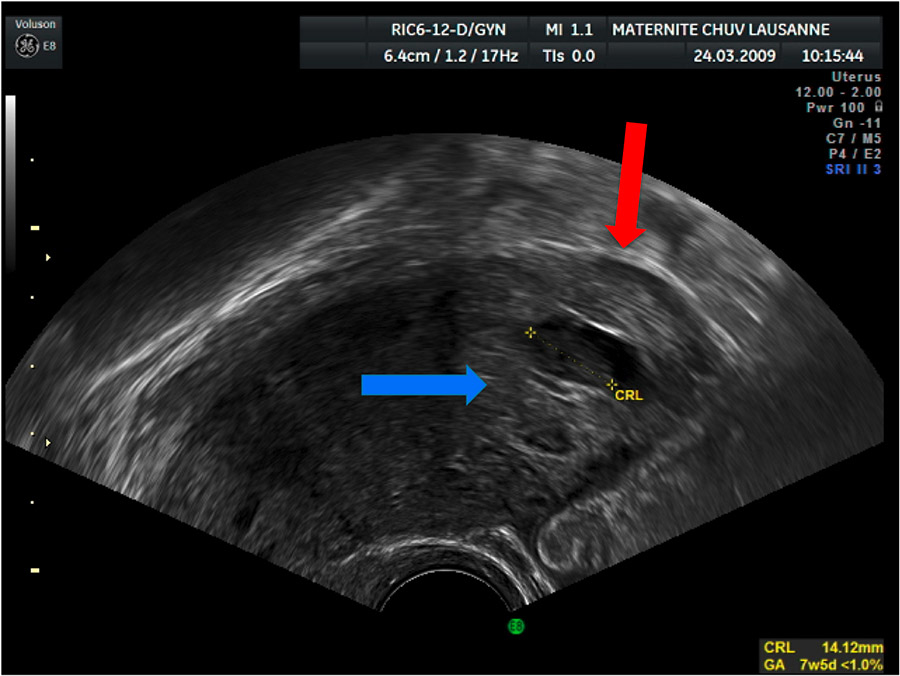
Figure 1
TVUS sagittal image showing an eccentric GS, blue arrow, surrounded by a thin myometrial layer, red arrow.
DOI: https://doi.org/10.4414/smw.2013.13736
Interstitial pregnancy (IP) is defined as a pregnancy where the product of conception is implanted in the intramural portion of the Fallopian tube. It is an uncommon kind of ectopic pregnancy (EP) accounting for around 2% of EP, i.e. one pregnancy for every 2,500–5,000 live births. It can be differentiated from angular and cornual pregnancy, where the product of conception is implanted in one of the lateral angles of the uterine cavity, medial to the uterotubal junction and round ligament, or in a horn of a bicornuate uterus, respectively. Most of the risk factors for IP are similar to those for tubal EP, i.e. a history of pelvic inflammatory disease, previous EP, pelvic surgery, smoking and a conception resulting from infertility treatment techniques. However, the use of intrauterine device as contraception is less frequent in patients with IP than in those with tubal or ovarian EP [1].
IP is a potentially life threatening pathology with a mortality rate of 2.5% [2] due to the risk of rupture in a highly vascularised area. The majority of ruptures are described at around 12 weeks of gestation, but they can happen between 7 and 12 weeks. This observation makes early diagnosis very important in order to offer a conservative treatment [3]. The aim of this review is to describe the management of IP in our institution between 2001 and 2011 and to define some general rules for the clinical practice.
A single institution retrospective study was conducted at the University Hospital of Lausanne (CHUV – Centre Hospitalier Universitaire Vaudois), Switzerland. Data on the diagnosis of IP, its management and patient follow up were collected from our computerised medical chart (DIAMM).
The diagnosis of IP was made either by transvaginal ultrasound (TVUS) or during a surgical diagnostic procedure. TVUS were performed in order to localise an early pregnancy or in case of symptoms, signs and laboratory results suspicious of EP or miscarriage.
The ultrasonographic criteria as described by Timor-Tritsch in 1992 [4] and later modified by Bourdel et al. [3] were used:

Figure 1
TVUS sagittal image showing an eccentric GS, blue arrow, surrounded by a thin myometrial layer, red arrow.
