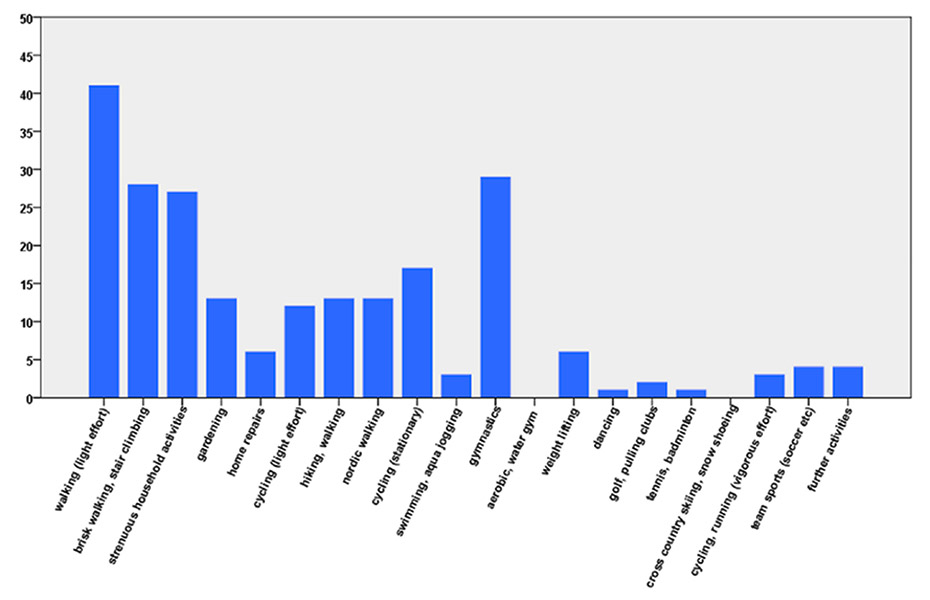
Figure 1
Number of patients who performed the physical activities in a typical week during the previous 2 months; data from questionnaire 1 (n = 48).
DOI: https://doi.org/10.4414/smw.2013.13752
Approximately 40% of all deaths in Switzerland can be attributed to cardiovascular diseases, which corresponds roughly to 40,000 life years lost prematurely per year [1]. Physical inactivity is an important and modifiable risk factor for myocardial infarction and recurrent events [2–3], whereas regular physical activity (PhA) slows down the progression of atherosclerotic lesions, and in some instances may even induce regression [4–5]. Regular PhA and improved fitness are associated with a reduction in long-term morbidity and mortality [6–8]. However, in Europe only around 15% of patients after an acute coronary syndrome or bypass surgery are physically active at least three times a week for 20 minutes [9].
PhA is multidimensional and there are still many unresolved issues concerning the relationship between PhA and coronary heart disease (CHD). Validated and inexpensive instruments are required to assess PhA in order to increase our knowledge in this field of research. The strengths and weaknesses of different assessment methods for PhA are comprehensively discussed in a review by Vanhees et al. [10]. Numerous studies assessing PhA express their results in metabolic equivalent (MET, 1 MET = oxygen uptake per kilogram of body mass per minute while sitting at rest = 3.5 mL.kg–1.min–1) time values, since extent and intensity of PhA seem to play a more important role than the actual type of activity in terms of prognosis in CHD [11–12].
Questionnaires are the most commonly used tool for evaluating PhA because of their low cost and relative ease of use [13]. They possess the quality of non-reactiveness and they can be validated against objective test methods [14]. Such questionnaires allow individual recommendations, which have been shown to increase the level of PhA [15]. In contrast to objective measurements, self-assessment is culture-dependent and it is therefore important to create questionnaires for specific population groups and in different languages, as applicable. To date, there is no questionnaire assessing PhA of cardiac rehabilitation patients in a German-speaking population.
The aim of this study was therefore to develop and validate a new PhA questionnaire in a population of German-speaking cardiac rehabilitation patients.
