Figure 1
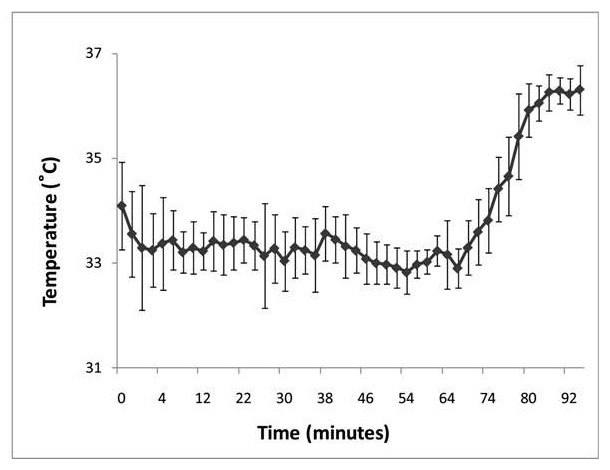
Mean rectal temperature (± SD) of the cohort during induction and maintenance of passive cooling and during rewarming.
DOI: https://doi.org/10.4414/smw.2013.13767
Therapeutic hypothermia has become the standard neuroprotective treatment modality for term newborns suffering from encephalopathy following perinatal asphyxia. All published randomised controlled studies in newborns have documented its long-term neurological benefit [1–6]. The technique of hypothermia induction and maintenance remains a subject of research. So far, all clinical studies in neonates have used active induction of hypothermia either by head cooling [2] or by total body surface cooling [1, 3–6]. However, the risk of hypothermic overshoot after initiation of active cooling has been well recognized in these studies. Uncontrolled and deeper hypothermia has been shown to accentuate hypothermia-induced side effects such as pulmonary hypertension, low cardiac output, dysrhythmia, coagulopathy, electrolyte disturbances, and sepsis [7]. Finally, it has been shown in experimental studies that severe over-cooling may abolish any neuroprotective effects of therapeutic hypothermia [8].
Cooling is a strong stimulator of thermal stress response not only in adults, but also in newborns [9]. Thermal stress response is mainly characterised by peripheral vasoconstriction and increase in metabolic rate, and it carries the risk of complete abolishment of the neuroprotective effect of hypothermia if not controlled [10]. Pharmacological agents such as sedatives and analgesics have been shown to decrease stress response by decreasing the endogenous thermal set point and therefore body temperature [7]. In addition, human newborns, like other mammalian newborns, react with an endogenous neuroprotective response to severe hypoxia, which is characterised by hypothermia and a reduced metabolic rate [11–12]. In previous experimental studies, we noticed that postischaemic neonatal rats were extremely prone to uncontrolled hypothermia in ambient temperatures [13–14]. We therefore speculated that in situations of hypothermia induced endogenously by neonatal hypoxia, further suppression of the thermal set point by pharmacological agents might suffice not only to quickly induce but also to maintain hypothermia over a prolonged period of time. Such a technique may be feasible without the need of cooling devices.
Three multidisciplinary neonatal and paediatric intensive care units in Switzerland agreed upon a common standard treatment protocol based on passive cooling throughout the necessary period of hypothermia on the one hand, and, on the other hand, to decrease body temperature with the aid of pharmacological agents or with ice packs as backup cooling methods. In the present report, we summarise preliminary data on the feasibility and safety of passive cooling for induction and maintenance of therapeutic hypothermia in asphyxiated infants. In addition, we compare our results of passive cooling with the results of active cooling from published and successful studies conducted by the National Institute of Child Health and Human Development (NICHD) neonatal research network [3, 15].
We retrospectively collected and analysed data on all asphyxiated newborns treated with therapeutic hypothermia in the neonatal and paediatric intensive care units of Zurich, Luzern and Bern between 1 April 2005 and 30 June 2007. All babies were treated according to a common standard protocol similar to the ones used by Eicher et al. [1] and by Shankaran et al. [3]. The institutional medical ethics committees approved the study; the requirement for informed consent was waived.