1 an eccentric gestational sac (GS) in regard to the uterus in a sagittal axis (fig. 1);
2 the absence of the double decidual sac sign around the GS;
3 less than 5 mm of myometrial layer around the GS (fig. 1);
4 the interstitial line sign, i.e. the presence of an echogenic line that joins the endometrial cavity to the GS in the cornual region.
We analysed the different treatment options, their results and the need for a second or third line treatment. Medical data of the different therapies are discussed as well as the follow up duration and its impact on the therapeutic approach.
The medical treatment adopted in our institution consisted either in systemic intramuscular Methotrexate (IM-MTX) injection (1 mg/kg) or direct Methotrexate injection (1 mg/kg) into the cornual region (IC-MTX) through laparoscopy or under TVUS, when the patient was haemodynamically stable and the pregnancy had been diagnosed as interstitial on TVUS. The follow up then included beta-HCG levels at days four and seven after MTX injection. If the decline of the beta-HCG level was less than 15% of the pre-MTX value, a second injection of MTX was performed; otherwise a weekly follow up was carried out until negativity. After two MTX injections a rise or a plateau in beta-HCG levels was an indication for laparoscopy.
When the clinical or haemodynamic condition of the patient did not allow conservative treatment, laparoscopy allowed cornuectomy or cornuostomy depending on the size of the IP and clinical situation.
Between 2001 and 2011, 1,334 patients were admitted with a suspicion of EP of which 758 (56.8%) were confirmed to have EP. Among these 758 patients, eleven had an IP (1.4% of EP).
The eleven patients with a diagnosis of IP had a median age of 33 years (range 25–39). The median gestity was four (range 1–8) and the median parity was one (range 0–5).
Symptomatic patients consulted the gynaecological outpatient unit for abdominal pain with or without associated spotting. Abdominal pain ranged from sudden cramps or sharp pelvic pain to dull abdominal discomfort. Asymptomatic patients were diagnosed with a cornual pregnancy during a routine ultrasound or, in case of in vitro fertilization, because of a suspicion of miscarriage or EP. TVUS allowed a definitive diagnosis of IP in seven out of 11 cases (64%), while in all the other cases a diagnostic laparoscopic procedure was necessary for a definitive diagnosis.
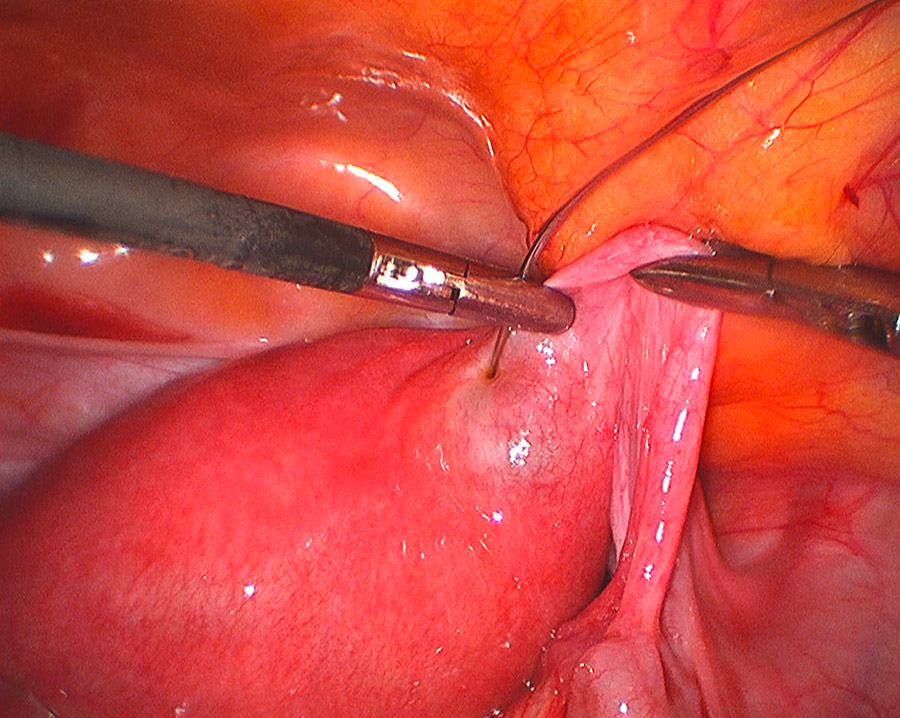
Figure 2
Laparoscopic intracornual MTX injection.
