Uptake of guidelines on prevention of mother-to-child transmission of HIV in rural Tanzania: time for change
DOI: https://doi.org/10.4414/smw.2013.13775
Anna
Gamell, Emili
Letang, Boniface
Jullu, Geoffrey
Mwaigomole, Angelo
Nyamtema, Christoph
Hatz, Manuel
Battegay, Marcel
Tanner
Summary
Guidelines on prevention of mother-to-child transmission (PMTCT) of human immunodeficiency virus (HIV) are inconsistently implemented in low-income countries. Strategies are needed to improve the uptake of these guidelines to prevent avoidable new HIV infections of infants. In 2010 the World Health Organisation presented its new PMTCT guidelines, offering two options for short courses of antiretroviral prophylaxis: Option A and Option B. Option A consists of antenatal prophylaxis with zidovudine followed by intrapartum and postpartum prophylaxis with single-dose nevirapine and zidovudine plus lamivudine. Option B recommends triple antiretroviral prophylaxis until after finishing breastfeeding. Tanzania has adopted Option A, and it is currently implementing it. A new option termed Option B+ has emerged recently, which recommends providing lifelong antiretroviral treatment to all HIV-positive pregnant women.
In this article, we discuss the likely impact of this last PMTCT strategy in rural Africa with an example of an observational cross-sectional analysis in a rural referral hospital in Tanzania aiming to assess the uptake of PMTCT recommendations. Gaps were identified at all steps of the PMTCT pathway.
Effective uptake of PMTCT guidelines has been shown to be extremely challenging in this setting. The continuously changing recommendations on PMTCT stress the need for a much simpler and effective approach. We argue in favour of implementing Option B+ in Tanzania. Financial challenges need to be faced, but Option B+ would help to overcome many barriers that prevent guidelines to be implemented in order to increase coverage and ultimately achieve the goal of ‘virtual elimination’ of mother-to-child transmission in sub-Saharan Africa.
Different options for preventing mother-to-child transmission of HIV
More than two million children under the age of 15 are infected with human immunodeficiency virus (HIV) worldwide, most of them in sub-Saharan Africa [1]. Mother-to-child transmission of HIV accounts for over 90% of these cases [1], the risk of transmission ranging from 25% to 48% in resource-limited settings [2].
Prevention of mother-to-child transmission (PMTCT) was shown to be effective in well-resourced settings through the administration of antiretroviral prophylaxis to the mother during pregnancy, labour and delivery and to the infant for the first six weeks of life [3]. Further evidence demonstrated that combined antiretroviral therapy (cART) given to the mother together with elective Caesarean section and avoidance of breastfeeding reduced mother-to-child transmission to less than 1% [4]. Consequently, guidelines were developed for high- and in low-income countries [5, 6].
The World Health Organisation (WHO) issued the first recommendations on PMTCT in resource-limited settings in 2000. These recommendations were revised in 2004 with the adoption of simplified and standardised regimens. In 2006 and later in 2010 [5], the guidelines were updated to incorporate new evidence and to be aligned with the global commitment to universal access.
Guidelines for resource-limited settings are difficult to implement, partly because of the fragility of health systems in these countries. Lack of infrastructure and constraints on human and financial resources contribute to a poor uptake of evidence-based interventions and, ultimately, to the impairment of clinical practice in these settings. Dissemination of changes in the guidelines is suboptimal and often takes time to take root among health workers and to change practices in rural sub-Saharan Africa [7]. According to the WHO, only 53% of pregnant women worldwide received any antiretroviral for PMTCT in 2009 [8], with substantial differences across countries in sub-Saharan Africa (i.e. 1% in the Democratic Republic of the Congo vs 52% in Mozambique) [9]. Importantly, having effective antiretroviral regimens and wide coverage is insufficient; effective delivery programmes are equally important [10, 11]. Simplification of the existing programmes is urgently needed in order to bridge the gaps observed with current recommendations.
In 2010 the WHO presented its new guidelines on PMTCT, recommending two PMTCT options: Option A and Option B. These two options include both treatment and prophylaxis components. In both options CD4 cell count is necessary to decide the eligibility of HIV-infected pregnant women for lifelong cART. For all women who have CD4 cell counts ≤350 cell/mm3 initiation of lifelong cART is recommended. For those women not eligible for lifelong cART, Option A recommends antenatal prophylaxis with zidovudine followed by intrapartum and postpartum prophylaxis with single-dose nevirapine and zidovudine plus lamivudine; Option B recommends triple antiretroviral prophylaxis until after finishing breastfeeding. Recently a new option has emerged, termed Option B+ [12]. This option is a single, universal regimen both to treat HIV-infected pregnant women and to prevent mother-to-child transmission. In Option B+ all HIV-positive pregnant women are provided with lifelong cART, regardless of the CD4 cell count. Option A might be difficult to implement because of the different drugs administered during antenatal, intrapartum and postpartum care. Options B and B+, although simplifying drug prescription, have a short-term drug cost greater than Option A. However, when taking into account maternal and infant life expectancy and lifetime healthcare costs, Option B is more effective and less expensive than Option A. Option B+ offers clinical benefits and economic value comparable to other widely used HIV interventions [13]. The three options are summarised in table 1.
Several countries in sub-Saharan Africa are currently considering modifying their PMTCT guidelines. Such a decision should be made on the basis of their implementation experience and a previous assessment of how they can better integrate, simplify and optimise the PMTCT programme in the existing HIV/AIDS care and treatment platform.
