Nanomaterials and the human lung: what is known and what must be deciphered to realise their potential advantages?
DOI: https://doi.org/10.4414/smw.2013.13758
Corinne
Jud, Martin James David
Clift, Alke
Petri-Fink, Barbara
Rothen-Rutishauser
Summary
Due to the constant expansion within the nanotechnology industry in the last decade, nanomaterials are omnipresent in society today. Nanotechnology-based products have numerous different applications ranging from electronic (e.g., advanced memory chips) to industrial (e.g., coatings or composites) to biomedical (e.g., drug delivery systems, diagnostics). Although these new nanomaterials can be found in many “everyday” products, their effects on the human body have still to be investigated in order to identify not only their risk, but also their potential benefits towards human health. Since the lung is commonly thought to be the main portal of entry into the human body for nanomaterials released within the environment, this review will attempt to summarise the current knowledge and understanding of how nanomaterials interact with the respiratory tract. Furthermore, the advantages and disadvantages of different experimental model systems that are commonly used to study this exposure route to the human body will be discussed.
Introduction
Throughout history major technical progress has always been accompanied by a fundamental change in the way of life of humans, including all the positive and negative factors that accompany it. With the invention of the computer, for example, the world moved from the industrial to the information age. The next technology that might have the potential to move humanity into a new age is nanotechnology, which explores the unique properties of nanomaterials. These differ tremendously from their larger sized counterparts with respect to their physical, chemical and mechanical properties [1]. Thanks to nanotechnology, the secret behind the lotus effect was solved [2] and self-cleaning bioinspired products (e.g. paints, sprays) have been marketed. In addition, to mention only a few other examples, the particular physicochemical characteristics of nanomaterials allowed the development of lighter and stronger construction materials [3], longer-lasting, biocompatibile medical implants [4], and highly sensitive sensors [5, 6]. However, apart from all the benefits that may be gained from such materials their potential risk to human health and the environment must also be considered.
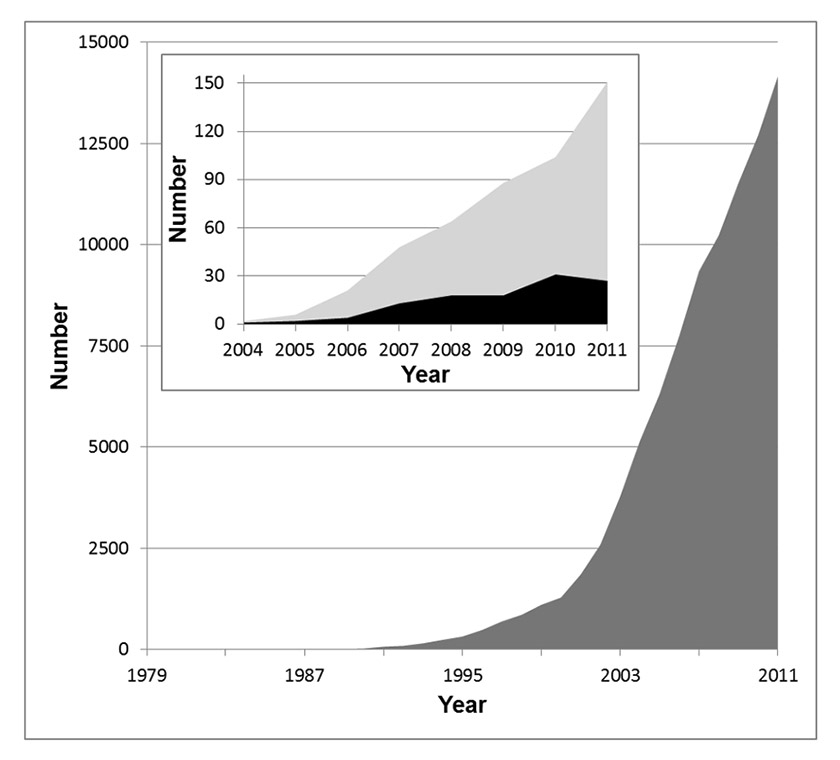
Figure 1
The number of publications in the field of nanotechnology is increasing exponentially. The dark grey area in the main graph depicts the publications per year listed in the ISI Web of Knowledge database (all databases) for the search term “nano”. In the inset, the light grey area shows the search result for the keyword “nanotoxicology” and the black area the hits for “nanotoxicology AND lung”.
Nanomaterials are usually defined as objects having at least one of their three dimensions between 1 and 100 nm; nanoparticles have all three dimensions in the nanoscale [7]. According to their origin, nanoparticles can be subdivided into three major groups: naturally occurring, unintentionally produced and engineered (manufactured for a specific purpose) [8]. Natural nanoparticles, for example soil colloids (e.g., silicate clay material), viruses, volcanic ash or airborne nanocrystals of sea salt are abundant in the environment. Unintended nanoparticles are most often generated as by-products of man-made processes such as grinding or combustion. Prominent members of this class are diesel exhaust, welding fumes or soot. Engineered nanoparticles are tailor-made materials that can be further subdivided into four categories: carbon-based (e.g., fullerene, carbon nanotubes), metal-based (e.g., metal oxides, nanosized metals, quantum dots), dendrimers (branched nanosized polymers with a high potential for medical applications) and composites (e.g., gold or titanium dioxide functionalised carbon dots) [9].
Switzerland plays a prominent role in the field of nanotechnology and Swiss industry produces various nanoparticles in considerable quantities. The following nanoparticles are the ones that are currently used in largest amounts by Swiss companies (>1000 kg per year): Ag, Al-Ox, Fe-Ox, SiO2, TiO2 and ZnO [10]. Worldwide, the volume of nanomaterials made up of different materials – so called nanocomposites – used in 2011 was estimated to be about 140,000 metric tons and is expected to reach about 330,000 metric tons in 2016 [11]. Annually, the global production of ultrafine TiO2 accounts for about 50,000 metric tons [12], and Swiss industry processes about 435 metric tons per year [10]. Hence, Switzerland uses about 0.87% of TiO2 produced worldwide, which is a considerable quantity, considering the size of the country. This overall nanotechnology boom is also well reflected in the exponential increase in scientific publications on the subject over the last 20 years, with a total of 90,719 hits for the search term “nano” between 1979 and 2011 (fig. 1). During the same period only 484 articles were published about “nanotoxicology” in general. Since the term “nanotoxicology” was coined only in 2004, early research that studied the interactions of ultrafine particles (which is the term usually used for combustion-derived nanoparticles) with the lung was not included in these hits. Between the years 2004 and 2011, the search term “nanotoxicology and lung” yields only 114 hits on the ISI Web of Knowledge database (inset fig. 1). Hence, this literature analysis clearly shows that there is a great need for research into potential health effects of nanomaterials.