The inclusion criteria were: A) clinical signs of hypoxia-ischaemia: severe acidosis (pH ≤7 or BE ≥ –12 mmol/l) at or within one hour after birth, or a perinatal event (uterine rupture, placental abruption, cord prolapse or rupture, late or variable decelerations, haemorrhage, cardiorespiratory arrest) combined either with Apgar scores at 5 or 10 minutes of ≤5 or with continuing need of assisted ventilation for more than 5 min after birth and: B) neurologic signs and symptoms of encephalopathy, i.e., the presence of postnatal seizures or clinical signs of encephalopathy corresponding to Sarnat stages 2 and 3. Exclusion criteria were inability to start hypothermia treatment within 6 hours after birth, gestational age <35 weeks, birth weight <2000 g, or severe congenital malformations.
Rectal temperature targets were chosen to be above 35 °C during initial resuscitation and transportation and, following admission to the intensive care unit, to be lowered to 33.0 ± 0.5 °C [1] or to 33.5 ± 0.5 °C [3]. The temperature target was to be reached within two hours of starting cooling. Rectal and toe temperatures were monitored continuously. Passive cooling was achieved by placing the uncovered infant in a primarily unheated neonatal open care unit. Room temperature of the intensive care units in Switzerland are maintained around 24 °C. In addition, pharmacological agents (barbiturates, opiates or benzodiazepines) were given not only to treat discomfort but also when core temperatures remained above target range despite passive cooling. When pharmacological agents did not suffice to reach target temperature, or if pharmacological agents were felt to be contraindicated by the intensivist in charge, active cooling with ice packs (put on head and groin) was added. Finally, as a last resort, a cooling mattress could be used. If core temperatures fell below target range, then the overhead heater from the open care unit was turned on as needed. After 72 hours of hypothermia, temperature was allowed to increase slowly by 0.5 °C per hour and kept at 36 ± 0.5 °C for another 48 hours. Breathing gas temperatures were kept normothermic at all times.
Infants received standard intensive care including extensive laboratory investigations, neurophysiological monitoring, and brain imaging. Arterial blood gases were corrected for body temperature. Physiological data were documented hourly. The start of hypothermia was defined as the first documented core temperature of ≤35°C. Criteria for cessation of therapeutic hypothermia were intracranial haemorrhage, or therapy-refractory shock or pulmonary hypertension.
For comparison of feasibility and safety of our new passive cooling protocol we used the data published in the successful NICHD studies [3, 15] and searched for matching data from the newborn group treated with the total body active cooling protocol. From both groups – our passively cooled group and the actively cooled NICHD-group – we compared time from birth to target temperature, incidence and degree of initial hypothermic overshoot, and variability of temperature during maintenance cooling. We also compared available data on perinatal characteristics, severity of illness or adverse events, and short term outcome.
Data are presented as mean ± standard deviation, if not otherwise stated. Normal distribution of data was checked. When comparing data between the presented study and the NICHD study, either one-sample or two-sample t-tests were used or the Fisher’s exact test with the aid of StatXact 9.0 (Cytel Inc, Cambridge, MA, U.S.A).
| Table 1: Perinatal characteristics and signs of asphyxia. | |||
| Passive hypothermia (n = 18) | NICHD study (n = 102) | ||
| Birthweight (g) | 3136 ± 404 | 3385 ± 617 | p = 0.10& |
| Gestational age (weeks) | 39.4 (±1.5) | not documented | |
| Born elsewhere (%) | 14 (78) | 48 (47) | p = 0.021* |
| Emergency Caesarean section (%) | 10 (56) | 72 (71) | p = 0.27* |
| pH ≤7.0 or BE ≥ –12 mmol/l | 16 (89) | not documented | |
| Apgar score ≤5 at 5 min (%) | 15 (83) | 92 (91) | p = 0.41* |
| Apgar score ≤5 at 10 min (%) | 14 (78) | 80 (84) | p = 1.00* |
| Time to spontaneous respiration ≥10 min | 14 (78) | 69 (71) | p = 0.58* |
| Mean ± SD; &Two-sample t-test; *Fisher’s exact test | |||
A total of 23 infants were treated with therapeutic hypothermia. Five newborns were excluded from data analysis because of protocol violations: two because the target temperature was chosen to be 34 °C, one because the hypoxic insult occurred two hours after birth, and two because cooling blankets were used from start. Overall, 72% of the 18 remaining newborns had defined perinatal events. At the start of the therapeutic hypothermia, 6 patients showed moderate encephalopathy (Sarnat 2), 2 babies had severe encephalopathy (Sarnat 3), and in 10 infants Sarnat stage was assumed to be at least moderate. Three infants presented with clinical seizures. Other perinatal characteristics of our patients were similar to those of the cooled NICHD study patients, except for a higher percentage of outborn infants in our study (table 1).