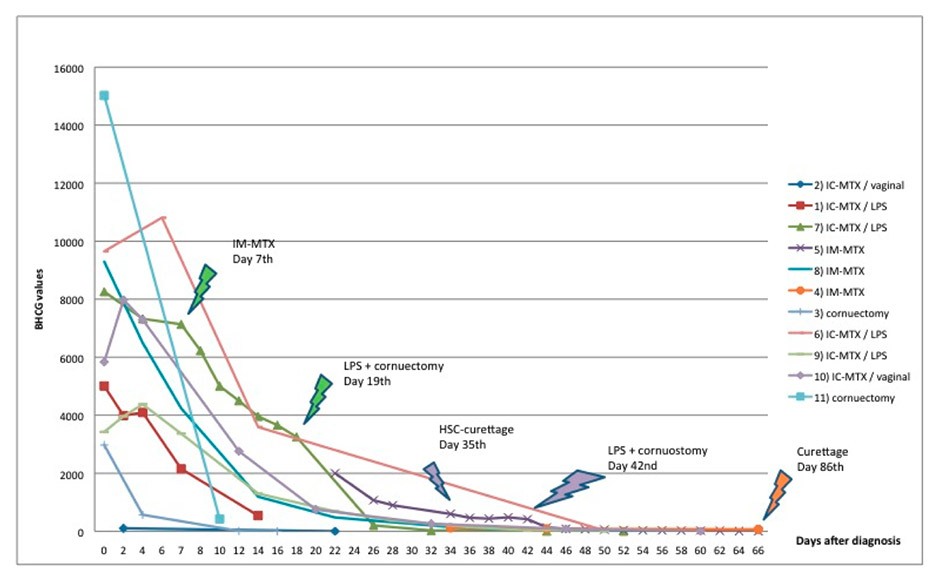
Figure 3
Beta-HCG level follow up after first, second and third line therapies.
The diagnosis was made at a median gestational age of seven weeks (range 5–10 weeks), with a median beta-HCG level of 5838 U/l (range 2,974–15,022).
With regard to IP risk factors, seven patients (64%) had a previous history of gynaecological surgery. Four of these had already had surgery for tubal EP: one case of salpingotomy, two salpingectomy and one had salpingotomy followed by a salpingectomy. The last three patients had, respectively, a left ovariectomy, one laparoscopic myomectomy and a bilateral salpingectomy.
Four of eleven IPs followed in vitro fertilization (IVF). None of the patients was using an intrauterine device at the time of the IP.
Nine patients were treated with MTX whereas two had a cornual resection during a diagnostic laparoscopy, converted to laparotomy in one case. Three out of nine patients were treated with intramuscular MTX (IM-MTX) and in the remaining six MTX was injected directly into the cornual region (IC-MTX): in four cases during a laparoscopy (fig. 2) and in two cases during a transvaginal ultrasound procedure.
The follow-up consisted in monitoring the beta-HCG level decline (fig. 3). The median time to beta-HCG resolution was 58 days (range 22–132 days). In the six patients treated with MTX and who did not need a second intervention, the time to beta-HCG resolution was 58 days for the only patient treated with IM-MTX and between 22 and 59 days for the five patients treated with IC-MTX (with a median of 52 days).
Three patients experienced post treatment side effects: one complained of sharp abdominal pain accompanied by nausea and vomiting on the day of IM-MTX injection and another reported an increase in abdominal pain seven days after a TVUS-guided IC-MTX. There was no evidence of uterine rupture in both cases. The third patient experienced weakness and dizziness the day after laparoscopic salpingectomy and cornuectomy for a ruptured IP, with a haemoglobin value of 94 g/l.
One patient asked repeatedly for a definitive surgical approach because of psychological exhaustion due to the stagnation of beta-HCG after three doses of IM-MTX administered in another institution and a hysteroscopy curettage performed in order to differentiate from an angular pregnancy. Another patient treated with IM-MTX needed a second intervention by curettage in order to treat heavy bleeding during the expulsion of the gestational sac 86 days after the IM-MTX injection. One more patient needed a second line intervention by laparoscopic cornuectomy and salpingectomy 21 days after laparoscopic IC-MTX because of a plateau in beta-HCG follow up. No case of cornual rupture was observed following conservative therapy.