Because of their use in a plethora of applications, interaction between nanomaterials and humans is inevitable – be it during their manufacturing, use or disposal. The specific routes by which nanomaterials may enter the human body, and potentially elicit adverse effects, are the lung via inhalation, the gastrointestinal tract via ingestion, and the skin and the bloodstream via intravenous injection. If nanoparticles manage to enter the blood stream, they can reach secondary organs which leads to their systemic distribution [8, 13, 14]. Since the lung is considered to be the most important area of interaction between nanomaterials that are released into the environment and the human body [8], this review attempts to summarise the current knowledge in this research field. Furthermore, it will give an overview on current in vitro lung models that are a promising alternative to in vivo and ex vivo experiments.
The human lung – from the trachea to the alveoli
The main task of the lung and other biological compartments is to act as a barrier between the “outside” and the “inside” [15]. Owing to its extensive internal surface area (>150 m2) and very thin air-blood tissue barrier (<1 μm) [16, 17], the lung is perfectly designed for optimal gas exchange by diffusion of oxygen and carbon dioxide between the air and the blood.
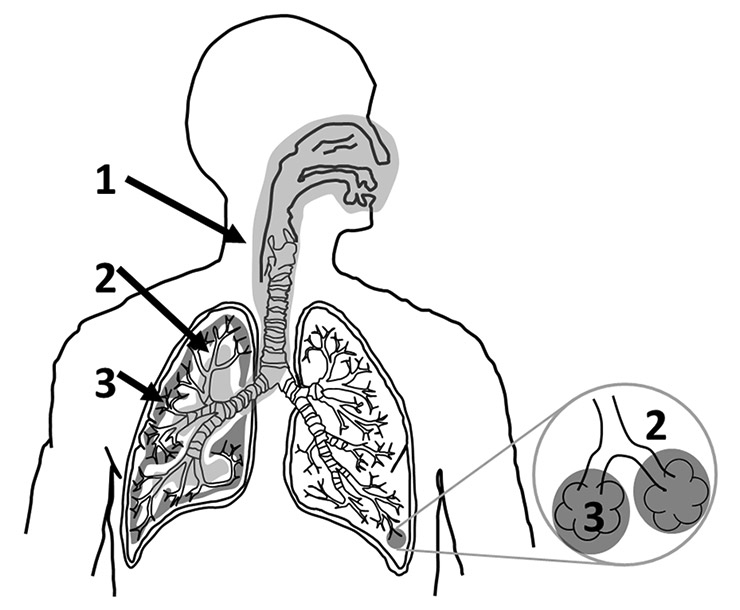
Figure 2
The human respiratory tract can be subdivided into three main structurally and functionally distinct areas. The air enters the thoracic region and continues to the tracheobronchiolar region (1, light grey). From there the air is conducted to the proximal part of the alveolar-interstitial region (2, white) before it reaches the distal part of the alveolar-interstitial region (3, dark grey).
The respiratory tract consists of three structurally and functionally distinct areas (fig. 2) [17, 18]. The air enters the respiratory tract via the most proximally located, extrathoracic region which consists of the nasal cavity, the mouth, the pharynx and the larynx. At the end of the extrathoracic zone, the air enters the tracheobronchiolar region which includes the trachea, the main bronchi, the bronchi, the bronchioles and the terminal bronchioles. During its passage through this first section (the extrathoracic and the tracheobronchiolar regions), the incoming air is humidified and temperature conditioned. Moreover, larger particulate material can be removed from the air by deposition in the airways and by subsequent mucociliary activity (fast particle clearance); it has been shown recently that nanomaterials can be trapped in human mucus [19]. The proximal part of the alveolar-interstitial region is composed of the respiratory bronchioli with only a few adjacent alveoli. Its task is air conduction, slow clearance of particulate material and a small amount of gas exchange. From there, the air reaches the third area, which is the distal part of the alveolar-interstitial region. This zone consists of the most peripheral airways, the alveolar ducts whose walls are completely covered with alveoli entrances, the alveolar sacs (alveolar ducts with alveoli closing the end of the terminal ducts) and the interstitial connective tissue. This area is mainly dedicated to the exchange of oxygen and carbon dioxide between the incoming air and the blood [20]. Particles that enter this deepest region of the lung are cleared very slowly [20].
With every breath, humans inhale not only air, but also millions of particles that deposit in the lung in a size-dependent manner [21, 22]: the smaller the particles, the deeper they can penetrate into the lung [8]. However, the respiratory tract is protected from both dangerous and inoffensive particulate matter by a series of structural and functional barriers [23]. The first barrier that particles encounter is a thin film of surfactant [24, 25] followed by an aqueous surface-lining layer including the mucociliary escalator [26]. The surface-active lipoprotein complex (surfactant) is predominantly produced by epithelial type II cells and consists of about 85–90% phospholipids [27] and about 10% surfactant proteins A, B, C and D [28, 29]. Its main function is to reduce the alveolar surface tension [17], but it also plays a crucial role in particle displacement in the lung because it wets particles by means of surface forces and then displaces them into the liquid phase (hypophase) [25, 30, 31]. This suggests that the particle surface is altered by components of the surfactant which can modify their effects on lung cells [28, 30–33]. A recent ex vivo study performed on rat lungs showed that particles suspended in 0.9% NaCl affect lung compliance by simply adsorbing surfactant. Particles precoated with surfactant, on the other hand, did not lower the maximal expiratory volume flow of the explanted lungs [34]. It has been shown that treating nanoparticles with surfactant protein A resulted in a higher uptake by macrophages [35]. Another study demonstrated a less efficient uptake of nanoparticles into alveolar macrophages and lung dendritic cells isolated from surfactant protein D deficient mice, compared with the uptake of these cells isolated from wild type mice. In a nutshell, their findings indicated an enhanced uptake of nanoparticles due to surfactant protein D adsorption on the particle surface, which in turn led to particle aggregation [36]. Furthermore, it was also observed that precoating of multiwalled carbon nanotubes with pulmonary surfactant significantly influences their potential to cause oxidative stress, cytokine/chemokine release and apoptosis [28]. Despite the relevance of surfactant proteins for the uptake of nanomaterials into phagocytic cells, they are only a minor component of surfactant. The major constituents of pulmonary surfactant are phospholipids. Recent findings demonstrated that surfactant lipids play an important role in modulating the interactions of nanoparticles with macrophages that are mediated by surfactant protein A or D [37]. Furthermore, phosphatidylserine-coated single-walled carbon nanotubes (SWCNT) were ingested at a significantly higher rate by alveolar macrophages upon their exposure to mice via pharyngeal aspiration as compared with non-coated SWCNT [38]. Nonetheless, additional studies are needed to elucidate how this interplay between surfactant proteins and lipids affects the interactions of nanomaterials with cells.