Figure 1
Mean rectal temperature (± SD) of the cohort during induction and maintenance of passive cooling and during rewarming.
Average temperature changes of the whole cohort during cooling and rewarming are shown in figure 1. Additional cooling characteristics are shown in table 2. When compared to the NICHD study, infants in the present study were cooled and reached target temperature significantly earlier, whereas the time from initiation of cooling to reaching target temperature was similar. Maximal overshoot during the cooling induction was significantly less than in the NICHD study. The standard deviations of the two average maintenance temperatures during cooling were similar when compared with the NICHD study. In the present study, four patients (22%) did not require any active cooling at all. The other 14 newborns (78%) were actively cooled with ice packs over a total of 9.6 ± 7.5 hours on average, corresponding to only 15 ± 10% of total cooling time being roughly 3.5 days (see fig. 1). Details on active cooling times were missing from one patient. However, cooling blankets were not used in any of the 18 study patients. All babies received opiates, and 39% of them were treated with additional sedatives.
In the present study, two newborns required no mechanical ventilation despite cooling and opiates. Seventeen (95%) of all babies had some form of circulatory support. There was no proven blood-stream infection, but empiric antibiotic treatment was started in 14 patients. Finally, cooling was stopped prematurely in six patients. In one infant, this was done because of cardiac arrhythmias and hyperkalaemia (potassium concentration of 7.2 mmol/l), which was easily treated with calcium administration. In another five patients, cooling was stopped prematurely because of redirection of care and withdrawal of life-sustaining therapy. Death occurred in six babies during the hospital course. Diverse markers of severity of illness were similar when compared with the cooled NICHD study patients, except for a higher percentage of patients needing cardiovascular drugs in our study (table 3).
| Table 2: Cooling characteristics. | |||
| Passive hypothermia (n = 18) | NICHD study (n = 102) | ||
| Postnatal age at start of cooling (min) | 242 ± 103 | 302 | p = 0.024+ |
| Postnatal age when reaching target temperature (min) | 319 ± 135 | 392 | p = 0.035+ |
| Induction time from cooling initiation to target temperature (min) | 71 ± 53 | 90 | p = 0.15+ |
| Maintenance temperature during cooling at target temperature At temperature target of 33.5 ± 0.5 °C At temperature target of 33.0 ± 0.5 °C | 33.4 ± 0.6 33.1 ± 0.5 | 33.4 + 0.9 | p = 1.00& |
| Maximal overshoot during induction in °C | –0.4 + 0.6 | –1.4 ± 0.6 | p <0.001& |
| Infants (%) with temperatures recorded <32.0 °C after reaching target temperature | 3 (17%) | 27 (27%) | p = 0.56* |
| Mean ± SD; +One sample t-test; &Two-sample t-test; *Fisher’s exact test | |||
| Table 3: Severity of illness during cooling. | |||
| Passive hypothermia (n = 18) | NICHD studies (n = 102) | Fisher’s exact test | |
| Use of cardiovascular drugs | 16 (89%) | 42 (42%) | p <0.001 |
| Platelet transfusion | 1 (6%) | 20 (20%) | p = 0.19 |
| Fresh frozen transfusion | 9 (50%) | Not reported | |
| Volume expanders | 14 (78%) | 55 (54%) | p = 0.073 |
| Nitric oxide inhalation | 2 (11%) | 17 (17%) | p = 0.74 |
| Seizures at 24 hours | 5 (28%) | 19 (20) | p = 0.74 |
| Cardiac arrhythmia | 1 (6%) | 1 (1%) | p = 0.28 |
| Hypoglycaemia+ | 12 (12%) | p = 0.21 | |
| Hepatic dysfunction& | 7 (39%) | 20 (20%) | p = 0.12 |
| Coagulopathy* | 3 (16%) | 18 (18%) | p = 1.00 |
| Persistent metabolic acidosis++ | 1 (6%) | 1 (1%) | p = 0.28 |
| Proven blood stream infection | 5 (5%) | p = 1.00 | |
| Death during hospital course | 6 (33%) | 19 (19%) | p = 0.21 |
| 1 Missing data for persistent acidosis in the “passive hypothermia” group; +glucose concentration <1.7 mmol/l; &aspartate aminotransferase >200 IU/I and alanine aminotransferase >100 IU/l, *2 out of 3 pathological values: prothrombin time, activated partial thromboplastin time or fibrinogen; ++pH <7.15 for more than 3 h after initiation of intervention | |||