With regard to fertility, six patients had further pregnancies (nine in total) and gave birth to healthy children in six of the nine cases. In the three other cases, one patient had a right tubal EP one year after the right IP, another patient had a voluntary termination of pregnancy and the third one has an ongoing pregnancy. Eight out of the nine pregnancies occurred after conservative treatment by MTX (either IM-MTX or IC-MTX) and one after cornuectomy. Three of the six patients needed IVF. All deliveries were by caesarean section. There were no cases of uterine rupture, in utero death or other remarkable complications.
| Table 1: Patient characteristics. | ||||||||||
| N. | Year | Age | Gestity Parity | Weeks of Gest. | ¥ | 1st ttt | 2nd ttt | 3rd ttt | History | Later pregnancies |
| 1 | 2001 | 30 years | 6G5P | 6 | Spont. | IC-MTX/LPS | – | – | R Salpingectomy EP | Cesarean 2002 (spont.) 1 voluntary abortion 2004 |
| 2 | 2003 | 32 years | 4G1P | 6 | Spont. | IC-MTX/ TVUS | – | – | L Ovariectomy (dermoid cyst) | R EP 2004 Twins- cesarean 2005 (IVF) |
| 3 | 2006 | 32 years | 6G0P | 6 | IVF | LPS/LPT cornuectomy | – | – | R salpingotomy EP R salpingectomy EP L salpingectomy EP | Cesarean 2010 (IVF) |
| 4 | 2007 | 34 years | 1G0P | 7 | IVF | Curettage/IM-MTX | Curettage under US Day-86 | – | Laparoscopic myomectomy | Cesarean 2008 (spont.) Cesarean 2010 (spont.) |
| 5 | 2008 | 39 years | 3G0P | 9 | IVF | IM-MTX (3X) | HSC-curettage Day-35 | LPS day-42 cornuostomy | L salpingectomy EP R salpingectomy hydrosalpinx Laparoscopic myomectomy | – |
| 6 | 2009 | 33 years | 4G1P | 5 | Spont. | IC-MTX/LPS | – | – | Cesarean 2010 (spont.) | |
| 7 | 2009 | 36 years | 3G1P | 7 | Spont. | IC-MTX/LPS | IM-MTX Day-7 | LPS day-21 cornuectomy and salpingectomy | – | – |
| 8 | 2009 | 28 years | 1G0P | 7 | Spont. | IM-MTX | – | – | – | – |
| 9 | 2010 | 25 years | 4G1P | 9 | Spont. | IC-MTX/LPS | – | – | L salpingotomy EP | – |
| 10 | 2011 | 33 years | 1G0P | 5 | IVF | IC-MTX/TVUS | – | – | Bilateral salpingectomy hydrosalpinx | Ongoing pregnancy |
| 11 | 2011 | 37 years | 8G2P | 10 | Spont. | LPS cornuectomy | – | – | – | – |
| Gest. = gestation; Spont. = spontaneous; ttt = treatment; IC-MTX = intra cornual methotrexate; LPS = laparoscopy; R = right; EP = ectopic pregnancy; TVUS = trans vaginal ultrasound; L = left; IVF = in vitro fertilization; LPT = laparotomy; IM-MTX = intra muscular methotrexate; US = ultrasound; HSC = hysteroscopy. | ||||||||||
Six of the eleven patients in our series had at least one of the risk factors for IP mentioned in the introduction. A high prevalence of previous EP, gynaecological surgery and of pregnancies resulting from in vitro fertilization was observed. The in vitro fertilization techniques can either represent an independent risk factor for IP or simply reflect the high prevalence of infertility caused by the other risk factors: indeed in our series all the patients who needed IVF had an history of previous gynaecological pelvic surgery.
These observations emphasize the importance of early ultrasound imaging in patients with a history of possible tubal damage since IP is often pauci-symptomatic until rupture occurs. The relatively high median age in our series can also be related to the high prevalence of prior tubal damage and the relative high gestity of our patients.
Although IP is often asymptomatic or pauci-symptomatic, it can present with symptoms and signs typical of EP, such as abdominal tenderness (from pelvic discomfort to strong abdominal pain in case of cornual rupture) associated with vaginal bleeding, even if this latter sign is less common in IP than in tubal EP [5]. These observations were confirmed in our series, where only one patient consulted because of vaginal bleeding, while five of the eleven presented with abdominal pain ranging from sudden cramps to dull abdominal discomfort.
