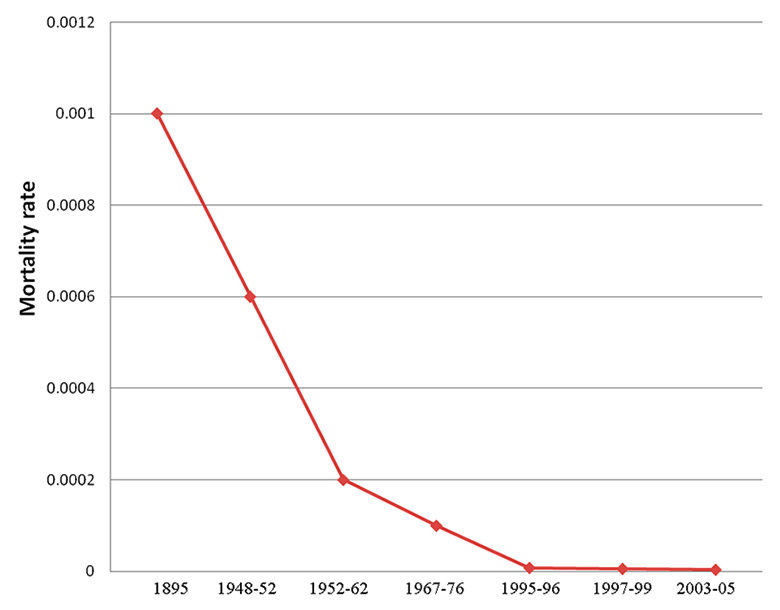
Figure 1
Historical evolution of improvements in anaesthesia-related mortality.
DOI: https://doi.org/10.4414/smw.2013.13770
In December 1999, the report of the Institute of Medicine (IOM) in America, “To err is human”, raised the alarm to the public that medical care could cause harm. It concluded that medical errors were responsible for up to 98,000 deaths and 1 million injuries each year in the United States [1]. Although extrapolations should be made with caution [2], related to the population of Switzerland, this would represent approximately 2,100 patients dying each year as a result of a medical error.
Such figures are appalling, and since the publication of the report, governmental bodies, hospitals and nonprofit organisations in many countries launched major initiatives to improve the safety of patient care [2–4]. Ten years later questions about the effectiveness of these quality improvement initiatives were raised, and a new study was performed in North Carolina [5]. The study conclusions were that, despite efforts, harm to patients remains common (18.1%) and that not all quality improvement interventions seem to be effective. Therefore, there is a need for a better understanding of effective approaches that are able to minimise risk and improve the overall safety of patient care.
Since the early beginnings of anaesthesia, anaesthetists have always been concerned with patient safety. They have developed a range of risk management strategies and significantly improved the safety record of anaesthesia. The specialty is often cited as a role model for its achievements in the field of patient safety improvements [6–7]. Analysing these strategies can provide clues as to the most effective approaches to improve overall safety of patient care.
This review will first define the concept of safety and how it should be understood in the context of patient care. It will then detail improvements made in anaesthesia, providing findings from landmark studies from the last 50 years. It will then identify the most common risk minimisation strategies used, according to a widely embraced model for accident prevention [8]. Future perspectives on patient safety improvements in healthcare will conclude the review.
Many meanings for the concept of patient safety have been provided; some emphasise the importance of harm caused to the patient by medical care and others see human error as a main contributor to patient harm within complex organisations [9–10]. As a result the concept includes several perspectives such as patient-, medication- and procedure-related injuries, as well as human errors and their occurrence within multifaceted organisational settings. All these dimensions have been nicely summarised by Cooper et al. in a consensus definition stating that patient safety is “the avoidance, prevention, amelioration of adverse outcomes or injuries stemming from the processes of health care. Patient safety should address events that span the continuum from what may be called errors and deviations to accidents. Patient safety is a subset of healthcare quality” [11].
The following section will provide results of prominent epidemiological studies in anaesthesia from recent past decades, showing the evolution of patient injuries related to anaesthesia care and human error.