The Tanzanian national guidelines on PMTCT were developed in 2004 and revised in 2007 [14]. New guidelines recommending WHO option A were developed in June 2012 and plans are being devised to implement them nationwide [15].
|
Table 1:Options for preventing mother-to-child transmission of human immunodeficiency virus. |
| |
Woman receives
|
Infant receives
|
| Treatment
(CD4 counts ≤350 cells/mm3) |
Prophylaxis
(CD4 counts >350 cells/mm3) |
| Option A |
Triple ARVs starting as soon as diagnosed and continued for life |
Antepartum: AZT starting as early as 14 weeks gestation
Intrapartum: at onset of labour, sdNVP and first dose of AZT/3TC
Postpartum: daily AZT/3TC until 7 days postpartum |
Mother received prophylaxis: daily NVP from birth until 1 week after cessation of all breastfeeding; if not breastfeeding until age 4‒6 weeks
Mother is on treatment: daily NVP until age 4–6 weeks |
| Option B |
Triple ARVs starting as soon as diagnosed and continued for life |
Triple ARVs starting as early as 14 weeks gestation and through childbirth if not breastfeeding, or until 1 week after cessation of all if breastfeeding |
Irrespective of mode of infant feeding: daily NVP or AZT from birth until age 4–6 weeks |
| Option B+ |
Same for treatment and prophylaxis |
Irrespective of mode of infant feeding: daily NVP or AZT from birth until age 4–6 weeks |
| Regardless of CD4 count, triple ARVs starting as soon as diagnosed and continued for life |
| Triple ARVs means the use of one of the recommended three-drug fully suppressive treatment options.
ARVs = antiretrovirals; AZT = zidovudine; sdNVP = single-dose nevirapine; 3TC = lamivudine; NVP = nevirapine. |
A cross-sectional survey in a referral hospital in Tanzania assessing the uptake of the current PMTCT recommendations: an example of problems encountered in rural sub-Saharan Africa
In this review we illustrate the problems of PMTCT with findings of a recently performed survey conducted in March 2012 in a referral hospital in Tanzania. Data on PMTCT services delivered between January 2010 and December 2011 at St Francis referral hospital in Ifakara, Kilombero district, Morogoro region, Southern Tanzania, were collected. Table 2 summarises the antiretroviral regimens recommended by the Tanzanian national guidelines on PMTCT before, during and after delivery. In this article, we will use this as a case-study to discuss the limitations of the implementation of guidelines, to identify the gaps to be bridged, and to envisage potential solutions, including the adoption of newer PMTCT strategies.
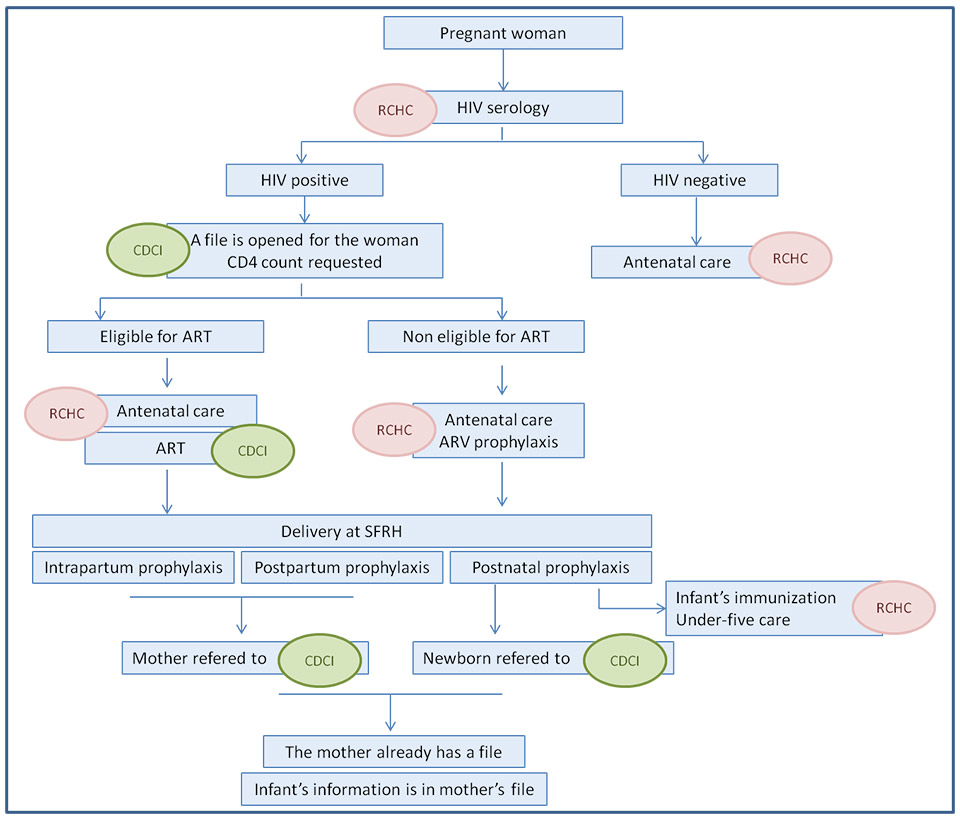
Figure 1
Theoretical care pathway for prevention of mother-to-child transmission (PMTCT) of human immunodeficiency virus (HIV) at St Francis Referral Hospital (SFRH).