Nanomaterials that reach the hypophase can interact with the next lung barrier level, which is composed of macrophages (professional phagocytes) [39, 40], the epithelial cellular layer with tight junctions as well as adherens junctions between the cells [41, 42], and a network of dendritic cells inside and underneath the epithelium [43, 44]. The role of the dendritic cells is to maintain the fragile equilibrium between raising an active immune response against a potentially dangerous pathogen and inducing tolerance against inoffensive substances. Hence, their main task is to engulf foreign material and to present antigen-derived peptides to T-cells. In their immature state, dendritic cells have a high endocytic activity but a low potential to stimulate T-cells. Once they have endocytosed an antigen, they transform into mature dendritic cells with a low capacity to uptake further pathogens and a high potential to stimulate T-cells. The activated dendritic cells then migrate to the draining lymph nodes along a chemokine gradient [45, 46]. In the lymph node, they interact with naïve T-cells by presenting them antigen-derived peptides. This stimulation results in clonal expansion of T-cells and their differentiation into various kinds of effector T-cells [47]. Some of these effector T-cells might then leave the lymph node and migrate to the inflamed tissue where they will fight the pathogen that triggered the immune response [48]. Nanomaterials could interact with dendritic cells in two ways: either the material could be taken up by macrophages and subsequently presented to dendritic cells in an antigen-like manner, or they could interfere with the presentation of another antigen. Although the uptake of fluorescently labelled ovalbumin into monocyte-derived dendritic cells was not altered by the presence of polyvinyl alcohol – supramagnetic oxide nanoparticles (PVA-SPIONs), subsequent antigen-processing and antigen-presentation were down-regulated. As a consequence, CD4+ T-cell activation was reduced and cytokine profiles were altered, suggesting that particle exposure reverted dendritic cells to a more immature-like state [49].
After the lung epithelial cells, the structural barriers are completed by the basement membrane [50], connective tissue [51] and capillary endothelium [52, 53].
Toxicokinetics and toxicodynamics of nanomaterials
There is published evidence that nanomaterials are able to cross the air-blood barrier in the lung in animals, which gives them access to the circulatory system [54, 55]. In humans, only one study so far has described a rapid and significant translocation of inhaled nanoparticles (99mTechnetium-labeled carbon) to the systemic blood circulation and their subsequent translocation to other organs [56]. In contrast, most other studies could detect only a low degree of translocation for iridium [57] or carbonaceous nanoparticles [58, 59]. Once the nanoparticles crossed the air-blood barrier, they were transported via the circulation to secondary organs such as the liver and the heart [57, 60]. Besides the translocation of nanomaterials through the blood stream, there are also indications that inhaled nanomaterials can reach the brain [61] along or inside neurones that project from the nasal epithelium [62]. Indeed, recent results obtained from whole body exposures in the rat confirm the existence of neuronal translocation pathways: it was shown that inhaled ultrafine manganese oxide particles (30 nm) could translocate along the neuronal olfactory route to the olfactory bulb and other regions of the central nervous system. It was deduced from a predictive particle deposition model that around 11.5% of the amount deposited on the olfactory mucosa reached the olfactory bulb [63]. Similar results were obtained for the neuronal translocation of ultrafine elemental 13C particles (36 nm). More than 50% of these nanoparticles were deposited in the nasopharyngeal region during nasal breathing. From this fraction, about 20% reached the olfactory bulb in rats [61]. These findings are also in line with early results obtained in nonhuman primates for the translocation of polioviruses (30 nm) and silver-coated gold nanoparticles (50 nm) to the olfactory bulb [64–66].
As mentioned above, nanomaterials can be taken up by various cell types [67–70]. Once they have penetrated into the cell, they may elicit several biological responses ranging from the enhanced expression of proinflammatory cytokines [71] to the generation of reactive oxygen species (ROS) [72] or DNA strand breaks [73]. Oxidative stress has been widely reported to play a key role in the mechanisms that underlie the adverse health effects related to exposure to particulate matter [74]. Moreover, oxidative stress has been linked to inflammatory responses that are also known to result in decreased cellular functions [75–77], which in turn is strongly associated with the onset of adverse health effects after exposure to ultrafine particles [78, 79]. Prior to understanding such effects however, it is essential to understand the lethal dose (LD50) as well as the inhibitory and effective doses (IC50 and EC50, respectively) of any nanomaterial in the biological system used, so as to determine whether or not a nanomaterial may elicit an effect that is separate from a cytotoxic response (IC50) [80–82]. Ultimately, these effects have been associated with the onset of genotoxicity which might lead to cancer [76, 83], which is known to be a consequence of accumulated alterations in the genetic code. Hence assays measuring genotoxicity have been introduced to investigate the potential carcinogenic risk of poorly soluble particles such as engineered nanomaterials and diesel exhaust particles [84].
Although a great deal of effort has been dedicated to unravelling why and how nanomaterials may elicit adverse health effects, the precise mechanisms of nanomaterial toxicology are still not fully understood. Further research into realistic nanomaterial exposure scenarios is still urgently needed to assess the factors highlighted previously. Today, many investigations into nanomaterials are based on the hypothesis that oxidative stress [83] underlies the adverse cellular effects they elicit. However, besides oxidative stress, the fibre paradigm [85] and the theory of genotoxicity [86] have also been suggested to play an important role in toxicological effects of nanomaterials. A recent study focusing on the fibre paradigm showed that long, straight and stiff multiwalled carbon nanotubes injected into the peritoneal cavity of mice elicited increased granuloma (small areas of tissue inflammation) formation in vivo. Most importantly, these lesions were phenotypically similar to those caused by long, straight and stiff asbestos fibres [87]. Nevertheless, as already indicated by its name, the fibre paradigm can only be applied to nanofibres and in particular to high aspect ratio nanomaterials (i.e., long, narrow and biopersistent) [88]. Therefore, this paradigm is not approprate for the large number of spherical nanoparticles. The theory of genotoxicity, on the other hand, fits both spherical and fibrous nanoparticles [89]. However, the weak point of this theory is that it is based to a large extent on studies that used particles bigger than 100 nm. Only a few genotoxicity testing strategies are based on nanoparticles (<100 nm). Hence much more work is needed to decipher and understand the genotoxic, mutagenic and carcinogenic potential of nanomaterials.
Where and how do nanomaterials interact with lung cells?