The study of anaesthesia-related mortality, as this outcome has been systematically analysed since the early beginning of the specialty, provides an interesting example of the evolution of patient safety in anaesthesia. However, these figures need to be interpreted with caution as there are some methodological limitations to the analysis. The first is a wide variety of definitions of anaesthesia-related mortality. For some authors, this term includes mainly perioperative death to which human error on the part of the anaesthesia provider has contributed [12–13]. For others, anaesthesia-related mortality refers to all potential causes of deaths occurring during or following anaesthesia, including those associated with both anaesthetic and surgical factors [14–15]. There is also a lack of consensus about the overall period of time after anaesthesia that defines anaesthetic mortality. Depending on the study, this period can vary from 24 hours to 30 days after an anaesthetic procedure. As a result, there is some controversy about the extent of improvement made by the specialty [16–17]. However, the historical analysis of the incidence of anaesthesia-related mortality, defined as patients dying under or following the care of an anaesthetist, provides compelling figures.

Figure 1
Historical evolution of improvements in anaesthesia-related mortality.
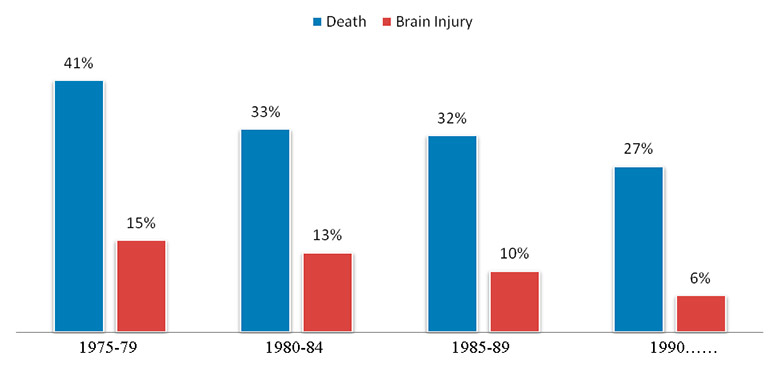
Figure 2
Claims for death and brain injuries as a proportion of all claims recorded.
At the end of the 19th century, for example, 1/900 patients died as a result of their anaesthesia [18]. In the late 1950s, anaesthesia-related mortality was much lower at 1/1560 to 1/3966 [19–20]. In the sixties, it decreased to 1/6789 [21]. However, major improvements were mainly observed during recent past decades, with mortality figures continuously dropping to 1/124,212 in the nineties [12], and to 1/146,341 five years later [22], reaching 1/249,321 in 2005 [23]. This represents a 100-fold decrease in the anaesthesia mortality rate between the 1950s and today Therefore, anaesthesia is often cited as the only speciality in healthcare to have reached the 6 sigma defect rate, which is used to describe a 99.99966% defect-free process (3.4 defects per million] and is often seen as the critical target to be reached by any manufacturing process or transport industry [24]. Figure 1 summarises the evolution of anaesthesia-related mortality from the end of the nineteenth century to 2005.
Anaesthesia-related morbidity, which includes all injuries caused by anaesthesia (except death), has largely followed the same trend. Even though data on morbidity are less trustworthy than mortality data (those most currently used are based on closed claims files), they clearly show that the rate of complaints for brain, heart and nerve damage has significantly decreased in the United States in the last thirty to forty years, as illustrated in figure 2 [25]. For example, complaints for injuries such as cardiac arrests following neuraxial anaesthesia have halved between 1970 and 1990 [26]. The same is true for awareness during obstetric anaesthesia, which decreased from 1.3% to 0.4% between 1982 and 1989 [27]. Claims for brain damage represented 15% of all claims in 1975, 13% in 1980, 10% in 1985 and 6% in 1990 [25, 28]. Data from the National Audit Projects (NAP) in the UK and from Swiss closed claims show, however, that brain and nerve injuries associated with difficult intubation and locoregional anaesthesia, respectively, still remain a challenge that has to be addressed [29–30].
These successes have been largely attributed to the systematic introduction of pulse oximetry into operating theatres in the late seventies, which allowed anaesthetists to measure hypoxaemia very early (before clinical signs appeared) and to respond appropriately [31–32]. However, with more careful analysis of the overall picture of patient safety improvements made in anaesthesia, it becomes evident that pulse oximetry is only a small part of a comprehensive risk management strategy developed by the specialty. It includes all categories of tools available to minimise human error and risk for patients. These categories of tools can be considered part of a hierarchical model as developed by industrial safety experts and known as the safety hierarchy model.