The PhA questionnaire (appendix) was based on a German PhA questionnaire, which is applicable to a healthy population [16]. Types of physical activities included in the questionnaire were those that were performed weekly by more than one of 250 cardiac patients selected from the Swiss national registry AMIS Plus (Acute Myocardial Infarction in Switzerland), who were interviewed by phone. To further adapt the questionnaire to patients in a cardiac rehabilitation setting, three experienced sports therapists and four cardiac rehabilitation patients were interviewed qualitatively. On the basis of the results of the qualitative content analysis, major revisions were made to the introduction detailing the goal of the survey, to the presentation of the listed activities and to the reference timeframe.

Figure 1
Number of patients who performed the physical activities in a typical week during the previous 2 months; data from questionnaire 1 (n = 48).
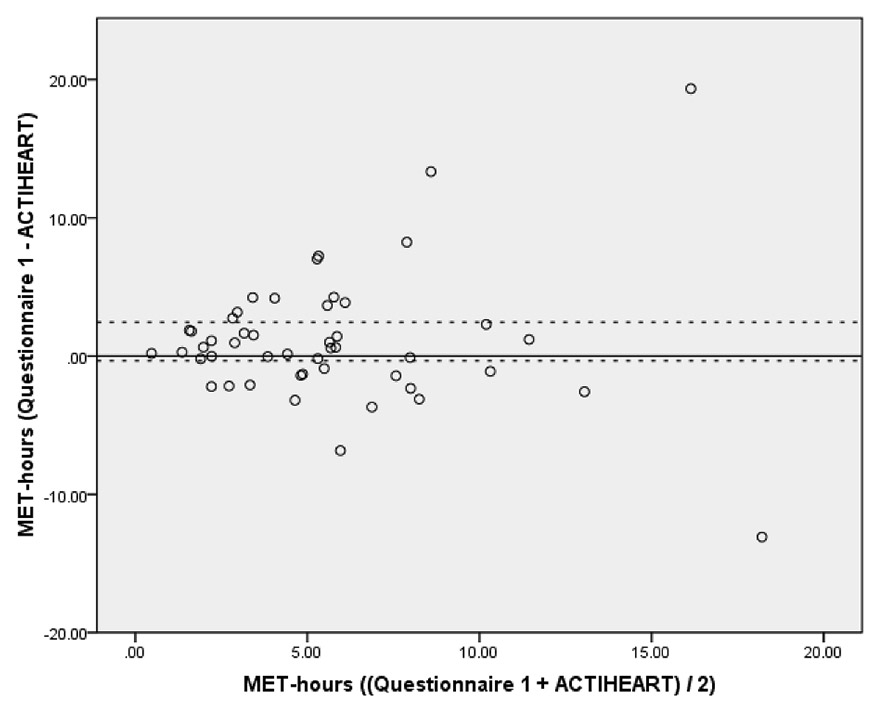
Figure 2
Bland-Altman plot of self-reported (questionnaire 1) minus measured (ACTIHEART) MET-hours (vertical axis) against mean of self-reported and measured MET-hours (horizontal axis). The solid horizontal line corresponds to the mean difference (1.05 MET-hours) while the dashed horizontal lines correspond to the 95% confidence interval (–0.34 to 2.44 MET-hours) (n = 48).
The PhA questionnaire includes 19 activities and 2 open answers for activities not on the list (fig. 1). Various activities included in the German PhA questionnaire have been omitted (child care, haying, chopping wood, stacking wood, singing, using public transport, driving a car or motorcycle, scuba diving, skiing downhill, water-skiing, squash, judo, karate, skating), and other activities were added (household activities, home repairs, nordic walking, cycling (stationary) and water aerobics). Patients had to specify the frequency (in times per week), duration (in minutes) and intensity (Borg’s subjective rating of perceived exertion) of the activities that they performed in a typical week in the previous 2 months.
Combined accelerometry and heart rate measurement using an ACTIHEART-monitor (CamNtech, UK, http://www.camntech.com ) as an objective measure, and a PhA diary as a subjective measure, were used to assess the validity of the PhA questionnaire. To examine the reliability of the PhA questionnaire, it was filled in twice with a 3-week interval.
