bar) | 0.002 2 | ||
| Organ failure support [solid open, hematologic malignancies closed bar; data given as %]4 | |||
| Mechanical ventilation | 0.002 2 | ||
| Vasopressor support | 0.012 2 | ||
| Renal replacement therapy | 0.017 3 | ||
| Complications on ICU [solid open, hematologic malignancies closed bar; data given as %]4 | |||
| Cardiovascular5 | 0.4662 | ||
| cardiac lung edema | 0.3713 | ||
| ACS | 0.4003 | ||
| lnfection | 0.1092 | ||
| Pneumonia | 0.026 3 | ||
| SIRS, sepsis, septic shock | 0.1322 | ||
| lnfection during aplasia | 0.1593 | ||
| Neurological deterioration | 0.5742 | ||
| Renal (incl. ARF due to sepsis) | 0.1962 | ||
| Respiratory6 | 0.2472 | ||
| Others (GIT, metabolic) | 0.1552 | ||
| * Specifications cf lable 1 1 Pvalue calculated using Wilcoxon Rank-Sum Test 2 Pvalue calculated using CHI-squared test 3 Pvalue calculated using Fisher's exact test 4 Organ failure (=SOFA score ≥ 3) within day 1- 10 on ICU 5 Al cardiovascular complications not relaled to SIRS / sepsis / septic shock 6 Excluding pneumonia and cardial induced lung edema | |||
| Table 4:Prediction of Fatality by number of organs in failure1: | ||||
| Prediction | Sensitivity | Specificity | ||
| Type of malignancy | Solid* | Hematologic* | Solid* | Hematologic* |
| 0organ in failure | 1 | 1 | ||
| 1organ in failure | 1 | 1 | 0.53 | 0.22 |
| 2organs in failure | 0.67 | 0.93 | 0.86 | 0.56 |
| 3organs in failure | 0.58 | 0.93 | 1 | 0.78 |
| 4organs in failure | 0.25 | 0.71 | 1 | 0.83 |
| 5organs in failure | 0.08 | 0.36 | 1 | 0.89 |
| > 5organs in failure | 0.21 | 1 | 1 | |
| 1 Data basing receiver operator curve shown in Figure 1C * Specifications cf table 1 | ||||
1 Kress JP, Christenson J, Pohlman AS, Linkin DR, Hall JB. Outcomes of critically ill cancer patients in a university hospital setting. Am J Respir Crit Care Med. 1999;160:1957–61.
2 Groeger JS, Lemeshow S, Price K, Nierman DM, White P, Jr., Klar J, et al. Multicenter outcome study of cancer patients admitted to the intensive care unit: A probability of mortality model. J Clin Oncol. 1998;16:761–70.
3 Staudinger T, Stoiser B, Mullner M, Locker GJ, Laczika K, Knapp S, et al. Outcome and prognostic factors in critically ill cancer patients admitted to the intensive care unit. Crit Care Med. 2000;28:1322–8.
4 Maschmeyer G, Bertschat FL, Moesta KT, Hausler E, Held TK, Nolte M, et al. Outcome analysis of 189 consecutive cancer patients referred to the intensive care unit as emergencies during a 2-year period. Eur J Cancer. 2003;39:783–92.
5 Soares M, Salluh JI, Spector N, Rocco JR. Characteristics and outcomes of cancer patients requiring mechanical ventilatory support for >24 hrs. Crit Care Med. 2005;33:520–6.
6 Evison J, Rickenbacher P, Ritz R, Gratwohl A, Haberthur C, Elsasser S, et al. Intensive care unit admission in patients with haematological disease: Incidence, outcome and prognostic factors. Swiss Med Wkly. 2001;131:681–6.
7 Kroschinsky F, Weise M, Illmer T, Haenel M, Bornhaeuser M, Hoeffken G, et al. Outcome and prognostic features of intensive care unit treatment in patients with hematological malignancies. Intensive Care Med. 2002;28:1294–300.
8 Benoit DD, Depuydt PO, Vandewoude KH, Offner FC, Boterberg T, De Cock CA, et al. Outcome in severely ill patients with hematological malignancies who received intravenous chemotherapy in the intensive care unit. Intensive Care Med. 2006;32:93–9.
9 Schapira DV, Studnicki J, Bradham DD, Wolff P, Jarrett A. Intensive care, survival, and expense of treating critically ill cancer patients. JAMA. 1993;269:783–6.
10 Groeger JS, White P, Jr., Nierman DM, Glassman J, Shi W, Horak D, et al. Outcome for cancer patients requiring mechanical ventilation. J Clin Oncol. 1999;17:991–7.
11 Epner DE, White P, Krasnoff M, Khanduja S, Kimball KT, Knaus WA. Outcome of mechanical ventilation for adults with hematologic malignancy. J Investig Med. 1996;44:254–60.
12 Huaringa AJ, Leyva FJ, Giralt SA, Blanco J, Signes-Costa J, Velarde H, et al. Outcome of bone marrow transplantation patients requiring mechanical ventilation. Crit Care Med. 2000;28:1014–7.
13 Faber-Langendoen K, Caplan AL, McGlave PB. Survival of adult bone marrow transplant patients receiving mechanical ventilation: A case for restricted use. Bone Marrow Transplant. 1993;12:501–7.