The model can be represented as a five step approach to minimise risk and prevent accident, from the most to the least effective strategy. It is described in figure 3.
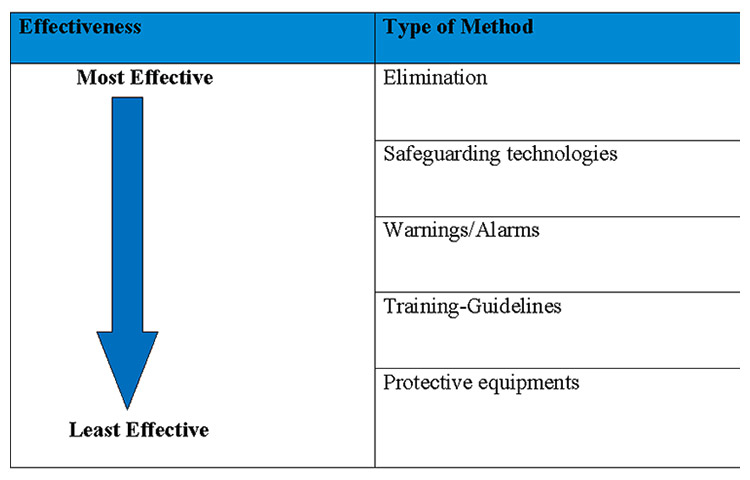
Figure 3
Safety hierarchy model.
The first and most effective approach is to remove the hazardous factor itself. A good example is tobacco smoking. Eliminating tobacco from restaurants and cafes has resulted in fewer hospitality workers and customers being exposed to passive smoking. The second approach is the use of safeguarding technologies. Such technologies have been widely developed in the transport industry, for example in trains. At regular intervals, a train driver needs to press on a specific button to maintain the engine function. If he does not, after a short time the train will automatically stop. This avoids accidents caused by a train driver falling asleep.
The third strategy is warning signs and alarms. This is one of the most commonly used methods to guarantee the safety of road traffic. Streets, motorways and country roads are full of warning signs, raising drivers’ awareness of potential hazards such as cross-roads, sharp curves or animal crossings. The fourth strategy relies on training and procedures. A good example of this approach is the driving license. It includes a specific training period complemented by the learning of specific rules. Finally, the last and least effective method is the use of personal protective equipment, such as helmets on motorcycles or life-jackets on boats. Both have a protective effect, but in the case of major crashes, they offer little protection.
All categories of methods included in the safety hierarchy model have been systematically used for decades by anaesthetists to minimise the risk of anaesthetic procedures for patients. Some of the most well-known strategies are summarised in table 1.
Throughout the development of the specialty, anaesthetists have carefully selected drugs and techniques that are easy and safe to use. Hazardous medications or procedures, identified by systematic audit of practice, have been progressively eliminated [33–34]. This is how halothane has come to be replaced by less hepatotoxic halogenated drugs, such as isoflurane or sevoflurane, and general anaesthesia replaced by neuraxial anaesthesia techniques in the field of obstetrics, to limit the risk of bronchoaspiration by pregnant women [35–37].
A large number of safeguarding technologies have also been developed since the late seventies. The first targeted the delivery system for anaesthesia, the anaesthetic machine. These included interlocks to prevent the delivery of hypoxic gas mixtures, noninterchangeable oxygen / nitrous oxide connections, and an emergency oxygen supply on anaesthetic machines [38]. Others targeted the drug administration process, with the development of prefilled syringes, avoiding the risk of errors in drug preparation [39]. There is currently also a debate about the introduction of ISO 80369 standards which would prevent inadvertent connection of, for instance, a feeding tube with liquid nutrients to an intravenous (iv) line. Each catheter or line (iv, intrathecal, nasogastric] would have specific male and female components of non-Luer connectors that join specifically together but do not allow the connections to be interchanged (e.g. iv to intrathecal) [40].