IP diagnosis is usually made by TVUS, which is the key to an accurate localisation of IP. An accurate and early localisation of the pregnancy by TVUS is a pre-requisite for a conservative treatment in asymptomatic or pauci-symptomatic patients [6–10] and in our series the earliness of the diagnosis was the factor that allowed a conservative treatment in the majority of cases. IP diagnosis and its differentiation from angular pregnancy nevertheless remain challenging and in the literature the ultrasound diagnosis of IP is accurate in 71.4% of cases [10].
Progress in 3D TVUS makes this technique a good and precise diagnostic tool. In particular, the 3D reconstruction in a coronal sectional plane allows localisation of the GS in relation to the uterine cavity, therefore helping in differentiating the IP from an angular pregnancy [11–13] (fig. 4).
In very specific situations (e.g. in case of obesity, when TVUS diagnosis can be equivocal) magnetic resonance imaging (MRI) can be used [3, 14, 15]. None of our patients needed MRI.
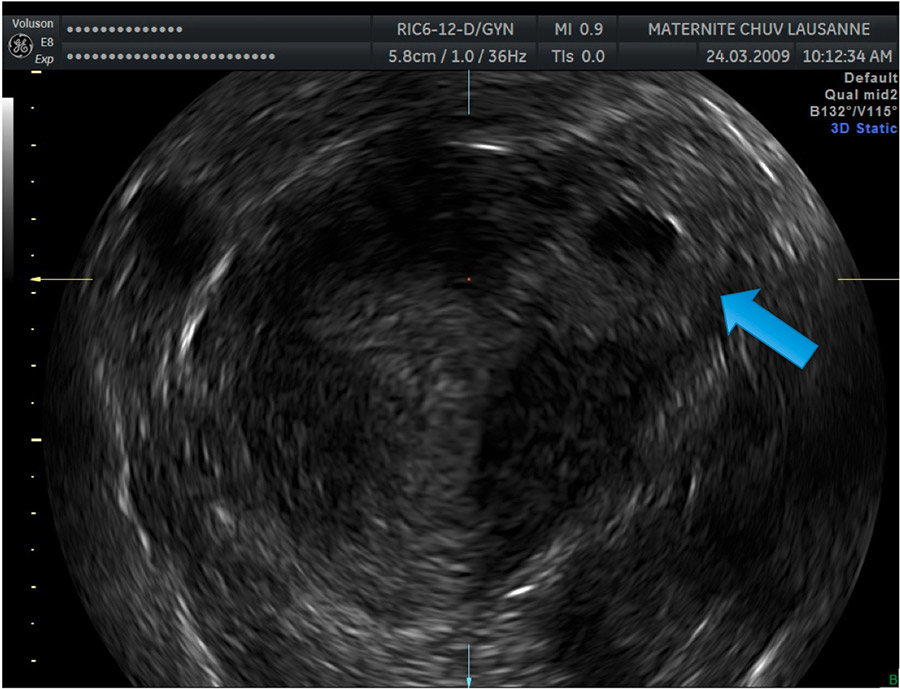
Figure 4
Image showing a left IP 3D reconstruction in a coronal sectional plane. The blue arrow indicates the lateral GS surrounded by a thin myometrial layer.
Unlike other kinds of EP, the initial level of beta-HCG in IP has no real impact on diagnosis and management. The follow up of beta-HCG levels is nevertheless fundamental in allowing a second line therapy in case of rising or plateau before the onset of major complications. However, beta-HCG can persist over a long period of time and this must be taken into account due to its possible psychological impact on patients. Furthermore, the length of beta-HCG fall could make the follow up schedule applied to EP not suitable for IP. In our series, in two of the three patients who needed a second line treatment, the decision was based on the plateau of the beta-HCG level. However, in the third patient, the third line treatment was based more on the kinetic of the beta-HCG fall, which was estimated to be too slow, than on the presence of a real plateau. That kinetic was very similar to those of other patients who did not receive additional treatments. Possibly the acceptance of a slower rate in beta-HCG level fall could have avoided a surgical treatment in this patient. Other studies also reported a long decline in beta-HCG levels, often exceeding 8 weeks [16, 17].
The treatment of IP depends on many factors, i.e. the clinical conditions of the patient, the wish for future pregnancies, the week of gestation at presentation, the diameter of the GS, the physician’s experience and the possibility of performing a long follow up.