RCHC = Reproductive and Child Health Clinic; CDCI = Chronic Disease Clinic of Ifakara; ART = antiretroviral therapy; ARV = antiretroviral.
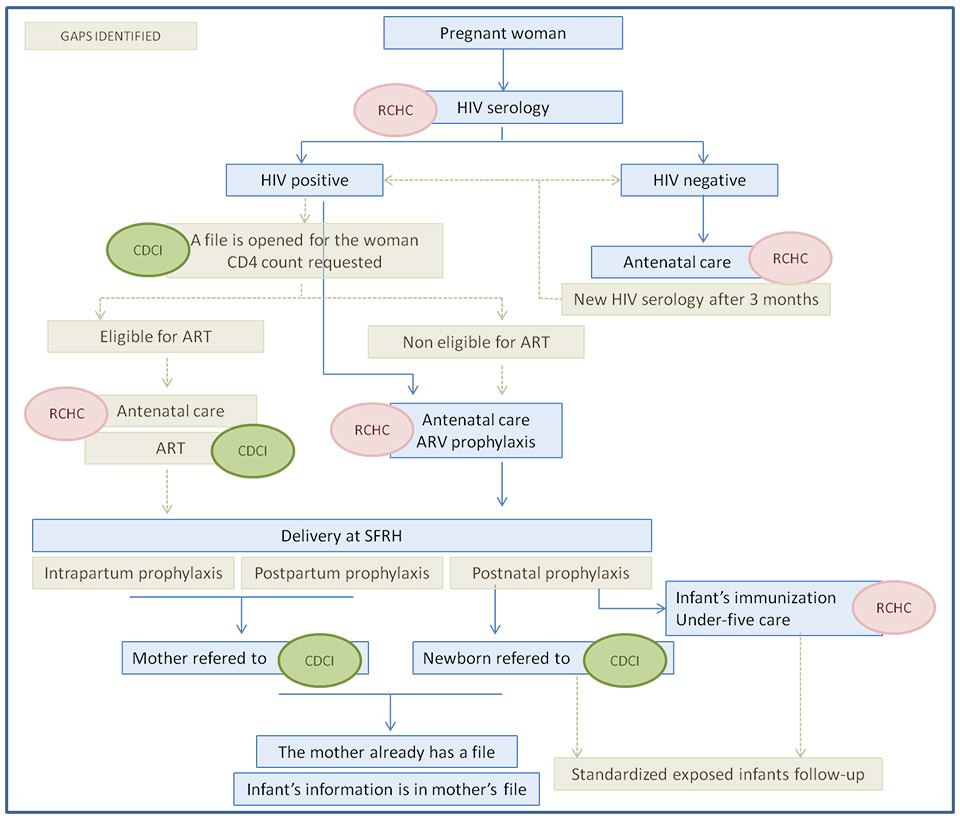
Figure 2
Main gaps identified in the care pathway for prevention of mother-to-child transmission (PMTCT) of human immunodeficiency virus (HIV) at St Francis Referral Hospital (SFRH).
The gaps are coloured in brown. RCHC = Reproductive and Child Health Clinic; CDCI = Chronic Disease Clinic of Ifakara; ART = antiretroviral therapy; ARV = antiretroviral.
Setting at a rural referral hospital
St Francis referral hospital has 370 beds and is the most important healthcare facility in the Kilombero district. PMTCT services are provided by several departments in this hospital, including the Reproductive and Child Health Clinic; the Chronic Diseases Clinic of Ifakara; the Obstetrics department; and the Neonatology unit.
The Reproductive and Child Health Clinic provides antenatal care to all pregnant women, as well as vaccinations and outpatient care for children under five years old. Care and treatment for all HIV-positive patients is provided at St Francis referral hospital in accordance with the National AIDS Control Programme through the Chronic Diseases Clinic of Ifakara. The Chronic Diseases Clinic of Ifakara works in cooperation with the Ifakara Health Institute, the Swiss Tropical and Public Health Institute and the Departments of Infectious Diseases and Hospital Epidemiology of the University Hospitals of Basel and Bern, Switzerland. All patients attending the Chronic Diseases Clinic of Ifakara since late 2004 are asked for informed consent to be enrolled at the Kilombero and Ulanga Antiretroviral Cohort (KIULARCO) [16]. This cohort comprises almost 6000 patients and is the largest peripheral HIV cohort in Tanzania.
The Obstetrics department attends approximately 5000 deliveries per year and works closely with the Neonatology unit.
PMTCT Circuit at St Francis referral hospital and gaps identified
Figure 1 shows the theoretical PMTCT pathway at St Francis referral hospital. The departments involved in this process are the following: Reproductive and Child Health Clinic, Chronic Diseases Clinic of Ifakara, Obstetrics, and Neonatology. The following key steps of the PMTCT pathway were identified: (1.) HIV counselling and testing; (2.) CD4 count measurement and eligibility for cART; (3.) antenatal antiretroviral prophylaxis; (4.) intrapartum and postpartum prophylaxis; (5.) postnatal prophylaxis for the infant; (6.) follow-up of HIV-positive mothers; and (7.) follow-up of HIV-exposed infants.