For the validation study, patients with sufficient knowledge of the German language, who were aged between 30 and 75 years, and who had either registered to take part in the out-patient cardiac rehabilitation programme or had completed the rehabilitation programme more than 3 months previously, were invited to participate. To avoid interference of pacemakers and defibrillators with the ACTIHEART, patients with such devices were excluded, as were patients with chronic heart failure or dementia.
Data collection for the main study was preceded by a pilot phase involving nine cardiac patients. On the basis of these results a power analysis was performed, which showed that a sample of 40 patients would be needed. Fifty-five patients from the Cardiovascular Prevention and Rehabilitation Unit of the University Hospital in Bern agreed to participate and were included in this study.
The validation study was approved by the local Ethics Committee in Bern. All patients included signed an informed consent form.
To calculate the volume of PhA, MET values were taken from the compendium published by Ainsworth et al. [17], and were allocated to the activities which are listed in the questionnaire. The MET value multiplied by the frequency (per week) and duration (minutes were converted to hours) per time resulted in MET-hours for the corresponding category, which were used to quantify the volume of weekly PhA. Furthermore, the numbers of hours in which the patients exercised with an intensity of <3 METs (light PhA), 3–6 METs (moderate PhA) or >6 METs (vigorous PhA) were calculated [18]. All results were then expressed as energy expenditure per day.
The ACTIHEART is a device with a combined heart rate and accelerometer monitor which provides reliable information on the intensity, frequency and duration of PhA, thus determining energy expenditure [19]. The ACTIHEART was validated against indirect calorimetry in field tests [20–21], as well as in the laboratory [22]. Correlation between energy expenditure measured using the ACTIHEART and measured by means of indirect calorimetry was high for activities of light, moderate and high intensities (Pearson’s r = 0.79, 0.72 and 0.80, respectively) [22]. For the purpose of this study, an ACTIHEART device was attached to the chest with the aid of two latex-free electrodes (Ambu Blue Sensor VL) [23]. The device was programmed to record heart rate and movements at 1-minute intervals for 1 week. Patients were asked not to remove the ACTIHEART during the 1-week monitoring. In case the ACTIHEART did not adhere after extended bathing or swimming, patients were given additional electrodes for replacement. A validated algorithm converted the data into MET levels. The algorithm discriminates, in a first step, between “activity” and “no activity”, depending on the acceleration measured. In a further step, different heart rate thresholds are applied in the presence and absence of activity, respectively. Detailed information can be found at http://www.camntech.com/files/The_Actiheart_User_manual.pdf . [24–25]. The algorithm was based on the following data, fed into the ACTIHEART in advance: age, sex, height and weight. All MET levels <3.0 were excluded as these data were neither collected in the questionnaire nor in the PhA diary. Finally, the MET-minutes were converted into MET-hours and then divided by the number of days monitored.
The PhA diary included questions concerning the time, type and duration of activity, and Borg’s subjective rating of perceived exertion. Data from the PhA diary was transformed manually into MET-hours: each activity performed was allocated a MET value taken from the compendium of Ainsworth et al. [17] and multiplied by the number of hours. All results were divided by the number of days recorded.
After the ACTIHEART was attached to the chest and recording quality was tested, monitoring was initiated. Patients received instructions on how to fill in the PhA diary over the subsequent week.
Seven days later, the ACTIHEART was removed, data downloaded and the PhA diary was collected. Patients were asked to fill in the PhA questionnaire for the first time (questionnaire 1 http://www.smw.ch/fileadmin/smw/pdf/SMW-13752_Fragebogen_SWISSPAQ_2012.pdf ). Three weeks after the second visit patients were asked to fill in the PhA questionnaire for a second time (questionnaire 2 http://www.smw.ch/fileadmin/smw/pdf/SMW-13752_Fragebogen_SWISSPAQ_2012.pdf ).