Many important innovations have also been developed in the area of warnings/alarms. Monitoring systems, such as capnography and pulse oximetry, have allowed anaesthetists to detect oesophageal intubation or hypoxia, two frequent and major anaesthetic risks [31]. Awareness is another anaesthetic hazard that can nowadays be measured using portable monitoring system that integrates into a unique value, the bispectral index, the many measurements derived from an electroencephalogram [41]. Additional steps included the use of colour labels for syringes, oxygen and other gas components to minimise the risk of drug confusion [42–43]. Other developments in the area of training-guidelines, such as crisis management, were instituted in the 1990s in order to expose anaesthetists to rare but life-threatening events, like difficult airway management [44]. The systematic use of guidelines and monitoring systems have become a standard of anaesthetic practice, which has been recently reemphasised by the European Society of Anaesthesiology in its Helsinki declaration on patient safety [45]. Finally, injuries to patients for which none of these strategies could be used have been addressed through the use of direct patient personal protection measures such as closing the eyelids with tape and re-covering them with plastic eye covers, using bite blocks to prevent tongue or orotracheal tube bites, and protecting against complications such as pressure ulcers and nerve damage with arm, head and body protective foam [46].
Since the publication of the IOM report, many other specialties have started to address patient safety issues using the methods described in the safety hierarchy model, particularly training/guidelines and safeguarding technologies. Some examples include: computerised provider order-entry systems to reduce the risk of drug prescription errors [47]; strict policies for standardisation of hand washing and disinfection methods to minimise the rate of nosocomial infection during peripheral or central venous catheter line insertion [48–49]; and patient safety checklists before surgery to reduce the rate of postoperative mortality [50]. All these strategies have a demonstrated effectiveness and their comprehensive use, as in anaesthesia, results in a clear and measurable improvement in the overall safety of patient care.
| Table 1:Classification of anaesthesia risk management strategies according to the safety hierarchy model. | |
| Category | Examples |
| Elimination | Neuraxial anaesthesia for pregnant patients Elimination of halothane/chloroform |
| Safety technologies | Noninterchangeable oxygen and nitrous oxide connections Prefilled syringes for anaesthetic drugs Emergency oxygen supply on anaesthetic machines Proportioning systems to prevent delivery of hypoxic gas mixtures Mcgraph laryngoscope Videolaryngoscopes |
| Warnings/alarms | Gas analysers Bispectral index measurement Colour coding of syringes content Colour coding of oxygen and other gas components Pulse oximetry and capnography Alerts from national incident reporting systems |
| Training guidelines | Difficult airway and other emergencies management algorithms Anaesthetic equipment checklists |
| Protective equipment | Eye protections Bite blocks Protection from positional related injuries on operating tables |
Not all these strategies are easy to implement. This is particularly the case for guidelines and policies. There are a significant number of barriers to the systematic use of guidelines in hospitals. First, guidelines and policies inherently challenge professional autonomy and are sometimes viewed as ‘bureaucratic’ or ‘cook-book’ medicine [51]. As a result, they are rarely embraced by all professionals. Secondly, their implementation largely relies on passive diffusion, and many studies have shown that this is often a very poor way to encourage changes within organisations [52].
Furthermore, clinical work is often complex and many aspects of clinical practice are difficult to integrate into guidelines. Finally, not all clinicians are convinced of the benefits of using guidelines and protocols, and these become effective only if they are implemented and effectively used by practitioners.
Another limitation of the strategies described in the safety hierarchical model is that, to be usable, most require clear identification of the working process. In many hospital environments, processes are not always systematically standardised and results of safety improvement initiatives are not easy to follow because of a lack of long-term outcome or reliable indicators.
Another limitation of the strategies described in the safety hierarchical model is that they were designed mainly to limit human error during the interactions of clinicians within their working environments. However, nowadays medicine is increasingly practiced in complex environments such as hospitals and clinics. Organisational issues have become a major cause of adverse outcomes, particularly those related to poor teamwork and communication. These factors contribute to 43% to 65% of sentinel events occurring in operating theatres (e.g. operation on the wrong side, transfusion error, incorrect administration of potassium chloride) [53]. Teamwork has been shown to be inadequate in 62% of deaths following surgery, mainly owing to communication breakdown or poor supervision [54–55]. As a result, global strategies to improve overall communication and teamwork should also be considered.
Although the comprehensive use of tools integrated into the hierarchical accident prevention model can have a clear impact, as demonstrated in anaesthesia, on overall patient safety, further efforts should also be made to improve both teamwork and communication.