According to the WHO and Tanzanian national guidelines, all pregnant women attending the Reproductive and Child Health Clinic for antenatal care have to be offered HIV counselling and testing. Between January 2010 and December 2011, 4027 pregnant women attended the Reproductive and Child Health Clinic. Forty-four (1%) were already known to be HIV infected. The remainder were offered HIV testing, with a rate of acceptance of 90.4% (3606/3983). Two hundred and seven of these 3606 women tested positive. Thus, the HIV prevalence among pregnant women attending antenatal services at St Francis referral hospital during the study period was 6.9% (251/3647). Current Tanzanian guidelines recommend HIV seronegative women to be retested after three months in order to avoid false negative diagnoses during the ‘window period’ after acute infection, but there was no information concerning the proportion of seronegative women who were retested. Information could only be extracted from randomly selected individual pregnancy cards, and none of the seronegative women assessed had a second test registered.
Those women who are newly diagnosed with HIV infection at St Francis referral hospital should be referred to the Chronic Diseases Clinic of Ifakara. However, only 25.6% (53/207) of newly diagnosed women visited the clinic during 2010–2011 and were assessed for cART eligibility. Seventy-four percent (154/207) of HIV-infected women were not registered at the Chronic Diseases Clinic and thus cART eligibility criteria were not assessed. Consequently, all HIV-positive pregnant women with unknown CD4 count were prescribed antenatal antiretroviral prophylaxis in the Reproductive and Child Health Clinic, regardless of their eligibility for initiation of cART. According to the labour ward PMTCT log book, the antenatal prophylaxis prescribed to all HIV-positive women was monotherapy with nevirapine, which was not a recommended option (table 2). This is inconsistent with the information collected at the Reproductive and Child Health Clinic regarding antenatal prophylaxis prescribed during the same period, which was recorded as zidovudine.
All HIV-infected pregnant women are advised to deliver at St Francis referral hospital to ensure optimal intrapartum and postpartum antiretroviral prophylaxis for the mother, and postnatal prophylaxis for the infant. Between 20/03/2011 and 03/05/2011, 570 women delivered at the hospital. Almost 5% (28/570) and 80% (456/570) were known to be HIV-positive and HIV-negative, respectively. The HIV serostatus was unknown for 14.4% (82/570) of women. Of those, 2.4% (2/82) were tested during labour, with one testing HIV-positive. In total, 5.9% (29/486) of all women with a known HIV serostatus giving birth at St Francis referral hospital during that period tested positive.
Concerning intrapartum prophylaxis, there was conflicting information in the register books and the interviews. The nurses interviewed did not appear to be aware of the latest recommendations on intrapartum prophylaxis, which include three drugs to be given at different intervals (table 2). In contrast, 86% (25/29) of the HIV-positive women were recorded as having correct prophylaxis with three drugs, and no information was registered for the remaining four women.
Regarding postpartum prophylaxis, no information was recorded for any women. The interviews with staff members of the labour and the obstetric wards suggest a lack of clarity with regards to the service responsible for its administration.
As postnatal prophylaxis, 83% (24/29) of the babies born from HIV-seropositive women were given single-dose nevirapine, one (3.4%) received single-dose nevirapine plus zidovudine for one week and no data was collected for the remaining four babies. This represents another important gap in this pathway, since the postnatal prophylaxis recommended in the guidelines includes both single-dose nevirapine plus zidovudine, very rarely administered at St Francis referral hospital during the period assessed.
After delivery, mother and child should be referred to the Chronic Diseases Clinic of Ifakara for follow-up. During 2010–2011 only one woman with an infant younger than two months was registered at the Chronic Diseases Clinic. There is no information with regards to the number of the HIV-exposed infants attending the clinic during the same period. There is neither active case-finding nor a standardised follow-up for HIV-exposed infants in place. Consequently, the infant is visited together with the mother and information related to the exposed noninfected child is not collected in any database.
Figure 2 illustrates the gaps identified in the PMTCT algorithm. For every gap identified at different steps of the PMTCT pathway, the possible causes were assessed and potential solutions were suggested. Table 3 summarises the most likely causes and the proposed solutions to close the specific gaps.
|
Table 2:Recommended combination antiretroviral prophylaxis regimens to prevent mother-to-child transmission of HIV according to the 2007 Tanzanian national guidelines for the Prevention of Mother-To-Child Transmission of HIV. |
|
Regimen
|
Antenatal
|
Intrapartum
|
Postpartum
|
Postnatal
|
| Recommended |
AZT 300 mg twice daily starting at 28 WG or as soon as possible thereafter |
AZT 300 mg at onset of labour and every 3 hours until delivery
sdNVP 200 mg at onset of labour
3TC 150 mg at onset of labour and every 12 hours until delivery |
AZT 300 mg twice daily for 7 days
3TC 150 mg twice daily for 7 days |
sdNVP 2 mg/kg immediately after birth
AZT 4 mg/kg twice a day for 7 days |
| Recommended if mother presents during labour |
|
AZT at 300 mg at onset of labour and every 3 hours until delivery
sdNVP 200 mg at onset of labour
3TC 150 mg at onset of labour and every 12 hours until delivery |
AZT 300 mg twice daily for 7 days
3TC 150 mg twice daily for 7 days |
sdNVP 2 mg/kg immediately after birth
AZT 4 mg/kg twice daily for 28 days |
| Recommended if mother tests HIV positive immediately after delivery |
|
|
Refer to Care and Treatment Clinic, do not give any antiretroviral |
sdNVP 2 mg/kg immediately after birth
AZT 4 mg/kg twice daily for 28 days |
| Recommended if mother was on ART before pregnancy |
Continue ART prescribed before pregnancy. In the first trimester replace EFV with NVP |
AZT 4 mg/kg twice a day for 7 days |
| AZT = zidovudine; WG = weeks of gestation; sdNVP = single-dose nevirapine; 3TC = lamivudine; ART = antiretroviral therapy; EFV = efavirenz; NVP = nevirapine. Source: Tanzanian national guidelines for the Prevention of Mother-To-Child Transmission of HIV. The United Republic of Tanzania, Ministry of Health and Social Welfare, 2007 [14] |
|
Table 3:Prevention of mother-to-child transmission (PMTCT) care pathway: observed gaps, possible causes, and potential solutions. |
|
Step involved
|
Observed gap
|
Possible causes
|
Potential solutions
|
| HIV testing at RCHC |
Not all seronegative pregnant women are retested 3 months after the first HIV-negative serology. |
Unawareness of the ‘window period’ after the primary HIV infection. |
CME. Refresher seminars for all the staff involved in antenatal care.