Once nanomaterials have passed the surfactant film and are located in the aqueous lining layer, they come in to close contact with cellular structures, such as the outer plasma membrane. The plasma membrane can be seen as a checkpoint because it segregates the cytoplasm from the extracellular environment, and it coordinates the entry and exit of variously sized molecules. Since the cell entry mechanisms that will be addressed here are common and not specific to lung cells, this section will be more general and not only focused on the respiratory tract.
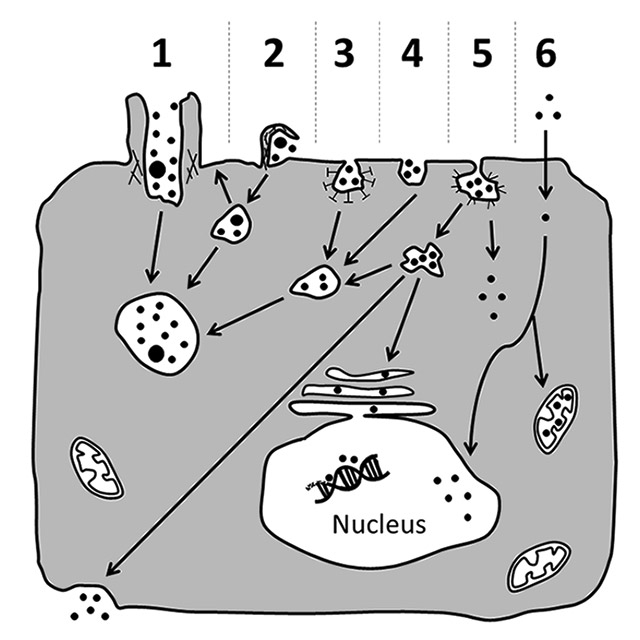
Figure 3
Possible mechanisms for cellular uptake of nanomaterials and their subsequent intracellular trafficking. Nanomaterials may be actively incorporated via phagocytosis (1), macropinocytosis (2), clathrin-dependent endocytosis (3), clathrin- and caveolae-independent endocytosis (4) or caveolae-mediated endocytosis (5). Particles that were internalised via active uptake are commonly transported in vesicular structures that then fuse to phagolysosomes or endosomes (1–5). Sometimes, they might be exocytosed upon macropinocytosis (2). Alternatively, they may also be carried to the cytosol, or be transported via caveosomes to the endoplasmic reticulum, or cross the cell as part of transcytotic processes (5). Besides active transport, nanoparticles may also enter the cell passively via diffusion through the plasma membrane (6). From the cytoplasm they may then gain access to subcellular compartments such as the nucleus and mitochondria (6). However, further research is needed to clarify if a particular entering mechanism or a certain intracellular localisation elicit specific cellular responses.
Figure and figure legend have been modified from reference [101].
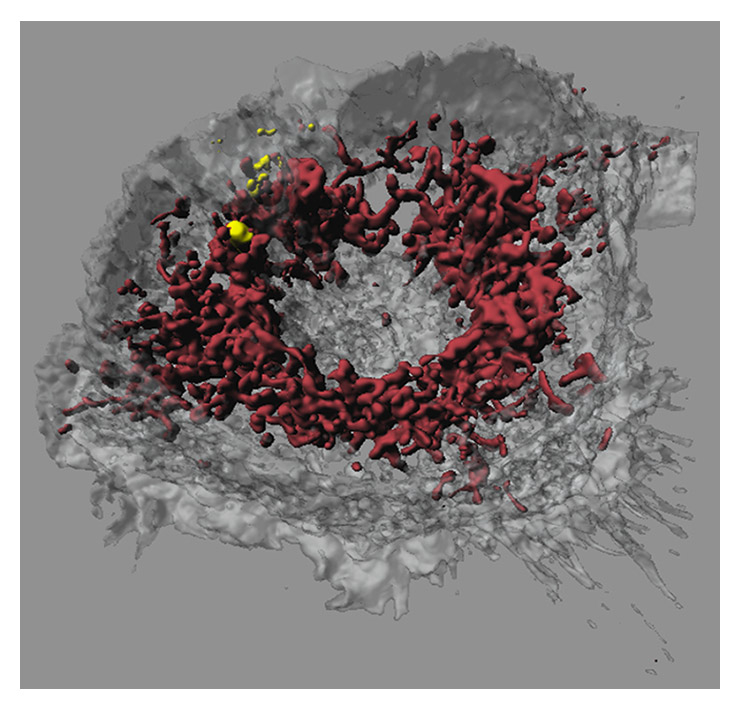
Figure 4
Confocal laser scanning microscopy image of a macrophage that had taken up iron-platinum nanoparticles. Fluorescent labelling of mitochondria using MitoTracker (bordeaux) reveals that the fluorescently labelled polymer-coated iron-platinum nanoparticles (yellow) do not colocalise with mitochondria in macrophages (white, transparent).
Image adapted and reproduced with permission from: Lehmann AD, Parak WJ, Zhang F, Ali Z, Röcker C, Nienhaus GU, et al. Fluorescent–magnetic hybrid nanoparticles induce a dose-dependent increase in proinflammatory response in lung cells in vitro correlated with intracellular localization. Small. 2010;6(6):753-62 [100].
Small molecules (e.g., ions, carbohydrates, amino acids) are essentially able to cross the plasma membrane through the action of channels or pumps that span the membrane. Macromolecules (e.g., proteins, polysaccharides), on the other hand, have to be internalised via an active form of uptake by the cell, known as endocytosis [90]. To form the endocytic vesicles, the plasma membrane first invaginates, engulfing the macromolecules, and then detaches from the remaining plasma membrane. Two main types of endocytosis can be distinguished: pinocytosis (“cell drinking”) and phagocytosis (“cell eating”). Pinocytosis is the ingestion of extracellular fluid and small molecules. During this process only very small vesicles with a diameter up to about 0.15 µm are formed [91]. Phagocytosis, on the other hand, is dedicated to the uptake of large particles such as microorganisms and cell debris. It is actin-dependent and often receptor mediated [92]. In contrast to pinocytosis, phagocytosis forms large vesicles (so-called phagosomes) that have in general a diameter of about 0.25 µm [91]. Another difference between the two uptake mechanisms is that phagocytosis is performed mainly by specialised phagocytic cells such as macrophages or dendritic cells, whereas pinocytosis occurs continuously in all eukaryotic cells [90].
Figure 3 summarises different possible mechanisms for cellular entry and intracellular trafficking of nanomaterials. Professional phagocytes such as macrophages engulf particles because this serves as a defence mechanism that removes foreign material from the organism. However some studies show that phagocytosis is not the only possible way for particles to enter the cells. The uptake of fine polystyrene particles (1 μm) by macrophages could be blocked by cytochalasin D, but this was not the case for polystyrene nanoparticles (78 nm). Since cytochalasin D is a potent inhibitor of actin polymerization, these findings indicate that nanoparticles can also cross the macrophage membrane via actin-independent processes [67].