Two of the most well-known methods to address these issues are Crew Resource Management (CRM) and Simulation. Both methods have been developed in aviation and are increasingly used in healthcare. The first formal CRM programme was implemented in commercial aviation in 1981 and is now a mandatory part of aviation crew training.
CRM is designed to improve collaboration and communication in accordance with the following concept: “Training crews to reduce pilot errors by making better use of the human resources on the flight deck” [56]. CRM technique aims, therefore, at developing shared behaviours to improve patient safety, and favour the use of team resources rather than individual resources. This is achieved through formal teaching on human factors and related issues, particularly in the area of leadership, stress and communication management. There is increasing evidence for the success of CRM in improving communication and team collaboration in emergency medicine, obstetrics and surgery [57].
Simulation is another method widely used to improve teamwork and communication. It can be used to train both technical and nontechnical skills such as teamwork, coordination and communication. Clinical ‘scenarios’ that replicate routine or unusual critical situations are used to teach participants about issues related to human factors. It also allows participants to question their own behaviour within the team and their communication skills. Used in obstetric units and neonatology units, simulation has been shown to improve the participants’ abilities to coordinate as a team and, ultimately, to improve patient outcome. A recent study has shown that improving nontechnical skills could reduce by as much as 50% the rate of complications following difficult deliveries [58].
Finally, the overall management of the organisation can impact on patient safety. Two recent reports showed that organisational and management factors contributed to 26% of deaths and severe morbidity. These included: inadequate matching between required resources and the patient’s condition, poor surgical planning, inadequate response to production pressure and inappropriate night call organisation [22, 59].
Since the publication of the report “To err is human”, many improvements have been made to address the issue of medical error and patient safety. The close analysis of methods used in anaesthesia shows the effectiveness of approaches that comprehensively integrate all dimensions of the accident prevention model. It is therefore not surprising that insurance premiums for anaesthetists have remained stable or have even decreased in European and North American countries during recent years. However, as clinical practice increasingly takes place in complex organisations, patient safety should be prioritised further and efforts to address human error, teamwork and communication, and optimisation of organisations themselves should be promoted. As a result, efforts toward improvements in patient safety should be a process of consecutive steps: the first being the safety hierarchy model, followed by improvements in teamwork and communication, and other steps to be defined. The way forward is clear, only additional time is needed to implement all these improvements.
1 Donaldson MS, Kohn LT, Corrigan J. To err is human: building a safer health system. Washington, D.C.: National Academy Press; 2000.
2 Hogan H, Healey F, Neale G, Thomson R, Vincent C, Black N. Preventable deaths due to problems in care in English acute hospitals: a retrospective case record review study. BMJ Qual Saf. 2012;21(9):737–45.
3 Barbour GL. Development of a quality improvement checklist for the Department of Veterans Affairs. Jt Comm J Qual Improv. 1994;20(3):127–39.
4 McCannon CJ, Hackbarth AD, Griffin FA. Miles to go: an introduction to the 5 Million Lives Campaign. Jt Comm J Qual Patient Saf. 2007;33(8):477–84.
5 Landrigan CP, Parry GJ, Bones CB, Hackbarth AD, Goldmann DA, Sharek PJ. Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363(22):2124–34.
6 Leape LL, Berwick DM, Bates DW. What practices will most improve safety? Evidence-based medicine meets patient safety. JAMA. 2002;288(4):501–7.
7 Gaba DM. Anaesthesiology as a model for patient safety in health care. BMJ. 2000;320(7237):785–8.
8 Barnett rl, Brickmann db. Safety Hierarchy. Journal of Safety Research. 1986;17(2]:49–55.
9 Layde PM, Cortes LM, Teret SP, Brasel KJ, Kuhn EM, Mercy JA, et al. Patient safety efforts should focus on medical injuries. JAMA. 2002;287(15):1993–7.
10 McNutt RA, Abrams R, Arons DC. Patient safety efforts should focus on medical errors. JAMA. 2002;287(15):1997–2001.
11 Cooper JB, Gaba DM, Liang B, Woods D, Blum LN. The National Patient Safety Foundation agenda for research and development in patient safety. MedGenMed. 2000;2(3):E38.