SOP. |
| CDCI |
After being diagnosed HIV-positive, most pregnant women do not attend the CDCI, so CD4 count is not measured and ART eligibility is not assessed. |
Pregnant women do not seem to feel comfortable attending the CDCI. According to the nurses’ interviews, they may feel ashamed because of stigma. |
Integrate the care of HIV-positive pregnant women into the RCHC.
Option B+ |
| Antenatal prophylaxis |
There are inconsistencies between the ARV drugs prescribed in the RCHC and the ARV drugs recorded in the labour ward books. |
Probably mistakes when filling the books at the labour ward. |
CME. SOP.
Option B+. |
| Delivery |
There is not enough information to assume that intrapartum prophylaxis is correctly administered. |
Doses and frequency of the ARV are not easy to remember. |
CME. SOP.
Poster with the intrapartum prophylaxis regimen.
Identify a staff member responsible for checking the availability of ARV drugs in the labour ward.
Option B+. |
| After delivery |
There are no data showing that postpartum prophylaxis is being administered in the hospital. |
The staff do not appear to know clearly who is supposed to administer it (labour ward or obstetric ward).
Lack of availability of ARV drugs in labour ward / obstetric ward.
Doses and frequency of the ARV are not easy to remember. |
Clearly assign responsibilities and duties
SOP
Identify a staff member responsible for checking the availability of ARV drugs in the labour ward/obstetric ward
Poster with the postpartum prophylaxis. Counsel the mother about ARV prophylaxis.
Option B+. |
| Perinatal period |
Postnatal prophylaxis is not always correctly administered after discharge. |
The staff do not seem to know clearly who is supposed to administer it (labour ward, obstetric ward or neonatal unit)
Lack of availability of ARV syrups.
Doses and frequency are not easy to remember. |
Because the sooner the dose is given the greater the protective effect, the labour ward should be the responsible.
Identify a staff member responsible for checking the availability of ARV syrups in the labour ward.
Poster with the postnatal prophylaxis regimen. Counsel the mother about ARV prophylaxis for the baby.
SOP.
Option B+. |
| Exposed infants follow-up |
There is no standardised follow-up for the exposed infants. |
The Guidelines do not specify where the follow up should be done, what frequency, etc. |
Integrate the follow-up of HIV-exposed infants into the existing under-five health services.
SOP.
Generate special files for the exposed infants. Attach these files to the mothers’ CDCI files. |
| RCHC = Reproductive and Child Health Clinic; CDCI = Chronic Disease Clinic of Ifakara; HIV = Human Immunodeficiency Virus; CME = continuing medical education; SOP = Standard Operating Procedure; ART = Antiretroviral Therapy; ARV = Antiretroviral; AZT = zidovudine; 3TC = lamivudine; NVP = nevirapine; |
Proposals to increase the uptake of current and forthcoming PMTCT guidelines: time for change
The uptake of the guidelines in sub-Saharan Africa is known to be poor. The aim of this case presentation was to illustrate the quality of PMTCT provision at a rural Tanzanian referral hospital by providing a snapshot of the reality in the field, and to identify existing gaps and potential ways to bridge them at an operational level. Our findings should help to develop new potential strategies to be tested in a prospective fashion with the aim of improving the uptake of PMTCT guidelines in rural Tanzania and other similar settings in sub-Saharan Africa. Moreover, simpler and more effective approaches need to be considered.
In St Francis referral hospital, all the services and most resources needed for a proper functioning of the Tanzanian PMTCT programme were in place. However, several gaps were observed that prevented the current Tanzanian recommendations to be implemented optimally. This is worrisome, since it may ultimately result in an increasing rate of HIV infection among infants.
The most important gaps identified in the PMTCT care pathway were: (a) no retesting of seronegative pregnant women in late pregnancy; (b) suboptimal follow-up of HIV-infected pregnant women, including irregular assessment of cART eligibility; (c) inconsistencies in the prescription of antiretroviral prophylaxes; and (d) lack of a standardised follow-up of HIV-exposed infants.