Statistical analyses were performed using SPSS® software (Version 17.0). To detect whether variables were normally distributed, Kolmogorov-Smirnov normality tests were used [26]. Differences between the results of the different PhA assessment methods were examined using t-tests for parametric or Wilcoxon signed rank tests for non-parametric data. Fisher’s exact test was applied to categorical variables. Correlations between MET-hours from the different measuring tools were examined using Pearson correlation coefficients. To express the correlations between the number of hours in each intensity group (<3, 3–6, >6 METs), Spearman correlation coefficients were used. Bland-Altman plots were used to illustrate agreement between the questionnaire and ECG-accelerometry as well as for the reproducibility of the PhA questionnaire [27].
| Table 1: Baseline characteristics of the patients (n = 48). | |
| Age (years), mean ± SD | 60 ± 8 (range 42–75) |
| Females, n (%) | 11 (23) |
| Height (cm), mean ± SD | 170 ± 10 |
| Weight (kg), mean ± SD | 78 ± 12 |
| Body Mass Index (kg.m–2), mean ± SD | 27 ± 4 |
| Cardiac diagnosis | |
| Myocardial infarction, n (%) | 36 (75) |
| Coronary heart disease without an acute event, n (%) | 9 (19) |
| Valvular heart disease, n (%) | 2 (4) |
| Aortic aneurysm, n (%) | 1 (2) |
| Cardiac interventions(more than one answer possible) | |
| Percutaneous coronary intervention, n (%) | 39 (81) |
| Coronary artery bypass grafting, n (%) | 8 (17) |
| Other cardiac surgery*, n (%) | 3 (6) |
| * Mitral valve repair, aortic valve replacement, aortic replacement surgery. | |
Of the 55 patients selected for initial inclusion, seven (12.7%) had to be excluded. One patient experienced tachycardia during ACTIHEART monitoring, two patients had allergic skin reactions and four patients had either failed or incomplete (<3 days) ECG-accelerometry measurements. Thus, 48 patients (37 male, 11 female) were finally included. Baseline characteristics, including diagnosis and type of intervention, are shown in table 1. Twenty-four (50%) of the patients belonged to a maintenance heart group, nine patients (18.8%) were included at admission to the cardiac rehabilitation programme, and 15 patients (31.3%) were tested 3 months after completion of the 12-week rehabilitation program.
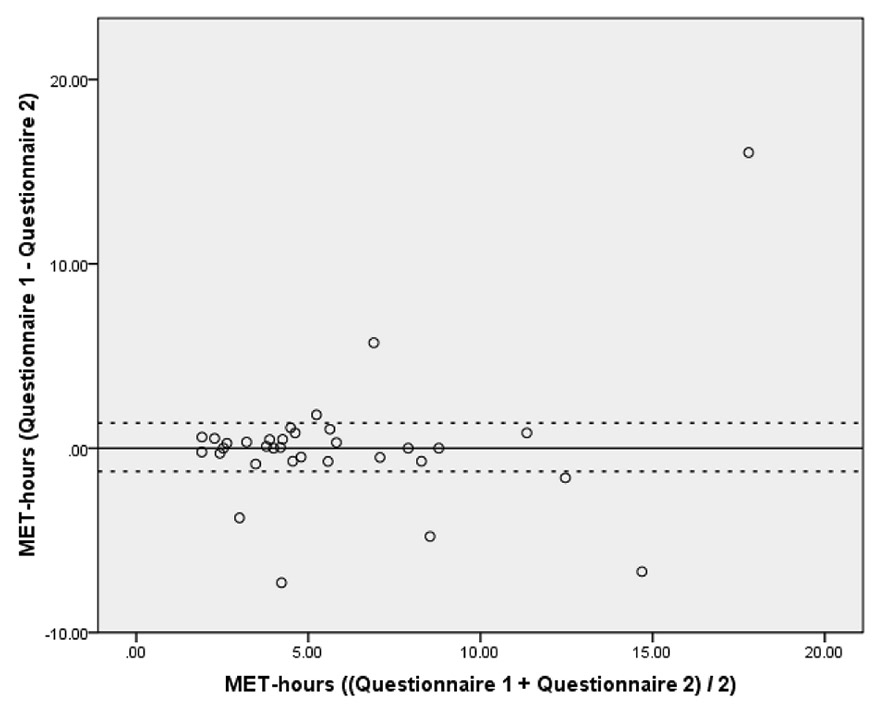
Figure 3
Bland-Altman plot of questionnaire 1 minus questionnaire 2 (vertical axis) against mean of questionnaire 1 and questionnaire 2 measured in MET-hours (horizontal axis). The solid horizontal line corresponds to the mean difference (0.06 MET-hours) while the dashed horizontal lines correspond to the 95% confidence interval (–1.26 to 1.37 MET-hours) (n = 33).