A conservative treatment can be performed by IM-MTX [18] or by intra-cornual MTX through a laparoscopic or US-controlled vaginal approach [19–21]. IM-MTX allows successful treatment without the need for an additional procedure in 80–94% of cases [7, 18] thus lowering the cost and the possible complications of surgery, but it is hampered by the risk of cornual rupture with subsequent catastrophic haemorrhage [22, 23]. For this reason, a close follow up and a 24 hour access to medical and surgical care are necessary. Moreover, the fall in beta-HCG levels seems to be longer than after IC-MTX injection, as is suggested in our series. A recently published paper reports the successful management of two IPs with IM-MTX combined with Mifepristone 600 mg, but the eventual advantages over IM-MTX alone remain to be established [24].
IC-MTX, through laparoscopy or TVUS-guided procedure, has a higher success rate than IM-MTX, similar to that of cornuostomy (86–100%) [7]. On the other hand, it is also hampered by the risk of cornual rupture, it implies the performance of an associated procedure, including all the consequent risks (anaesthesia, haemorrhages, infections, injuries to other organs), it requires special expertise and the results are operator dependent. Taking this into account, IC-MTX should be used when laparoscopy is necessary to establish the definitive diagnosis and to look for and eventually treat associated pathologies or complications. In our series one of six patients treated with IC-MTX and two of three patients treated by IM-MTX needed a second and third line treatment, without a significant difference in the gestational age at diagnosis (between six and nine weeks). We can thus conclude that in our experience IC-MTX seems to be more efficacious than IM-MTX and that IC-MTX under TVUS guidance can be a good alternative to IM-MTX due to its higher efficacy coupled with the absence of the risks related to laparoscopy. These observations confirm those of several other studies [7, 16, 25].
When a second injection of MTX is needed, it is usually performed IM and can be associated with a selective uterine artery embolization, so as to interrupt the blood supply and obtain a spontaneous necrosis of IP. This technique nevertheless remains experimental until more information on safety and efficacy is gathered [26–28].
It must be noted, however, that the use of MTX, IC or IM, implies a long follow up of beta-HCG level resolution and of the patient’s clinical condition. For this reason, the ability of the patient to show up at follow up controls, her level of understanding of the danger of her condition and her psychological compliance must be evaluated and should, when possible, be discussed with the patient when choosing the appropriate treatment.
When the patient’s clinical conditions, the persistence of trophoblastic material or the impossibility of respecting the follow up protocol do not allow a conservative treatment, a surgical approach is indicated, with salpingectomy or hysterectomy as a last option in case of uncontrolled bleeding [5]. In our series two of the eleven patients were treated by cornuectomy as a first line treatment; in the first case a diagnostic laparoscopy was converted to laparotomy because of persistent bleeding from the ruptured uterine horn and in the second case a cornuectomy was performed because of massive bleeding from the ruptured IP. Two other patients needed a surgical approach after the failure of conservative management: one could be treated by a laparoscopic cornuostomy while the local conditions of the uterine horn required a laparoscopic cornuectomy and salpingectomy in the other patient.
Cornual resection is thus considered the classical intervention, but with pregnancies <40 mm in diameter a simple linear incision over the interstitial pregnancy and expression of the gestational tissue (cornuostomy) can be attempted. Laparoscopy is the preferred approach in well equipped centres with trained surgical and reanimation teams [8, 29–31].
Different case reports describe techniques to improve haemostasis control during laparoscopic procedures (Endoloop, automatic staplers such as Endo GIA Auto Suture, uterine artery ligation, advanced bipolar coagulation) [32–36].
The treatment should also aim at preserving fertility, if this represents the wish of the patient. In our series, all patients were clinically stable at diagnosis and none needed hysterectomy. Five patients had pregnancies after conservative treatment by MTX and one after cornuectomy. Independent of the kind of treatment performed all patients were delivered by elective caesarean section because of the risk of uterine rupture [37, 38].