As mentioned, the Tanzanian Government has adopted Option A for the new PMTCT Guidelines. Lower short-term costs might justify the adoption of this strategy in Tanzania. However, drug prices have been reduced since 2010, when the WHO recommendations were published, and they are expected to further decrease, narrowing the cost difference between Options A and B. There are concerns about the lack of simplification when switching from the former recommendations to Option A. Even if the higher CD4 count threshold for cART eligibility, the earlier initiation of antenatal prophylaxis and the longer postnatal prophylaxis for breastfeeding infants are definitely welcome modifications, the complexity of the regimen remains high and might prevent correct implementation in the real world, as shown in the case presented. Simpler strategies are needed to increase the coverage and the efficacy of these programmes. Better infrastructure and human resources must accompany these new strategies to avoid logistic problems such as drug stock-outs [17] and overburdened health staff.
In 2011, Malawi adopted Option B+, for its ease of implementation and potential prevention benefit [18]. Likewise, other countries, such as Uganda and Swaziland, are also considering moving to Option B+. Using the case presented as a starting point, we are now going to discuss every gap observed and envisage what would be the situation if Option B+ was the adopted PMTCT recommendation in Tanzania.
HIV testing was done once and not repeated during the third trimester among those women who were initially seronegative. In a South African study [19], 3% of initially seronegative pregnant women were found to be HIV-infected in late pregnancy, showing the relevance of retesting during the third trimester. Acute HIV infection during pregnancy is associated with high rates of vertical transmission owing to the extremely high HIV ribonucleic acid (RNA) levels during this phase of the infection, which is associated with higher probabilities of vertical transmission [20]. Option A, B or B+ would not bridge this gap, since the awareness of the importance of retesting has to be spread through education.
Regarding the assessment of the cART eligibility, the current Tanzanian guidelines during the time the analysis was performed recommended offering cART to all pregnant women with CD4 counts ≤200 cells/mm3 or ≤350 cells/mm3 in case of WHO stage 3 or 4 disease. However, our analysis shows that after being found to be HIV-positive, most pregnant women were not assessed for cART eligibility. Instead, most were offered antiretroviral prophylaxis regardless of CD4 cell count. We have to take into account the possibility that some women attended a clinic other than the Chronic Disease Clinic of Ifakara, but we do not consider this is a common or representative occurrence. With Option B+, all HIV-positive pregnant women would receive cART, avoiding suboptimal antiretroviral prophylaxis for women in need of cART for their own health.
For the antenatal prophylaxis prescribed, inconsistencies between the antiretroviral regimens administered and the records in the different services were observed. Concerning intrapartum prophylaxis, the Tanzanian PMTCT guidelines recommended using a combination of zidovudine, lamivudine, and nevirapine. Our findings again show irregularities in this crucial step. According to a study conducted in Malawi, skilled attendance at birth appeared to be an important determinant of correct intrapartum prophylaxis [21]. In our setting, interviews with midwives revealed a suboptimal knowledge of the recommended regimens and doses. Postpartum and postnatal prophylaxes are poorly implemented in sub-Saharan Africa [22–24]. Likewise, data on postpartum prophylaxis were nonexistent in our hospital and only 1 out of 29 newborns received correct postnatal prophylaxis. When antenatal and intrapartum prophylaxis is correctly administered, between one-third and one-half of all mother-to-child transmission of HIV is estimated to occur during the postpartum period, mostly through breastfeeding [25]. Thus, a good and updated knowledge on postpartum and postnatal prophylaxes among staff working on maternity wards would make possible to maximise the chance that mother-baby pairs get appropriate and timely medications. However, frequent staff turnover and attendance by junior clinicians present additional obstacles to the capacitation and continuing medical education of attending staff.
With Option B+ the differences between antenatal, intrapartum, and postpartum prophylaxis disappear. From the moment the pregnant woman is diagnosed as HIV-positive, she is prescribed lifelong cART, including pregnancy, delivery and breastfeeding periods. This is much simpler than the current guidelines and the forthcoming Option A. By adopting Option B+, the gaps arising from the complexity of the drug combinations for different periods of prophylaxis would be avoided, as well as many infant infections derived from the poor uptake of the more complex recommendations. Concerns about drug compliance have been raised because some women will be asymptomatic and feel healthy. As seen in a Brazilian study, we expect pregnant women to be highly motivated to protect their babies, especially if they are periodically counselled and a simple regimen with not many pills per day is prescribed [26]. Extra counselling focused on minimising the possibility of treatment interruption between pregnancies and its consequences might be necessary for asymptomatic mothers after the delivery and the breastfeeding period.
Similarly, postnatal prophylaxis would be simplified with Option B+. With this option, all HIV-exposed infants receive antiretroviral prophylaxes for four to six weeks, regardless of the feeding method. In contrast, with the current recommendations and with the forthcoming coming Option A, there are different regimens depending on the feeding method, which again add complexity to their implementation in the field.
Follow-up of HIV-exposed infants is notoriously weak in sub-Saharan Africa and it leads to under-diagnosis of HIV [27], and our analysis confirms these findings in our setting. According to data from the WHO, in 2010, in 65 reporting countries only 28% of the HIV-exposed infants had an HIV test within the first two months of life [28]. Concurring with other studies from Kenya and Mozambique [29, 30], a recent study from Ethiopia showed that slightly more than 50% of HIV-exposed infants were followed-up at six weeks and less than one-third had a documented HIV test result. Remarkably, this low coverage of HIV testing occurred in the frame of an immunisation coverage of more than 80% among HIV-exposed infants [31], suggesting specific problems related to HIV testing. Integration of the follow-up of HIV-exposed infants into the existing under-five child health clinics would maximise the opportunities for prompt diagnosis and timely treatment for HIV-infected children. Unfortunately, the lack of a standardised follow-up of HIV-exposed infants cannot directly be bridged by implementing Option B+. However, by providing Option B+ it is probable that the follow-up of HIV-exposed infants will improve, since more mothers will get medical care. Moreover, with this option, fewer infants are expected to be infected, so the number of undiagnosed infants will decrease.