12 Arbous MS, Grobbee DE, van Kleef JW, de Lange JJ, Spoormans HH, Touw P, et al. Mortality associated with anaesthesia: a qualitative analysis to identify risk factors. Anaesthesia. 2001;56(12):1141–53.
13 Chopra V, Bovill JG, Spierdijk J. Accidents, near accidents and complications during anaesthesia. A retrospective analysis of a 10-year period in a teaching hospital. Anaesthesia. 1990;45(1):3–6.
14 Eagle CJ, Davies JM. Current models of “quality” – an introduction for anaesthetists. Can J Anaesth. 1993;40(9):851–62.
15 Warden JC, Borton CL, Horan BF. Mortality associated with anaesthesia in New South Wales, 1984–1990. Med J Aust. 1994;161(10):585–93.
16 Lagasse RS. Anesthesia safety: model or myth? A review of the published literature and analysis of current original data. Anesthesiology. 2002;97(6):1609–17.
17 Cooper JB, Gaba D. No myth: anesthesia is a model for addressing patient safety. Anesthesiology. 2002;97(6):1335–7.
18 Blake JB. An examination of some recent statistics in regard ether, and a consideration of some present methods of administration. Boston Medical Surgery Journal. 1895;132(559):590.
19 Beecher HK, Todd DP. A study of the deaths associated with anesthesia and surgery: based on a study of 599, 548 anesthesias in ten institutions 1948–1952, inclusive. Ann Surg. 1954;140(1):2–35.
20 Memery HN. Anesthesia mortality in private practice. A ten-year study. JAMA. 1965;194(11):1185–8.
21 Clifton BS, Hotten WI. Deaths Associated with Anaesthesia. Br J Anaesth. 1963;35:250–9.
22 Lienhart A, Auroy Y, Pequignot F, Benhamou D, Warszawski J, Bovet M, et al. Survey of anesthesia-related mortality in France. Anesthesiology. 2006;105(6):1087–97.
23 Australian and New Zealand College of Anaesthetists. Safety of Anaesthesia in Australia. In: P.Mackay, editor. A review of Anaesthesia related mortality 1997–1999. Melbourne: Australian and New Zealand College of Anaesthetists; 2002.
24 Amalberti R, Auroy Y, Berwick D, Barach P. Five system barriers to achieving ultrasafe health care. Ann Intern Med. 2005;142(9):756–64.
25 Domino KB. Trends in anaesthesia litigation in the 1990s:monitored anesthesia care claims. ASA Newsletter 1997;61(2):15–7.
26 Lee LA, Domino KB. The Closed Claims Project. Has it influenced anesthetic practice and outcome? Anesthesiol Clin North America. 2002;20(3):485–501.
27 Lyons G, Macdonald R. Awareness during caesarean section. Anaesthesia. 1991;46(1):62–4.
28 Cheney FW, Posner KL, Lee LA, Caplan RA, Domino KB. Trends in anesthesia-related death and brain damage: A closed claims analysis. Anesthesiology. 2006;105(6):1081–6.
29 Staender S, Schaer H, Clergue F, Gerber H, Pasch T, Skarvan K, et al. A Swiss anaesthesiology closed claims analysis: report of events in the years 1987–2008. Eur J Anaesthesiol. 2011;28(2):85–91.
30 Royal College of Anaesthetists. National Audit Projects (NAP) 2011; Available from: http://www.rcoa.ac.uk/clinical-standards-and-quality/national-audit-projects. Accessed 18 December 2012.
31 Cooper JB, Cullen DJ, Nemeskal R, Hoaglin DC, Gevirtz CC, Csete M, et al. Effects of information feedback and pulse oximetry on the incidence of anesthesia complications. Anesthesiology. 1987;67(5):686–94.
32 Tinker JH, Dull DL, Caplan RA, Ward RJ, Cheney FW. Role of monitoring devices in prevention of anesthetic mishaps: a closed claims analysis. Anesthesiology. 1989;71(4):541–6.
33 Derrington MC, Smith G. A review of studies of anaesthetic risk, morbidity and mortality. Br J Anaesth. 1987;59(7):815–33.