Another uptake mechanism is caveolae-mediated endocytosis. As the name implies, one of the characteristics of this type of endocytosis is the presence of caveolin proteins in the 50–100 nm omega-shaped invaginations of the plasma membrane [93, 94]. Once assembled, caveolar membrane microdomains remain stable during vesicular trafficking [95]. Clathrin-dependent endocytosis is a type of pinocytosis that occurs in virtually all mammalian cells. This receptor-mediated process is very well studied and leads to vesicles of about 100 nm in diameter, which are coated by a protein complex that mainly consists of clathrin. In contrast to caveolin-mediated transport, the vesicle coat does not remain stable during clathrin-dependent endocytosis. Once the vesicles have detached from the plasma membrane, the clathrin coat is disassembled and the clathrin triskelia are recycled back to the plasma membrane where they assemble again around a new vesicle bud [96]. The importance of these two processes for nanomaterial uptake needs further investigation. By means of inhibition of specific endocytotic pathways, the uptake of nanomaterials can be studied in detail and it could be shown that caveolin, as well as clathrin-dependent uptake, are the main mechanisms for pegylated-gold nanoparticles (15 nm) [97]. Again, the mechanism depends on cell as well as particle type and needs to be investigated in more detail.
The endocytic pathways described above have one feature in common: particles are localised in intracellular vesicles after internalisation. However, several studies indicate the existence of alternative pathways for particles to enter the cells that allow nanomaterials to remain nonmembrane bound [67, 68, 98]. The authors of these studies suggest, among other mechanisms, passive or receptor-mediated diffusion of nanomaterials through membrane pores, as well as so-called adhesive interactions that are mediated by van der Waals or steric interactions [99].
Although a lot of research in recent years has been directed toward gaining a better understanding of the cellular uptake of nanomaterials, many questions remain that need to be answered both in vitro and in vivo. The particular chemical and physical properties of the nanomaterial and of the membranes that are responsible for the translocation of nanomaterials into the cells and subsequent compartments (e.g., nucleus, mitochondria) need to be elucidated. Intracellular trafficking studies using quantitative transmission electron microscopy have shown that the preferred localisation for pegylated-gold nanoparticless (15 nm) in A549 alveolar epithelial cells are vesicles of different sizes [97]. Another study using laser scanning microscopy combined with digital image restoration showed that polymer-coated gold and iron oxide nanoparticles co-localised with lysosomes but not with mitochondria or the cell nuclei (fig. 4) [100].
In recent years, several reviews have discussed different mechanisms by which nanomaterials can be taken up by cells and their resultant mode of intracellular trafficking [101–103]. The speed of these processes seems to be strongly dependent on the surface properties of the nanomaterials and on their in vivo surface modifications (e.g., by endogenous proteins or lipids found in surfactant or plasma) [8]. These observations led to the formulation of the “corona” theory, which states that, in a biological environment (e.g., surfactant, blood, mucus), the particle surface is covered by biological macromolecules (e.g., proteins, lipids). This corona can be further subdivided into a “soft” and “hard” part. The former is characterised by a dynamic exchange of macromolecules between the particle surface and the biological surrounding, whereas the latter consists of biological molecules that are strongly attached to the particle [37, 104, 105]. Although it is clear that the surface properties of nanoparticles are essential for their interaction with cells, it is still debated as to which characteristics are leading to which cellular responses. In addition, the cell types investigated also differ with regard to uptake and defence mechanisms [100, 106]. A general conclusion that all cells may react similarly after exposure to the same nanomaterial should be avoided.
Models used to assess the interactions of nanomaterials with the lung: in vivo, ex vivoandin vitro
So far, three approaches have been used to study the effects of particles on the respiratory tract under controlled conditions: in vivo experiments on animals, ex vivo studies on biopsies or isolated lungs and in vitro experiments using more or less complex cell culture systems [107]. Of course, all three strategies have advantages and disadvantages that must be considered. Moreover, they all require profound methodological knowledge and an interdisciplinary approach in order to assess the risk of nanomaterials and their interactions with cells.
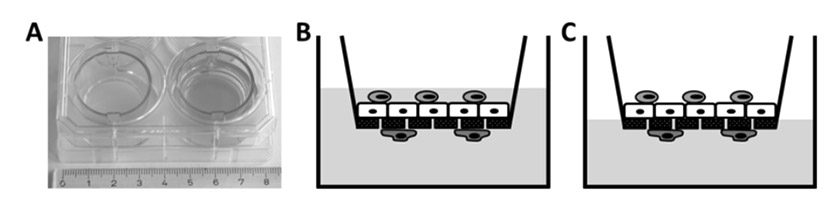
Figure 5
Two chamber cell culture system.
A conventional two chamber cell culture system in a six-well culture dish. Cells are grown on a porous polyethylene terephthalate (PET) membrane. For reference, the well on the left does not contain medium. B/C Triple cell co-culture model of the human air-liquid barrier consisting of macrophages (light gray, top), epithelial cells (white, middle) and dendritic cells (dark grey, bottom). The cells can be kept submerged (B) or at the air-liquid interface (C).
In vivo exposure procedures with nanomaterials can be subdivided into three main categories: whole body, head/nose/mouth-only or lung-only exposures [108–110]. During whole body exposure, animal suffering is clearly lowest compared with the other two methods, and it mimics environmental, occupational or intended exposure most realistically. Since this exposure type is less stressful for the animals because it requires neither anaesthesia nor surgery, it is ideally suited for studies of chronic exposure. The quality of the results obtained using whole body exposure, however, depends strongly on an equal distribution of the particles in the exposure chamber. Moreover, relatively large amounts of test material are needed to fill the volume of the exposure chamber, which might limit its usefulness when expensive materials have to be tested.
Head/nose/mouth-only exposures are more stressful for animals because their food and water supplies are cut off during the exposure. Nonetheless, the big advantage of this delivery method is that the particle administration is very efficient and doses can be well controlled. As with whole body exposure, head/nose/mouth-only exposure does not require anaesthesia and surgery [111–113].
The third way to study the effects of inhaled aerosolised nanomaterials is lung-only exposure. This method is technically much more challenging because it requires intubation or tracheotomy for intratracheal or orotracheal instillation, respectively. With this exposure technique very precise dosages can be administered but the significance of the results may be obscured by the lack of reaction of the systemic and autonomous nervous systems due to anaesthesia. Moreover, this technique can lead to local tissue damage and uneven distribution of the applied substances in the lung [111, 114, 115].