Based on our findings, several programmatic solutions can be proposed to increase the uptake of current and forthcoming PMTCT guidelines. The implementation of the guidelines should be combined with a comprehensive package including educational and logistic interventions to be easily and affordably implemented in St Francis referral hospital and other similar settings in sub-Saharan Africa. Analogously, Youngleson et al. implemented a ‘change package’ to improve the PMTCT programme in a sub-district of Western Cape, South Africa, including maximising the use of existing resources, reducing duplication, and developing patient-centred approaches. As a consequence of this programme, significant improvements were achieved, with a decrease in the perinatal transmission rate from 7.6% to 5% [32]. The logistics of implementation would be easier if Option B+ were adopted, mainly resulting from a reduction in the training requirements. As a consequence the likelihood of successful implementation would be greatly increased.
Furthermore, apart from the simplification of the PMTCT programme and its better implementation, lifelong cART for HIV-infected pregnant women (Option B+) has additional benefits. By adopting this strategy, future pregnancies will be protected from conception, and sexual transmission of HIV to a seronegative partner will be significantly reduced [33]. All women will require cART at some point, with Option B+ they will merely start it earlier. In this view, Option B+ can be placed in the frame of the idea of ‘treatment as prevention’, which has attracted tremendous interest and hope [33, 34].
Conclusions
In summary, we strongly believe that it is time to move forward and argue in favour of the Option B+ strategy in Tanzania and other sub-Saharan Africa countries. Option B+ is one of the most exciting developments in the prevention of vertical transmission and paediatric HIV in the recent years. Financial and operational challenges will have to be overcome and the feasibility, the cost-benefit and the public health impact will need to be assessed in those countries implementing Option B+. Data from solid scientific studies are still needed to support Option B+ definitively. Achieving the aim of the WHO for the ‘virtual elimination’ of paediatric HIV, aiming to attain a mother-to-child transmission risk of less than 5% for the year 2015, will be, even in the best scenario, hard to do. Option B+ gives us for the first time the opportunity to think that this goal is achievable at a global scale. Strong political will and strong support from governments and public health authorities will be needed to achieve this milestone in the history of the HIV pandemic.
References
1 UNAIDS. Joint United Nations Program on HIV/AIDS (UNAIDS). Global Report: UNAIDS report on the global AIDS epidemic 2010. Geneva, UNAIDS, 2010. Available from: http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf.
2 De Cock KM, Fowler MG, Mercier E, de Vincenzi I, Saba J, Hoff E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA: the journal of the American Medical Association. 2000;283(9):1175–82. Epub 2000/03/07.
3 Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O’Sullivan MJ, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331(18):1173–80. Epub 1994/11/03.
4 European Collaborative Study. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40(3):458–65.
5 WHO. New guidance on prevention of mother-to-child transmission of HIV and infant feeding in the context of HIV. Geneva, World Health Organization, 2010. Available from: http://www.who.int/hiv/pub/mtct/PMTCTfactsheet.pdf.
6 European AIDS Clinical Society Guidelines (2011). Available from: http://www.europeanaidsclinicalsociety.org/images/stories/EACS-Pdf/EACSGuidelines-v6.0-English.pdf.
7 Nkonki LL, Doherty TM, Hill Z, Chopra M, Schaay N, Kendall C. Missed opportunities for participation in prevention of mother to child transmission programmes: simplicity of nevirapine does not necessarily lead to optimal uptake, a qualitative study. AIDS research and therapy. 2007;4:27. Epub 2007/11/24.
8 WHO. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress report 2010. Geneva, World Health Organization, 2010. Available from: http://www.who.int/hiv/pub/2010progressreport
9 UNAIDS. Joint United Nations Program on HIV/AIDS (UNAIDS). AIDSinfo Country fact sheets. Geneva, UNAIDS. Available from: http://www.unaids.org/en/dataanalysis/tools/aidsinfo/countryfactsheets/.
10 Barker PM, Mphatswe W, Rollins N. Antiretroviral drugs in the cupboard are not enough: the impact of health systems’ performance on mother-to-child transmission of HIV. Journal of acquired immune deficiency syndromes. 2011;56(2):e45–8. Epub 2010/11/19.
11 Ciaranello AL, Perez F, Keatinge J, Park JE, Engelsmann B, Maruva M, et al. What will it take to eliminate pediatric HIV? Reaching WHO target rates of mother-to-child HIV transmission in Zimbabwe: a model-based analysis. PLoS medicine. 2012;9(1):e1001156. Epub 2012/01/19.
12 WHO. Programmatic Update. Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. April 2012. Geneva, World Health Organization, 2012. Available from: www.who.int/hiv/PMTCT_update.pdf.
13 Ciaranello AL, Perez F, Engelsmann B, Walensky RP, Mushavi A, Rusibamayila A, et al. Cost-effectiveness of World Health Organization 2010 Guidelines for Prevention of Mother-to-Child HIV Transmission in Zimbabwe. Clin Infect Dis. 2012. Epub 2012/12/04.