14 Price KJ, Thall PF, Kish SK, Shannon VR, Andersson BS. Prognostic indicators for blood and marrow transplant patients admitted to an intensive care unit. Am J Respir Crit Care Med. 1998;158:876–84.
15 Soubani AO, Kseibi E, Bander JJ, Klein JL, Khanchandani G, Ahmed HP, Guzman JA. Outcome and prognostic factors of hematopoietic stem cell transplantation recipients admitted to a medical icu. Chest. 2004;126:1604–11.
16 Khassawneh BY, White P, Jr., Anaissie EJ, Barlogie B, Hiller FC. Outcome from mechanical ventilation after autologous peripheral blood stem cell transplantation. Chest. 2002;121:185–8.
17 Bach PB, Schrag D, Nierman DM, Horak D, White P, Jr., Young JW, et al. Identification of poor prognostic features among patients requiring mechanical ventilation after hematopoietic stem cell transplantation. Blood. 2001;98:3234–40.
18 Ewig S, Torres A, Riquelme R, El-Ebiary M, Rovira M, Carreras E, et al. Pulmonary complications in patients with haematological malignancies treated at a respiratory icu. Eur Respir J. 1998;12:116–22.
19 Pene F, Aubron C, Azoulay E, Blot F, Thiery G, Raynard B, et al. Outcome of critically ill allogeneic hematopoietic stem-cell transplantation recipients: A reappraisal of indications for organ failure supports. J Clin Oncol. 2006;24:643–9.
20 Azoulay E, Alberti C, Bornstain C, Leleu G, Moreau D, Recher C, et al. Improved survival in cancer patients requiring mechanical ventilatory support: Impact of noninvasive mechanical ventilatory support. Crit Care Med. 2001;29:519–25.
21 Ferra C, Marcos P, Misis M, Morgades M, Bordeje ML, Oriol A, et al. Outcome and prognostic factors in patients with hematologic malignancies admitted to the intensive care unit: A single-center experience. Int J Hematol. 2007;85:195–202.
22 Darmon M, Thiery G, Ciroldi M, de Miranda S, Galicier L, Raffoux E, et al. Intensive care in patients with newly diagnosed malignancies and a need for cancer chemotherapy. Crit Care Med. 2005;33:2488–93.
23 Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (saps ii) based on a european/north american multicenter study. JAMA. 1993;270:2957–63.
24 Moreno R, Vincent JL, Matos R, Mendonca A, Cantraine F, Thijs L, et al. The use of maximum sofa score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working group on sepsis related problems of the esicm. Intensive Care Med. 1999;25:686–96.
25 Moreno R, Vincent JL, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The sofa (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med. 1996;22:707–10.
26 Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the sofa score to predict outcome in critically ill patients. JAMA. 2001;286:1754–8.
27 Buchheidt D, Hummel M, Engelich G, Hehlmann R. Management of infections in critically ill neutropenic cancer patients. J Crit Care. 2004;19:165–73.
28 Thiery G, Azoulay E, Darmon M, Ciroldi M, De Miranda S, Levy V, et al. Outcome of cancer patients considered for intensive care unit admission: A hospital-wide prospective study. J Clin Oncol. 2005;23:4406–13.
29 den Boer S, de Keizer NF, de Jonge E. Performance of prognostic models in critically ill cancer patients – a review. Crit Care. 2005;9:R458–63.
30 Lamia B, Hellot MF, Girault C, Tamion F, Dachraoui F, Lenain P, et al. Changes in severity and organ failure scores as prognostic factors in onco-hematological malignancy patients admitted to the icu. Intensive Care Med. 2006;32:1560–8.
31 Merz TM, Schar P, Buhlmann M, Takala J, Rothen HU. Resource use and outcome in critically ill patients with hematological malignancy: A retrospective cohort study. Crit Care. 2008;12:R75.
32 Soares M, Carvalho MS, Salluh JI, Ferreira CG, Luiz RR, Rocco JR, et al. Effect of age on survival of critically ill patients with cancer. Crit Care Med. 2006;34:715–21.
33 Silfvast T, Pettila V, Ihalainen A, Elonen E. Multiple organ failure and outcome of critically ill patients with haematological malignancy. Acta Anaesthesiol Scand. 2003;47:301–6.
34 Sculier JP, Paesmans M, Markiewicz E, Berghmans T. Scoring systems in cancer patients admitted for an acute complication in a medical intensive care unit. Crit Care Med. 2000;28:2786–92.
35 Azoulay E, Moreau D, Alberti C, Leleu G, Adrie C, Barboteu M, et al. Predictors of short-term mortality in critically ill patients with solid malignancies. Intensive Care Med. 2000;26:1817–23.
36 Blot F, Guiguet M, Nitenberg G, Leclercq B, Gachot B, Escudier B. Prognostic factors for neutropenic patients in an intensive care unit: Respective roles of underlying malignancies and acute organ failures. Eur J Cancer. 1997;33:1031–7.
Funding / potential competing interests: Swiss National Science Foundation subsidy (#PZ00P3_136639/1) to RAS / no competing interests to be declared.