Although lung-only exposure is frequently used to study the toxicology of nanomaterials, it should not be regarded as an adequate substitute for inhalation studies because it is not representative of environmental and occupational exposure scenarios [111]. In a comparison study in mice, inhalation of single-walled carbon nanotubes (SWCNTs) elicited a stronger inflammatory response and increased oxidative stress than instillation of an equivalent mass. Although the trends were similar in both exposure models, inhalation of the dry powder was more potent for SWCNTs than instillation of the suspension [116]. In rats the opposite has been observed: inhaled ultrafine TiO2 particles (21 nm) led to a decreased pulmonary response compared with a similar dose of instilled particles. These results might be explained by differences between the two methods in particle distribution, dose rate, or clearance [117]. However, another study in rats comparing the two administration routes for TiO2 particles gave consistent toxicity data for inhalation and instillation [118]. Hence, under certain circumstances and for specific materials, instillation might be a cost-effective procedure for initial safety screening.
In vivo, temperature, humidity and pressure in the lung are tightly controlled.. Thus, it is important to remember this in order to obtain meaningful data from ex vivo experiments. The most common ex vivo method is the use of isolated perfused lungs. Separating the lung from the body has the advantage that experimental parameters can be better controlled and monitored. However, it has the disadvantage that it is difficult to maintain physiological conditions ex vivo, which limits the lifespan of the lung under artificial conditions to only a few hours. Moreover, this approach is technically very demanding and requires a profound knowledge of surgery [108, 119]. Isolated perfused lungs have not only been used to study the transport of pharmaceutically relevant substances through the air-blood barrier, but also to study the translocation of nanoparticles through this barrier [120, 121]. A study investigating the transport of 18 nm iridium particles through isolated rat lungs has shown that they do not translocate to the perfusate under normal conditions. However, pretreating the lungs with either H2O2 (to mimic oxidative stress) or histamine allowed particles to translocate across the air-blood barrier [121]. These results confirm earlier findings where no translocation of ultrafine polystyrene particles (24, 110 or 190 nm) through isolated rabbit lungs could be measured under physiological conditions [120]. Although these findings confirm each other, they should be interpreted carefully because they conflict with certain in vivo results. These include the finding that albumin (80 nm, coated with 99mTc) [54] and ultrafine carbon particles (18 nm) [122] translocate across the lung barrier in vivo. These contrasting findings were explained by the absence of the lymph flow, haemodynamic factors and inflammatory cells in the explanted lungs [120].
Besides isolated perfused lungs, precision-cut lung slices can also be used as an ex vivo model. Murine lung slices, for example, have been used to study the suitability of solid lipid nanoparticles as a drug delivery system [123].
Although both in vivo and ex vivo models are valuable tools for the study of the effects and toxicokinetics of nanomaterials in the lung, they have certain limitations. To understand the toxicodynamics, i.e., how nanomaterials interact with individual cells and which pathways they influence inside cells, a zoom-in is required. Because of the complex nature of the lung architecture, in vitro models are more suited to the study of these complex parameters in a simplified set-up. It goes without saying that a good in vitro model should mimic as many characteristics of the corresponding region in the respiratory tract as possible. Culturing human or animal cells according to strict standardised protocols and following guidelines for good cell culture practice allows results to be obtained that are much more reproducible than in vivo data [124–126]. This high data consistency, the relatively low costs and the short experimental time span mean that in vitro methods are particularly suitable for high throughput screening. However, in vitro data may differ from in vivo results because cell culture systems are isolated from the physiological context. Hence, at a certain point, in vitro findings have to be confirmed in animals and humans. Moreover, species differences might be more significant than often assumed. Disappearance kinetics of hydrophilic molecules, for example, differ quite tremendously between species [108, 127, 128]. Certain drugs, might be effective in animals but not in humans. For example, in mice, 3-methyladenine (3-MA) has been shown to reduce acute lung injury triggered by polyamidoamine dendrimers (a nanomaterial developed for clinical applications) [129]. In humans, however, this autophagy inhibitor is not stable [130]. Additionally, there is evidence that there is an important difference between the aquaporin distribution in human and rodent airways [131]. Thus a big advantage of cell culture experiments is that they can be performed using cells from human origin, yielding data that are eventually more meaningful for humans than those obtained from animal experiments. For this reason, and because most studies in the field of nanotoxicology have used human cells [110], we will focus here on human in vitro systems only. Table 1 summarises the currently available cell culture models mimicking the human lung.
Cells used for in vitro experiments can stem either from a continuous cell line (secondary cultures) or freshly isolated tissues (primary cultures). Cell lines have the advantage that they are very homogenous. If they are used properly, they yield very reproducible results. However, they retain only little phenotypic differentiation compared with the initial cell type in vivo. Primary cultures, on the other hand, are very heterogeneous, consisting of several cell types with cells at various stages of differentiation. Besides, they need fairly complex media to be maintained in culture. Since these cells undergo senescence quite quicklyin vitro, they are only viable for a few passages. Moreover, each tissue isolate is unique owing to donor variation. This makes them difficult to standardise, which causes a higher variability of the results [124, 132]. Other limiting factors for primary cultures are that healthy human airway tissue is not easily available and that only a few cells are obtained per isolation [110].
Over the last three decades, protocols for the isolation of primary epithelial cells from both the tracheobronchiolar [133–136] and the alveolar region [137–141] have been established. Because of the ease of use of cell lines and the limitations of primary cells discussed above, most nanotoxicology studies have been performed using immortalised epithelial cells. The most popular tracheobronchiolar cell lines are Calu-3 [142], 16HBE14o- [143] and BEAS-2B [144]. These three cell lines are not only frequently used for drug absorption studies [145], but also to assess particle-cell interactions [146] and to investigate the toxicity of particulate matter [147] or nanoparticles [148–150]. Permeability values observed in Calu-3 and 16HBE14o- cell lines appear to be predictive of absorption properties within intact lungs [108]. Besides these cell lines, NuLi-1 [151] seems to be another promising candidate for future nanotoxicity studies on human airway epithelial cells [108, 152]. The most widely used and best-characterised in vitro model of the human alveolar epithelium is the A549 cell line, which has many features of alveolar epithelial type II (ATII) cells [153–155]. However, there are marked differences in morphology and transepithelial electrical resistance (TEER) between primary human alveolar epithelial cells (hAEpCs) and A549 cells [156]. NCI-H441 is another in vitro model of the alveolar epithelium that was obtained from a human lung adenocarcinoma. This cell line has been described as having significant TEER values [157, 158] and the characteristics of not only ATII [159, 160] cells but also Clara cells (i.e., bronchiolar exocrine cells) [161, 162]. Recently, primary human ATII cells have been immortalised and used for latex particle (50 nm – 1 µm) uptake studies. This cell line displays an ATI phenotype and the immortal cells no longer express alkaline phosphatase, pro-surfactant protein C and thyroid transcription factor-1, but they do express increased calveolin-1 and the receptor for advanced glycation end products. This in vitro model of ATI cells might help us to understand the importance of this cell type for the translocation of particles [163] as compared with AT2 cells.