14 Tanzanian national guidelines for the Prevention of Mother-To-Child Transmission of HIV. The United Republic of Tanzania, Ministry of Health and Social Welfare, 2007.
15 Tanzanian national guidelines for comprehensive care of Prevention of Mother-To-Child Transmission of HIV services. United Republic of Tanzania, Ministry of Health and Social Welfare, June 2012.
16 Stoeckle M, McHomvu R, Hatz C, Battegay M, Aris EA, Mshinda H, et al. Moving up from 3 by 5. The Lancet infectious diseases. 2006;6(8):460–1. Epub 2006/07/28.
17 Pasquet A, Messou E, Gabillard D, Minga A, Depoulosky A, Deuffic-Burban S, et al. Impact of drug stock-outs on death and retention to care among HIV-infected patients on combination antiretroviral therapy in Abidjan, Cote d’Ivoire. PloS one. 2010;5(10):e13414. Epub 2010/10/27.
18 Schouten EJ, Jahn A, Midiani D, Makombe SD, Mnthambala A, Chirwa Z, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet. 2011;378(9787):282–4. Epub 2011/07/19.
19 Moses SE, Tosswill J, Sudhanva M, Poulton M, Zuckerman M. HIV-1 seroconversion during pregnancy resulting in vertical transmission. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2008;41(2):152–3. Epub 2007/12/07.
20 Garcia PM, Kalish LA, Pitt J, Minkoff H, Quinn TC, Burchett SK, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341(6):394–402. Epub 1999/08/05.
21 Kasenga F, Hurtig AK, Emmelin M. Home deliveries: implications for adherence to nevirapine in a PMTCT programme in rural Malawi. AIDS care. 2007;19(5):646–52. Epub 2007/05/17.
22 Kirsten I, Sewangi J, Kunz A, Dugange F, Ziske J, Jordan-Harder B, et al. Adherence to combination prophylaxis for prevention of mother-to-child-transmission of HIV in Tanzania. PloS one. 2011;6(6):e21020. Epub 2011/06/23.
23 Mirkuzie AH, Hinderaker SG, Morkve O. Promising outcomes of a national programme for the prevention of Mother-to-Child HIV transmission in Addis Ababa: a retrospective study. BMC health services research. 2010;10:267. Epub 2010/09/11.
24 Geddes R, Giddy J, Butler LM, Van Wyk E, Crankshaw T, Esterhuizen TM, et al. Dual and triple therapy to prevent mother-to-child transmission of HIV in a resource-limited setting – lessons from a South African programme. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2011;101(9):651-4. Epub 2011/09/17.
25 WHO, UNICEF, UNAIDS, UNFPA. HIV and infant feeding. Update. Based on the technical consultation held on behalf of the Inter-agency Task Team (IATT) on Prevention of HIV Infection in Pregnant Women, Mothers and their Infants; 2006 Oct 25–27. Available from: http://whqlibdoc.who.int/publications/2007/9789241595964_eng.pdf.
26 Vaz MJ, Barros SM, Palacios R, Senise JF, Lunardi L, Amed AM, et al. HIV-infected pregnant women have greater adherence with antiretroviral drugs than non-pregnant women. International journal of STD & AIDS. 2007;18(1):28–32. Epub 2007/03/01.
27 Braitstein P, Katshcke A, Shen C, Sang E, Nyandiko W, Ochieng VO, et al. Retention of HIV-infected and HIV-exposed children in a comprehensive HIV clinical care programme in Western Kenya. Tropical medicine & international health: TM & IH. 2010;15(7):833–41. Epub 2010/05/22.
28 WHO. Global HIV/AIDS response: epidemic update and health sector progress towards Universal Acccess. Progress report 2011. Geneva, World Health Organization, 2011. Available from: http://whqlibdoc.who.int/publications/2011/9789241502986_eng.pdf.
29 Cook RE, Ciampa PJ, Sidat M, Blevins M, Burlison J, Davidson MA, et al. Predictors of successful early infant diagnosis of HIV in a rural district hospital in Zambezia, Mozambique. Journal of acquired immune deficiency syndromes. 2011;56(4):e104–9. Epub 2011/01/27.
30 Azcoaga-Lorenzo A, Ferreyra C, Alvarez A, Palma PP, Velilla E, del Amo J. Effectiveness of a PMTCT programme in rural Western Kenya. AIDS care. 2011;23(3):274–80. Epub 2011/02/25.
31 Mirkuzie AH, Hinderaker SG, Sisay MM, Moland KM, Morkve O. Current status of medication adherence and infant follow up in the prevention of mother to child HIV transmission programme in Addis Ababa: a cohort study. Journal of the International AIDS Society. 2011;14:50. Epub 2011/10/25.
32 Youngleson MS, Nkurunziza P, Jennings K, Arendse J, Mate KS, Barker P. Improving a mother to child HIV transmission programme through health system redesign: quality improvement, protocol adjustment and resource addition. PloS one. 2010;5(11):e13891. Epub 2010/11/19.
33 Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. Epub 2011/07/20.
34 Shelton JD. HIV/AIDS. ARVs as HIV prevention: a tough road to wide impact. Science. 2011;334(6063):1645–6. Epub 2011/12/24.