In summary, our study showed that passive cooling without any additional cooling device was feasible for the entire treatment period in 4 out of 18 newborns suffering from encephalopathy after perinatal insult and in 14 newborns active cooling with ice packs was applied only during 15% of total cooling time. This is the first study demonstrating feasibility of passive cooling during 72 hours for therapeutic hypothermia. Spontaneous hypothermia in newborns has been known to occur for some time. Newborns suffering from perinatal asphyxia are especially likely to drop their body temperature after birth [16]. This phenomenon is part of an endogenous neuroprotective reaction also found in other mammalian newborns, and is characterised by hypothermia and hypometabolism [11–12]. In previous experimental studies with neonatal rats, we could confirm the occurrence of spontaneous postischaemic hypothermia [13–14]. All these observations suggest that spontaneous hypothermia in postischaemic human babies may be sufficient to fulfil the requirements of therapeutic hypothermia. Indeed, feasibility of passive cooling during neonatal transportation has recently been described [17]. In conclusion, postischaemic passive cooling is based upon a solid physiological background and, as illustrated by the results of our study, appears to be clinically feasible over 72 hours. We speculate that with increasing experience the percentage of active cooling time will further decrease.
To control overshooting of hypothermia, the overhead heating device of the open care unit was used. Two other published successful RCT’s of postischaemic hypothermia also used overhead heating devices [2, 6]. This counter-warming of some parts while cooling centrally or other parts of the body has been shown in adults to diminish the thermoregulatory stress response to hypothermia (shivering and vasoconstriction) independently from pharmacological interventions [18]. This is important since an uncontrolled thermoregulatory stress response may abolish the neuroprotective properties of hypothermia [10]. Therefore counter-warming has the potential to enhance the neuroprotective effects of cooling and appears to be an attractive adjunct to hypothermia protocols.
Compared with the cooled NICHD study patients [3], there was an earlier postnatal age at the start of cooling and earlier postnatal age when reaching target temperatures in our study. In experimental studies, time to initiation of cooling and time to reach target temperature have been shown to be crucial for neuroprotective efficacy of postischaemic hypothermia: the faster cooling is initiated and the faster target temperature is reached, the better the neuroprotection [13, 19]. We speculate that the simplicity of passive cooling may be an important reason why average target temperatures were reached one hour earlier in our study when compared to the NICHD study. Another reason could be that we did not need to get consent from parents as cooling was the clinical standard and therefore treatment could be started earlier. However, it should be noted that most infants were born elsewhere and required transport to our unit.
In addition, there was a smaller maximum overshoot during initiation of hypothermia suggesting that temperature control by nurses may be more reliable than by some high-tech cooling devices. Another reason for less overshooting may be the use of counter-warming measures and of pharmacological agents to reduce the thermoregulatory stress response. Decreased thermal stress response during cooling may lead to better peripheral vasodilatation with less central temperature pooling, and easier cooling initiation and stabilisation at hypothermic core temperatures, with less overshooting [7]. Two recent studies in neonates suffering from perinatal asphyxia have shown minimal overshooting during induction and maintenance phases of therapeutic hypothermia by using a new servo-controlled cooling device: mean maximal overshoot was only 0.3 °C [20–21]. However, neither study documented the consequences of the new cooling algorithm on induction time, where time to reach target temperature might become prolonged and may therefore theoretically impede neuroprotective effectiveness [13, 19].
We also tried to compare the severity of the perinatal hypoxic insults between our and the cooled NICHD study patients, since cooling effectiveness may depend upon severity of ischaemic insult. Precooling characteristics appear to be very similar except for the proportion of outborns, which was higher in our cohort and may have been associated with a more severe illness. Postcooling characteristics were also similar, except for a higher percentage of vasoactive drug use in our study. Theoretically, the latter may be due to the higher percentage of infants born elsewhere or due to the lower target temperature in our study. However, it may also be considered to be an adverse event secondary to the cooling method and the adjunct therapies used such as sedation and analgesia.