The majority of studies in the field of nanotoxicology have been performed with monocultures grown as monolayers on impermeable surfaces. Several studies have shown that cells that are grown this way after their isolation from the tissue undergo dedifferentiation and lose their specialised functions [164]. This might be because they lose their habitual three-dimensional (3D) environment and also their neighbours of different cell types. Since in vivo cells continuously crosstalk through intercellular signalling to maintain homeostasis and to coordinate immune responses [165], the absence of these neighbouring cells might influence the experimental outcome. Recent studies have shown that adding a third dimension to the cell’s environment [166–168], or co-culturing different cell types, significantly influences cellular characteristics, behaviour and responses to stimuli [169, 170]. Including these additional parameters into an in vitro model creates a greater similarity between the artificial system and the natural situation in the human body, which ultimately leads to more relevant results. Our research group has recently developed an in vitro model of the human airway barrier consisting of three different cell types. In our triple cell co-culture system, monolayers of either A549 [153], 16HBE14o- [152] or primary epithelial type I cells (hAEpCs) [171] are grown on a microporous membrane in a two-chamber system (fig. 5A). Once the monolayer is confluent, macrophages and dendritic cells derived from human blood monocytes are added to the apical and basal side of the epithelium, respectively (fig. 5B). After thorough evaluation, this model has already been successfully used to study cellular interplay and signalling, as well as the cellular responses of epithelial cells, macrophages and dendritic cells to airborne or suspended particles of different sizes (≤1 μm) and materials (polystyrene, titanium dioxide, gold, cerium oxide) [69, 110, 146, 172–174]. Particle translocation and cellular localisation were studied in parallel, and it could be shown that translocation of nanoparticles into the different cell types is different from their larger particle counterparts [69]. In addition to the triple co-culture described above, a quadruple co-culture model consisting of epithelial cells, macrophages, mast cells and endothelial cells has been established [175]. Another triple co-culture model of the human airways was made up of fibroblasts, monocyte-derived dendritic cells and epithelial cells [176]. In these three models, the cells are not just cultured together: they are built up on the porous support in such a way that the in vitro architecture reflects the specific in vivo surroundings that the model is mimicking. To come even closer to the lung environment in our body, dynamic microsystems have recently been developed that simulate blood circulation [177] and even breathing [178] or bronchoconstriction [179, 180]. It was shown that cells that were subjected to mechanical strain took up significantly more polystyrene nanoparticles (100 nm) than static cells [178].
|
Table 1: Human cell culture models mimicking epithelial barriers found in the human lung. |
|
Cell culture model
|
References
|
|
Airway epithelial cells
|
|
| Calu-3 (ATCC HTB-55) |
[232‑239] |
| 16HBE14o- (can be obtained from D.C. Gruenert) |
[152, 187, 194, 240, 241] |
| BEAS-2B (ATCC CRL-9609) |
[149, 150, 242‑245] |
| NuLi-1 (ATCC CRL-4011) |
[151] |
|
Primary airway epithelial cells
|
|
| hBEpC |
[133, 134, 246, 247] |
| Alveolar epithelial cell lines |
|
| A549 (ATCC CL-185): ATII phenotype |
[140, 154, 181, 183, 243, 247‑249] |
|
Immortalised human ATII cells with ATI phenotype
|
[163]
|
| NCI-H441 (ATCC HTB-174): ATII and Clara cell phenotype |
[157‑162] |
|
Primary alveolar epithelial cells
|
|
| hAEpC: ATII cells that differentiate in vitro into ATI-like morphology |
[137‑139] |
|
3D cultures
|
|
| 3D aggregates of A549 cells |
[166] |
| Bilayer coculture model: epithelial and endothelial cells |
[250‑253] |
| Bilayer coculture model: epithelial cells and fibroblasts |
[254] |
| Triple cell coculture model: epithelial cells, macrophages, dendritic cells |
[110, 172, 255, 256] |
| Triple cell coculture: epithelial cells, dendritic cells, fibroblasts |
[176] |
| Double, triple and quadruple cell coculture models: epithelial cells, endothelial cells, mast cells, macrophages |
[175] |
|
Biomimetic microsystems
|
|
| Breathing lung-on-a-chip: epithelial and endothelial cells |
[178] |
| Perfused chip: epithelial and endothelial cells |
[177] |
| Strain device: fibroblasts and epithelial cells |
[179, 180] |
| AT = alveolar type, table adapted from references [257], [80] and [126] |
A realistic in vitro model must be combined with an appropriate exposure system
Not only do in vitro models have to reflect the natural situation as closely as possible, the method of exposure to the nanomaterials must also be chosen carefully. To date, most experiments studing interactions between nanomaterials and lung cells have used nanomaterial suspensions that were applied to submerged cell cultures [69, 148, 181–183]. In vivo, however, lung epithelial cells are separated from the air by only a thin aqueous lining layer with a surfactant film at the air-liquid interface [24, 25]. As a consequence, the first barrier particles encounter in the lung after deposition on the epithelium is surfactant. The two-chamber system described earlier not only leads to increased differentiation of the epithelial cells [168], but it also allows them to be kept at the air-liquid interface. The medium can be removed from the upper chamber without any harm to the cells because they continue to be fed from the bottom (fig. 5C) [184–188]. The advantage of this technique is that the cells are still covered by a thin liquid film which is much closer to the in vivo situation. However, to mimic the natural situation it is not sufficient to remove the medium from the upper chamber only shortly before applying test substances, because lung epithelial cells need time to secrete surfactant [110, 186]. Taking these aspects into account, several recent studies investigated the effects of nanomaterials on the lung at the air-liquid interface using newly developed exposure systems [174, 189–194].
For safety reasons and to prevent contamination, air-liquid exposures are usually in a closed system. Nanomaterials are either directly produced in the vicinity of the cell culture dish (e.g., flame spray synthesis [174], combustion engine [192]) or they are nebulied in an exposure chamber and allowed to settle on the cells [186, 191, 195–197]. For a complete review on the currently available air-liquid exposure systems see Müller et al. [198] and Paur et al. [199].