34 Tiret L, Hatton F, Desmonts JM, Vourc’h G. The implications of a national study of risk of anaesthesia. Health Policy. 1988;9(3):331–6.
35 Davies JM, Strunin L. Anesthesia in 1984: how safe is it? Can Med Assoc J. 1984;131(5):437–41.
36 Soreide E, Bjornestad E, Steen PA. An audit of perioperative aspiration pneumonitis in gynaecological and obstetric patients. Acta Anaesthesiol Scand. 1996;40(1):14–9.
37 Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191–205.
38 Botney R. Improving patient safety in anesthesia: a success story? Int J Radiat Oncol Biol Phys. 2008;71(1 Suppl):S182–6.
39 Jensen LS, Merry AF, Webster CS, Weller J, Larsson L. Evidence-based strategies for preventing drug administration errors during anaesthesia. Anaesthesia. 2004;59(5):493–504.
40 Walker IA, Griffiths R, Wilson IH. Replacing Luer connectors: still work in progress. Anaesthesia. 2010;65(11):1059–63.
41 Myles PS, Leslie K, McNeil J, Forbes A, Chan MT. Bispectral index monitoring to prevent awareness during anaesthesia: the B-Aware randomised controlled trial. Lancet. 2004 29;363(9423):1757–63.
42 Merry AF, Webster CS. Labelling and drug administration error. Anaesthesia. 1996;51(10):987–8.
43 Currie M, Mackay P, Morgan C, Runciman WB, Russell WJ, Sellen A, et al. The Australian Incident Monitoring Study. The “wrong drug” problem in anaesthesia: an analysis of 2000 incident reports. Anaesth Intensive Care. 1993;21(5):596–601.
44 Howard SK, Gaba DM, Fish KJ, Yang G, Sarnquist FH. Anesthesia crisis resource management training: teaching anesthesiologists to handle critical incidents. Aviat Space Environ Med. 1992;63(9):763–70.
45 European Society of Anaesthesiology. Helsinki Declaration on Patient Safety in Anaesthesiology 2010; Available from: http://www.euroanaesthesia.org/sitecore/content/Publications/Helsinki%20Declaration.aspx http://www.euroanaesthesia.org/sitecore/content/Publications/Helsinki Declaration.aspx Accessed 20 December 2012.
46 Auerhammer J. Positioning of the patient for surgery. Anaesthesist. 2008;57(11):1107–24.
47 Bates DW, Gawande AA. Improving safety with information technology. N Engl J Med. 2003;348(25):2526–34.
48 Eggimann P, Pittet D. Overview of catheter-related infections with special emphasis on prevention based on educational programs. Clin Microbiol Infect. 2002;8(5):295–309.
49 Pittet D, Hugonnet S, Harbarth S, Mourouga P, Sauvan V, Touveneau S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356(9238):1307–12.
50 Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat AH, Dellinger EP, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360(5):491–9.
51 Davies HT, Harrison S. Trends in doctor-manager relationships. BMJ. 2003;326(7390]:646–9.
52 Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet. 1993;342(8883]:1317–22.
53 Joint Commission on Accreditation of Healthcare Organizations. Root cause analysis in health care: tools and techniques. 2nd ed. Oakbrook Terrace, IL: Joint Commission on Accreditation of Healthcare Organizations; 2003.
54 Gawande AA, Zinner MJ, Studdert DM, Brennan TA. Analysis of errors reported by surgeons at three teaching hospitals. Surgery. 2003;133(6):614–21.
55 Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186–94.
56 Sexton JB, Thomas EJ, Helmreich RL. Error, stress, and teamwork in medicine and aviation: cross sectional surveys. BMJ. 2000;320(7237):745–9.
57 Zeltser MV, Nash DB. Approaching the evidence basis for aviation-derived teamwork training in medicine. Am J Med Qual. 2010;25(1):13–23.
58 Halamek LP, Kaegi DM, Gaba DM, Sowb YA, Smith BC, Smith BE, et al. Time for a new paradigm in pediatric medical education: teaching neonatal resuscitation in a simulated delivery room environment. Pediatrics. 2000;106(4):E45.
59 Gibbs N, Rodoreda P. Anaesthetic mortality rates in Western Australia 1980–2002. Anaesth Intensive Care. 2005;33(5):616–22.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.