From the PhA questionnaire, the sum of moderate to vigorous PhA was calculated to be on average (mean ± standard deviation; SD) 6.14 ± 4.46 MET-hours per day. The MET-hours per day measured using ECG-accelerometry over 3–8 days (mean 6 days) were on average 5.08 ± 4.34. The MET-hours from the PhA questionnaire were on average 1.05 ± 4.79 (95% confidence interval (CI) of the difference = –0.34 to 2.44; p = 0.135) higher than the MET-hours from ECG-accelerometry. In the Bland-Altman plot, measurements above or below the dashed lines do not indicate good agreement between the two assessment methods (fig. 2). However the variability was mainly due to three outliers. The correlation between the MET-hours from questionnaire and ECG-accelerometry was r = 0.407 (p = 0.004).
The number of hours per day that a patient spent in a particular MET group (<3, 3–6, >6 METs) was calculated. No significant differences were found between the number of hours from the PhA questionnaire and those from ECG-accelerometry for the three MET groups (p = 0.525, 0.538 and 0.158, respectively) (table 2). However, the correlation was considerably better for the light-and moderate-activity MET groups than for the MET group reflecting vigorous PhA. Vigorous PhA (>6 METs) was performed on average for 4 ± 14 minutes per day. Over half of the patients spent no time on activities >6 METs.
A sub-analysis was conducted in patients with and without beta-blockers. The correlation of the MET-hours for patients without beta-blockers (n = 14) was r = 0.61 (p = 0.02), compared with r = 0.33 (p = 0.054) for patients with beta-blockers (n = 34).
PhA diary data were available for 46 (95.8%) patients. The average number of MET-hours per day was 6.60 ± 3.87. There was a high correlation between the PhA diary and PhA questionnaire (r = 0.412, p = 0.004).
Questionnaire 2 counted as the retest within the scope of validation. Thirty-three patients completed the questionnaire a second time on average 25 ± 8 days after questionnaire 1 (range 14‒51 days). The total MET-hours per day taken from questionnaires 1 and 2 did not significantly differ (p = 0.931). The correlation of the MET-hours from both PhA questionnaires was r = 0.624 (p <0.001). The Bland-Altman plot (fig. 3) shows that the mean difference between questionnaire 1 and questionnaire 2 was minimal and amounted to 0.06 MET-hours (95% CI of the difference = –1.26 to 1.37). However, the SD of 3.70 MET-hours was relatively high, which can mainly be ascribed to six outliers.
| Table 2: Comparison of the questionnaire 1 data with the ACTIHEART measurements (n = 48). | ||
| MET group | ||
| <3 METs | 3–6 METs | |
| Questionnaire 1 (hours per group), mean ± SD (median) | 22.5 ± 0.9 (22.8) | 1.4 ± 0.8 (1.3) |
| ACTIHEART (hours per group), mean ± SD (median) | 22.7 ± 1.1 (22.9) | 1.3 ± 1.0 (1.1) |
| Correlation between questionnaire 1 and ACTIHEART, r and 95% CI | 0.513 0.268–0.696 | 0.518 0.274–0.699 |
| p-value of correlation | <0.001 | <0.001 |
This study reports the validation of a PhA questionnaire to assess PhA in German-speaking cardiac rehabilitation patients. This PhA questionnaire represents a readily applicable method to assess PhA levels in cardiac rehabilitation patients. It provides a tool for physicians and therapists to monitor activity levels in their patients and to make recommendations accordingly.