There are several limitations of the retrospective data collection and analysis. The start of cooling was difficult to estimate retrospectively. In addition, frequency of temperature recording was not standardised in detail between the participating centres, but was at least hourly. Therefore, induction time and amount of overshooting might have been underestimated when compared with the NICHD study, in which temperature was recorded every 15 minutes for 4 hours, then hourly for 8 hours and thereafter every 4 hours. We estimate that the risk of underestimation is low since in all intensive care units measured physiological values out of target were routinely recorded.
In conclusion, our preliminary study suggests that passive cooling is feasible and effective to achieve and maintain target temperatures in therapeutic hypothermia in asphyxiated newborn infants. In addition, it appears to be associated with a lower risk of overshooting. These data support the continuation of this multicentre standard treatment protocol and the further investigation of the clinical feasibility and safety of passive hypothermia in asphyxiated neonates.
Acknowledgement: We thank RA Ammann, MD, for independent statistical analysis.
1 Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32(1):11–7.
2 Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–70.
3 Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–84.
4 Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–58.
5 Simbruner G, Mittal RA, Rohlmann F, Muche R, neo.nEURO.network Trial Participants. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010;126(4):e771–8.
6 Jacobs SE, Morley CJ, Inder TE, Stewart MJ, Smith KR, McNamara PJ, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165(8):692–700.
7 Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med. 2009;37(3):1101–20.
8 Weinrauch V, Safar P, Tisherman S, Kuboyama K, Radovsky A. Beneficial effect of mild hypothermia and detrimental effect of deep hypothermia after cardiac arrest in dogs. Stroke. 1992;23(10):1454–62.
9 Hey EN. The relation between environmental temperature and oxygen consumption in the newborn baby. J Physiol. 1969;200(3):589–603.
10 Tooley JR, Satas S, Porter H, Silver IA, Thoresen M. Head cooling with mild systemic hypothermia in anesthetized piglets is neuroprotective. Ann Neurol. 2003;53(1):65–72.
11 Miller JA. Factors in Neonatal Resistance to Anoxia. I. Temperature and Survival of Newborn Guinea Pigs Under Anoxia. Science. 1949;110(2848):113–4.
12 Gautier H. Interactions among metabolic rate, hypoxia, and control of breathing. J Appl Physiol. 1996;81(2):521–7.
13 Wagner BP, Nedelcu J, Martin E. Delayed postischemic hypothermia improves long-term behavioral outcome after cerebral hypoxia-ischemia in neonatal rats. Pediatr Res. 2002;51(3):354–60.
14 Adcock KH, Nedelcu J, Loenneker T, Martin E, Wallimann T, Wagner BP. Neuroprotection of creatine supplementation in neonatal rats with transient cerebral hypoxia-ischemia. Dev Neurosci. 2002;24(5):382–8.
15 Shankaran S, Pappas A, Laptook AR, McDonald SA, Ehrenkranz RA, Tyson JE, et al. Outcomes of safety and effectiveness in a multicenter randomized, controlled trial of whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2008;122(4):e791–8.
16 Robertson NJ, Nakakeeto M, Hagmann C, Cowan FM, Acolet D, Iwata O, et al. Therapeutic hypothermia for birth asphyxia in low-resource settings: a pilot randomised controlled trial. Lancet. 2008;372(9641):801–3.
17 Kendall GS, Kapetanakis A, Ratnavel N, Azzopardi D, Robertson NJ. Cooling on Retrieval Study Group. Passive cooling for initiation of therapeutic hypothermia in neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2010;95(6):F408–12.
18 Sweney MT, Sigg DC, Tahvildari S, Iaizzo PA. Shiver Suppression Using Focal Hand Warming in Unanesthetized Normal Subjects. Anesthesiology. 2001;95(5):1089–95.
19 Laptook A. Use of Therapeutic hypothermia for term infants with hypoxic-ischemic encephalopathy. Pediatr Clin N Am. 2009;56:601–16.
20 Strohm B, Azzopardi D. UK TOBY Cooling Register Study Group. Temperature control during therapeutic moderate whole-body hypothermia for neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2010;95(5):F373–5.
21 Hoque N, Chakkarapani E, Liu X, Thoresen M. A comparison of cooling methods used in therapeutic hypothermia for perinatal asphyxia. Pediatrics. 2010;126(1):e124–30.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.