In conclusion, we can recommend conservative treatment as a first line therapy in those patients who are stable at diagnosis, who can comply with a possibly long follow up and whose IP has been diagnosed at an early gestational age. We achieved better results with IC-MTX, which was performed in four of six cases during a laparoscopy. Nevertheless, because of the rarity of IP and the heterogeneity in managements and treatments, many questions still remain open. The sensitivity and specificity of currently available diagnostic tools (TVUS and 3D TVUS) should be increased and the exact role and benefits of TVUS-guided IC-MTX should be better established. At the same time, clear and unambiguous attitudes regarding initial treatment, follow up and second line therapy in case of persistent IP need to be defined. Considering the rarity of this pathology, multicentre studies are required to definitely answer these questions.
1 Bouyer J, Coste J, Fernandez H, Pouly JL, Job-Spira N. Sites of ectopic pregnancy: a 10 year population-based study of 1800 cases. Hum Reprod. 2002;17(12):3224–30.
2 Walker JJ. Ectopic pregnancy. Clin Obstet Gynecol. 2007;50(1):89–99.
3 Bourdel N, Roman H, Gallot D, Lenglet Y, Dieu V, Juillard D, et al. Interstitial pregnancy. Ultrasonographic diagnosis and contribution of MRI. A case report. Gynecol Obstet Fertil. 2007;35(2):121–4.
4 Timor-Tritsch IE, Monteagudo A, Matera C, Veit CR. Sonographic evolution of cornual pregnancies treated without surgery. Obstet Gynecol. 1992;79(6):1044–9.
5 Moawad NS, Mahajan ST, Moniz MH, Taylor SE, Hurd WW. Current diagnosis and treatment of interstitial pregnancy. Am J Obstet Gynecol. 2010;202(1):15–29.
6 Agdi M, Tulandi T. Surgical treatment of ectopic pregnancy. Best Pract Res Clin Obstet Gynaecol. 2009;23(4):519–27.
7 Lau S, Tulandi T. Conservative medical and surgical management of interstitial ectopic pregnancy. Fertil Steril. 1999;72(2):207–15.
8 MacRae R, Olowu O, Rizzuto MI, Odejinmi F. Diagnosis and laparoscopic management of 11 consecutive cases of cornual ectopic pregnancy. Arch Gynecol Obstet. 2009;280(1):59–64.
9 Tulandi T. New laparoscopic technique for interstitial pregnancy resection. J Reprod Med. 1998;43(2):159–60.
10 Tulandi T, Al-Jaroudi D. Interstitial pregnancy: results generated from the Society of Reproductive Surgeons Registry. Obstet Gynecol. 2004;103(1):47–50.
11 Nardozza LM, Moron AF. Three-dimensional transvaginal sonographic diagnosis of early and asymptomatic interstitial pregnancy. Arch Gynecol Obstet. 2007;275(3):207–10.
12 Araujo Júnior E, Zanforlin Filho SM, Pires CR, Guimarães Filho HA, Massaguer AA, Nardozza LM, et al. Three-dimensional transvaginal sonographic diagnosis of early and asymptomatic interstitial pregnancy. Arch Gynecol Obstet. 2007;275(3):207–10.
13 Valsky DV, Hamani Y, Verstandig A, Yagel S. The use of 3D rendering, VCI-C, 3D power Doppler and B-flow in the evaluation of interstitial pregnancy with arteriovenous malformation treated by selective uterine artery embolization. Ultrasound Obstet Gynecol. 2007;29(3):352–5.
14 Filhastre M, Dechaud H, Lesnik A, Taourel P. Interstitial pregnancy: role of MRI. Eur Radiol. 2005;15(1):93–5.
15 Parker RA 3rd, Yano M, Tai AW, Friedman M, Narra VR, Menias CO. MR Imaging Findings of Ectopic Pregnancy: A Pictorial Review. Radiographics. 2012;32(5):1445–60.
16 Jourdain O, Fontanges M, Schiano A, Rauch F, Gonnet JM. Management of other ectopic pregnancies (cornual, interstitial, angular, ovarian). J Gynecol Obstet Biol Reprod (Paris). 2003;32(7 Suppl):S93–100.
17 Klemm P, Koehler C, Eichhorn KH, Hillemanns P, Schneider A. Sonographic monitoring of systemic and local methotrexate (MTX) therapy in patients with intact interstitial pregnancies. J Perinat Med. 2006;34(2):149–57.
18 Jermy K, Thomas J, Doo A, Bourne T. The conservative management of interstitial pregnancy. BJOG. 2004;111(11):1283–8.
19 Timor-Tritsch IE, Monteagudo A, Mandeville EO, Peisner DB, Anaya GP, Pirrone EC. Successful management of viable cervical pregnancy by local injection of methotrexate guided by transvaginal ultrasonography. Am J Obstet Gynecol. 1994;170(3):737–9.