The PhA questionnaire primarily comprises everyday activities and activities of moderate intensity. Walking was the most common activity, which is consistent with other validation studies [28–29]. Participants spent approximately 1.5 hours per day performing physical activities of 3 to 6 METs. The high volume of activity in this study population could be due to the fact that most of these patients took part in a structured ambulatory rehabilitation program and that a considerable number of patients also took part in a maintenance heart group, where they remained aware of the importance of PhA.
The result of this validation study, with a correlation of r = 0.41 between the MET-hours of the PhA questionnaire and the ECG-accelerometry, is in line with those of other studies in elderly patients which cite correlations of r = 0.35 to r = 0.56 between subjective and objective instruments measuring PhA [28], and is considered acceptable [30]. However, because of the wide confidence intervals the results of this validation study have to be interpreted with caution.
In contrast to other questionnaires [31–32], the validity of our PhA questionnaire was better for physical activities with lower intensities than for higher intensities. Similar results were shown in the study by DuBose et al. [33]. One reason for this finding could be that the PhA questionnaire focuses strongly on moderate physical activity, which is more common in a population of cardiac patients, rather than vigorous physical activity. Another reason could be that cardiac medication such as beta-blockers directly influences heart rate at rest and during PhA, thereby affecting the calculation of PhA used in the combined heart rate and accelerometry measurements [34]. As the heart rate does not increase as sharply when performing moderate activity as it does during vigorous activity, it might have less impact on the calculation of METs using the ACTIHEART algorithm. A sub-analysis showed that MET-hours from the questionnaire and ECG-accelerometry correlated better in patients without beta-blockers than in patients with beta-blockers. Data derived from the PhA diaries did not correlate significantly with data from ECG-accelerometry. Practical problems such as time constraints, forgetting to fill in the diary properly, and the well-known tendency to overstate are the main reasons for this common finding.
In order to use the PhA questionnaire in a non-German speaking population, it would be necessary to translate it back and forth several times and to further test it qualitatively in order to adapt it to the cultural aspects and characteristics of the particular population group being studied.
The test-retest analysis of this study resulted in a correlation of r = 0.62, with a mean difference between questionnaire 1 and 2 of 0.06 MET-hours per day. This indicates that the reliability of the PhA questionnaire was moderate to good. However, the confidence interval was relatively wide, primarily owing to six outliers, approximately a fifth of the questionnaires.
Test-retest reliability has inherent limitations [35]. PhA levels may change between the test and the retest time period, especially if the time between filling in questionnaires was long. This would lead to an underestimation of reliability. On the other hand, the patient may remember his answers and repeat them rather than answer the questions truly, which would lead to an overestimation of reliability. The risk of this occurring is greater if the time period between completing questionnaires is short. We consider the timeframe of 3 weeks as intermediate, so that one error may balance the other.
Physical inactivity is an important cardiovascular risk factor. A recent study by Gerber et al. [36] found a strong inverse, gradual relationship between recreational PhA after a myocardial infarction and mortality risk, with regular PhA halving the risk of mortality. Steinacker et al. (2011) showed beneficial long-term effects in terms of morbidity and mortality for participants of either comprehensive in-patient or out-patient cardiac rehabilitation programmes [37].
Measuring PhA using an easily applicable questionnaire that is sufficiently valid and reliable is crucial for the planning, realisation and evaluation of intervention programs on PhA in both primary and secondary prevention.
Self-administered questionnaires on physical activities tend to overestimate PhA levels because of social desirability. Furthermore, our results are likely to be affected by selection (especially participation) bias, as the study was voluntary and half of the participants belonged to a maintenance heart group, thus presumably having a more structured and more active everyday life. The results may also be affected by recall bias. The volume of physical activities with higher intensities was very small in this study population, thereby reducing the validity of the PhA questionnaire for vigorous physical activities. As the reference timeframe of the PhA questionnaire was 2 months, seasonal aspects may have to be considered. The applicability of the PhA questionnaire is limited to patients who know the Borg scale of PhA intensity. Since the majority of the patients being studied were within normal weight ranges, the applicability of the PhA questionnaire to obese patients must be questioned. Furthermore, as patients with pacemakers and chronic heart failure were excluded, there is no solid evidence that the questionnaire may be valid in these patients. However, further validation of the questionnaire in such patient groups is planned.
The use of measurements of energy expenditure using a combined heart rate and movement monitor and the PhA diary to validate a questionnaire do not conform to the gold standard [10]. However, the simultaneous heart rate and movement measurement is considered a very useful technique to validate other field-based PhA assessment methods, especially those designed to measure the time spent in moderate or vigorous activity [38]. The patients could have been influenced by wearing the ACTIHEART, in that they could have been more conscious of their own physical activity levels. However, the ACTIHEART was not equipped with a display, so the patients themselves did not receive data on the physical activities performed. The assessed time period using the objective measuring method was 1 week, whereas the PhA questionnaire covered the previous 2 months. However, this limitation is likely to under- rather than overestimate the correlation between the PhA questionnaire and the ECG-accelerometry.
This study demonstrates acceptable validity and moderate-to-good reliability of the newly developed PhA questionnaire to assess PhA levels in German-speaking cardiac rehabilitation patients. Further studies would be needed to implement this questionnaire in different populations of cardiac patients under different circumstances.
Acknowledgement: The authors would like to thank Jenny Piket and Prisca Eser for proof-reading the manuscript.
1 Schweizerische Herzstiftung. Available at: http://www.swissheart.ch/d/service/facts.htm [Accessed February 2, 2009].
2 Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52.
3 Chow CK, Jolly S, Rao-Melacini P, Fox KA, Anand SS, Yusuf S. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary syndromes. Circulation. 2010;121(6):750–8.
4 Hambrecht R, Niebauer J, Marburger C, Grunze M, Kalberer B, Hauer K, et al. Various intensities of leisure time physical activity in patients with coronary artery disease: effects on cardiorespiratory fitness and progression of coronary atherosclerotic lesions. J Am Coll Cardiol. 1993;22(2):468–77.
5 Sato S, Makita S, Uchida R, Ishihara S, Majima M. Physical activity and progression of carotid intima-media thickness in patients with coronary heart disease. J Cardiol. 2008;51(3):157–62.
6 Richardson CR, Kriska AM, Lantz PM, Hayward RA. Physical activity and mortality across cardiovascular disease risk groups. Med Sci Sports Exerc. 2004;36(11):1923–9.
7 Hamer M, Stamatakis E. Physical activity and mortality in men and women with diagnosed cardiovascular disease. Eur J Cardiovasc Prev Rehabil. 2009;16(2):156–60.
8 Oguma Y, Shinoda-Tagawa T. Physical activity decreases cardiovascular disease risk in women: review and meta-analysis. Am J Prev Med. 2004;26(5):407–18.
9 Kotseva K, Wood D, De Backer G, De Bacquer D, Pyorala K, Keil U. EUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Eur J Cardiovasc Prev Rehabil. 2009;16(2):121–37.
10 Vanhees L, Lefevre J, Philippaerts R, Martens M, Huygens W, Troosters T, et al. How to assess physical activity? How to assess physical fitness? Eur J Cardiovasc Prev Rehabil. 2005;12(2):102–14.
11 Lee IM, Rexrode KM, Cook NR, Manson JE, Buring JE. Physical activity and coronary heart disease in women: is “no pain, no gain” passe? JAMA. 2001;285(11):1447–54.
12 Manson JE, Hu FB, Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;341(9):650–8.
13 Ainsworth BE, Bassett DR, Jr., Strath SJ, Swartz AM, O’Brien WL, Thompson RW, et al. Comparison of three methods for measuring the time spent in physical activity. Med Sci Sports Exerc. 2000;32(9 Suppl):S457–64.
14 Kriska A, Caspersen C. Introduction to a Collection of Physical Activity Questionnaires. Med Sci Sports Exerc. 1997;29(Supplement 6):5–9.
15 Kallings LV, Sierra Johnson J, Fisher RM, Faire U, Stahle A, Hemmingsson E, et al. Beneficial effects of individualized physical activity on prescription on body composition and cardiometabolic risk factors: results from a randomized controlled trial. Eur J Cardiovasc Prev Rehabil. 2009;16(1):80–4.