20 Frishman, GN. Transvaginal ultrasound-guided methotrexate injection of cornual ectopic pregnancy. J Am Assoc Gynecol Laparosc. 2004;11(1):1.
21 Morgan M, Aziz M, Mikhail M, Henein M, Atalla R. Ultrasound guided treatment of cornual ectopic pregnancy. Eur J Obstet Gynecol Reprod Biol. 2009;143(2):126.
22 Barnhart K, Spandorfer S, Coutifaris C. Medical treatment of interstitial pregnancy. A report of three unsuccessful cases. J Reprod Med. 1997;42(8):521–4.
23 Voigt RR, Van d, V, Karsdorp VH, Hogerzeil HV, Ketting BW. Treatment of interstitial pregnancy with methotrexate: report of an unsuccessful case. Hum Reprod. 1994;9(8):1576–9.
24 Gómez García MT, Aguarón Benitez G, Barberá Belda B, Callejón Rodríguez C, González Merlo G. Medical therapy (methotrexate and mifepristone) alone or in combination with another type of therapy for the management of cervical or interstitial ectopic pregnancy. Eur J Obstet Gynecol Reprod Biol. 2012 Jul 7. [Epub ahead of print]
25 Benifla JL, Fernandez H, Sebban E, Darai E, Frydman R, Madelenat P. Alternative to surgery of treatment of unruptured interstitial pregnancy: 15 cases of medical treatment. Eur J Obstet Gynecol Reprod Biol. 1996;70(2):151–6.
26 Deruelle P, Lucot JP, Lions C, Robert Y. Management of interstitial pregnancy using selective uterine artery embolization. Obstet Gynecol. 2005;106(5 Pt 2):1165–7.
27 Deruelle P, Closset E. Management of interstitial pregnancy using selective uterine artery embolization. Obstet Gynecol. 2006;107(2 Pt 1):427–8.
28 Ophir E, Singer-Jordan J, Oettinger M, Odeh M, Tendler R, Feldman Y, et al. Uterine artery embolization for management of interstitial twin ectopic pregnancy: case report. Hum Reprod. 2004;19(8):1774–7.
29 Grobman WA, Milad MP. Conservative laparoscopic management of a large cornual ectopic pregnancy. Hum Reprod. 1998;13(7):2002–4.
30 Api M, Api O. Laparoscopic cornuotomy in the management of an advanced interstitial ectopic pregnancy: a case report. Gynecol Endocrinol. 2010;26(3):208–12.
31 Ng S, Hamontri S, Chua I, Chern B, Siow A. Laparoscopic management of 53 cases of cornual ectopic pregnancy. Fertil Steril. 2009;92(2):448–52.
32 Sergent F, Le Cornec JB, Meilhaud MF, Marpeau L. Laparoscopic cornual excision with an automatic stapler for ruptured interstitial pregnancies. J Gynecol Obstet Biol Reprod. 2003;32(5):426–30.
33 Raheem M, Afifi Y. Laparoscopic selective ipsilateral uterine artery ligation for the management of a cornual ectopic pregnancy. J Minim Invasive Gynecol. 2008;15(3):260–1.
34 Khawaja N, Walsh T, Gill B. Uterine artery ligation for the management of ruptured cornual ectopic pregnancy. Eur J Obstet Gynecol Reprod Biol. 2005;118(2):269.
35 Rahimi, MA. A new laparoscopic approach for the treatment of interstitial ectopic pregnancy. J Am Assoc Gynecol Laparosc. 1999;6(2):205–7.
36 Zuo X, Shen A, Chen M. Successful management of unruptured interstitial pregnancy in 17 consecutive cases by using laparoscopic surgery. Aust N Z J Obstet Gynaecol. 2012;52(4):387–90.
37 Downey GP, Tuck SM. Spontaneous uterine rupture during subsequent pregnancy following non-excision of an interstitial ectopic gestation. Br J Obstet Gynaecol. 1994;101(2):162–3.
38 Weissman A, Fishman A. Uterine rupture following conservative surgery for interstitial pregnancy. Eur J Obstet Gynecol Reprod Biol. 1992;44(3):237–9.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.