16 Mader U, Martin BW, Schutz Y, Marti B. Validity of four short physical activity questionnaires in middle-aged persons. Med Sci Sports Exerc. 2006;38(7):1255–66.
17 Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–504.
18 Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273(5):402–7.
19 Strath SJ, Bassett DR, Jr., Swartz AM, Thompson DL. Simultaneous heart rate-motion sensor technique to estimate energy expenditure. Med Sci Sports Exerc. 2001;33(12):2118–23.
20 Brage S, Brage N, Franks PW, Ekelund U, Wareham NJ. Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur J Clin Nutr. 2005;59(4):561–70.
21 Crouter SE, Churilla JR, Bassett DR, Jr. Accuracy of the Actiheart for the assessment of energy expenditure in adults. Eur J Clin Nutr. 2008;62(6):704–11.
22 Barreira T, Kang M, Caputo J, Farley S, Renfrow M. Validation of the Actiheart Monitor for the Measurement of Physical Activity. International Journal of Exercise Science 2009;2(1):60–71.
23 Brage S, Brage N, Ekelund U, Luan J, Franks PW, Froberg K, et al. Effect of combined movement and heart rate monitor placement on physical activity estimates during treadmill locomotion and free-living. Eur J Appl Physiol. 2006;96(5):517–24.
24 Brage S, Brage N, Franks PW, Ekelund U, Wong MY, Andersen LB, et al. Branched equation modeling of simultaneous accelerometry and heart rate monitoring improves estimate of directly measured physical activity energy expenditure. J Appl Physiol. 2004;96(1):343–51.
25 Brage S, Ekelund U, Brage N, Hennings MA, Froberg K, Franks PW, et al. Hierarchy of individual calibration levels for heart rate and accelerometry to measure physical activity. J Appl Physiol. 2007;103(2):682–92.
26 Chakravarti IM, Laha RG, Roy J. Handbook of methods of applied statistics. New York,: Wiley; 1967.
27 Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10.
28 Harris TJ, Owen CG, Victor CR, Adams R, Ekelund U, Cook DG. A comparison of questionnaire, accelerometer, and pedometer: measures in older people. Med Sci Sports Exerc. 2009;41(7):1392–402.
29 Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288(16):1994–2000.
30 Orrell A, Doherty P, Miles J, Lewin R. Development and validation of a very brief questionnaire measure of physical activity in adults with coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2007;14(5):615–23.
31 Pettee Gabriel K, McClain JJ, Lee CD, Swan PD, Alvar BA, Mitros MR, et al. Evaluation of physical activity measures used in middle-aged women. Med Sci Sports Exerc. 2009;41(7):1403–12.
32 Wendel-Vos GC, Schuit AJ, Saris WH, Kromhout D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol. 2003;56(12):1163–9.
33 DuBose KD, Edwards S, Ainsworth BE, Reis JP, Slattery ML. Validation of a historical physical activity questionnaire in middle-aged women. J Phys Act Health. 2007;4(3):343–55.
34 Wonisch M, Hofmann P, Fruhwald FM, Kraxner W, Hodl R, Pokan R, et al. Influence of beta-blocker use on percentage of target heart rate exercise prescription. Eur J Cardiovasc Prev Rehabil. 2003;10(4):296–301.
35 Bühner M. Einführung in die Test- und Fragebogenkonstruktion. 2. ed. München: Pearson Studium; 2006. German
36 Gerber Y, Myers V, Goldbourt U, Benyamini Y, Scheinowitz M, Drory Y. Long-term trajectory of leisure time physical activity and survival after first myocardial infarction: a population-based cohort study. Eur J Epidemiol. 2011;26(2):109–16.
37 Steinacker JM, Liu Y, Muche R, Koenig W, Hahmann H, Imhof A, et al. Long term effects of comprehensive cardiac rehabilitation in an inpatient and outpatient setting. Swiss Med Wkly. 2011;140:w13141.
38 Strath SJ, Bassett DR, Jr., Thompson DL, Swartz AM. Validity of the simultaneous heart rate-motion sensor technique for measuring energy expenditure. Med Sci Sports Exerc. 2002;34(5):888–94.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.