In summary, air-liquid exposure is not only more physiological, but it is also mimics more realistically nanomaterial morphology encountered by the lung in the real world. In suspension, particle agglomeration and hence deposition behaviour might be changed by characteristics of the dispersion medium (e.g., pH [200, 201], ionic strength [200], protein content [202]). Hence, to get most meaningful results from in vitro systems one has to combine the proper cell culture model with the appropriate exposure method.
What needs to be done in the future for a better understanding of the interactions?
Although researchers worldwide have put a lot of effort into elucidating nanomaterial-(lung) cell interactions, the underlying mechanisms are still poorly understood. To shed further light on this, more systematic and interdisciplinary approaches are needed to gain a maximum of information about a specific nanomaterial. First of all, nanomaterials that are to be tested on a biological system have to be fully characterised and these data have to be made available to other scientists in publications, for later comparison. Particle characterisation should not be limited to classic parameters such as size, surface charge, surface structure, coating, chemical composition or particle shape, but should also include information about contaminants (e.g., endotoxins such as lipopolysaccharide or adjuvants) or their colloidal stability in the environment used later during the experiment [81, 203]. The impact of particle characteristics on cell uptake and cytotoxicity is an entire field of research, which has grown tremendously in recent years. In particular, the effects of particle size and surface charge on cellular uptake, cytotoxicity, and biodistribution have been studied extensively. For example, Chithrani and colleagues studied the uptake of gold nanoparticles and showed that uptake velocity and concentration varied with size [204]. They also showed that spheres were more readily and efficiently internalised than rods of the same size. However, at present it is very difficult to compare published data since not only materials, surfaces and cells, but also protocols, concentrations, controls or methods vary substantially. In addition, it is well known that biological fluids usually have a high ionic strength, which might screen possible repulsive forces between nanomaterials (owing to their identical charge). In consequence, nanomaterials might change their colloidal behaviour and agglomerate (be loosely bound) or even aggregate be firmly bound or even fused) in cell culture medium, for example. Eventually, however, they might also be more stable in a biological environment. In any case, changes in their colloidal stability will influence cellular uptake mechanisms and subsequent cellular responses [205]. Moreover, to avoid misinterpretation of the results due to experimental artefacts it is crucial that scientists of different fields, such as chemistry, physics and biology, collaborate closely [206]. The importance of an interdisciplinary approach is illustrated by a study that investigated the effects of gold nanoparticles (7 nm) on human dendritic cells. During a first attempt they found that in conventional laboratory surroundings, gold spheres activated dendritic cells. However, when the nanoparticles were sterile and endotoxin free, they had no maturation effect on dendritic cells. Combining the knowledge of material and biomedical scientists revealed that the observed activation of the dendritic cells during the first experiment was due to lipopolysaccharide contamination of the gold spheres [207]. This study also showed that standardisation of certain experimental procedures between laboratories is important because this greatly enhances the comparability of the results. For instance, choosing a certain number of methods to measure biochemical markers (e.g., reactive oxygen species production) would allow the effects of different nanomaterials to be compared and hence for them to be ranked by their potential adverse effects [208, 209]. Moreover, in order to double-check the results, at least two complementary techniques should be used to assess one parameter [203]. However, before a method or a kit is chosen, it is crucial to test whether the nanoparticles (or buffer components, etc.) interfere with it or not [210]. Hence, the probability of obtaining false-positive or false-negative effects can be minimised. Of course it is good laboratory practice to include appropriate positive and negative controls in each experiment [80, 211].
So far, it is still unclear as to which particle parameters determine which cell entry mechanism and which cellular effects. Hence it is of primary importance to perform systematic studies that change only one parameter step-by-step (e.g. size [212], exposure time [174], coating [97]). To be relevant, the range should be carefully chosen to mimic realistic exposure. In the long run, such studies will help to modulate the biological effects caused by nanoparticles by allowing fine-tuning of some of their parameters. In our daily life, however, we are not exposed to one kind of well-defined monodispersed particles but to a mixture of various particles that we inhale with every breath. As a consequence, future studies should also address this aspect. Again, a systematic approach would be helpful to decipher the contribution of individual components to the overall biological impact of the mixture [213]. Hence, as a first step all the constituents would have to be identified and characterised. Then they should be tested one by one for their toxicological potential before mixing them again stepwise. It is needless to say that such an approach is only feasible for relatively simple mixtures with only a few components. To overcome the huge amount of data that would have to be combined for complex systems, computer modelling could be an asset.
Conclusion
Despite various concerns about nanomaterials, they have various beneficial effects. They potentially offer not only the possibility to target (cancer) cells but might also help to fight other diseases such as Alzheimer’s disease [214] or illnesses caused by antibiotic-resistant bacteria [215]. Nanomaterials can also serve as an effective tool in diagnosis (e.g., increased contrast for magnetic resonance imaging, detection of pathogens or proteins) and life science research (e.g., fluorescent labels, purification of biological molecules and cells) [216]. However, risk assessment should not be neglected in the light of their great advantages.
In a nutshell, inhaled nanomaterials might cause inflammation or other potentially adverse cellular effects, depending on their properties and their rate of clearance from the respiratory tract [217]. Nanomaterials can be removed from the lung by mucocilliary clearance within the conducting airways, by macrophage and/or dendritic cell phagocytosis, or by translocation through the air-blood tissue barrier [218]. Over the last decade, there has been a great deal of research into the effects of nanomaterials on the respiratory tract. However, to obtain meaningful data it is important to use well characterised nanomaterials, a realistic exposure scenario system, and an appropriate and validated lung model. In vitro studies have many advantages over in vivo or ex vivo studies. For instance, they can be used for high-throughput screening, which allows the analysis of the effects of a large number of nanomaterials on the respiratory tract in a short time. Moreover, species differences can be ruled out when cells of human origin are used. Unfortunately, cell culture systems often do not exhibit all the characteristics of the corresponding native tissue. However, this issue can be minimized using 3D and co-culture models [186]. If an appropriate cell culture system is chosen – carefully weighing its advantages and limitations – in vitro models of the human lung are a powerful tool for addressing specific scientific questions. Ultimately, the development and use of more sophisticated and well-validated cell culture lung models will help to reduce animal experiments, which is desirable both ethically and financially. Yet, with all efforts taken, the perfect model for lung risk assessment of nanomaterials does not yet exist. Hence, data obtained in in vitro and animal studies, should always be compared with each other and, more importantly, with epidemiological and/or clinical studies and vice versa [219] because they all provide different pieces of the same research puzzle [220]. The relation between air pollution and adverse health effects has, for example, been reported in epidemiological studies [221–229], but animal and, more importantly, in vitro studies helped to pinpoint the cellular pathways that are activated by ultrafine particles [230]. In addition, animal as well as epidemiological studies allow the investigation of the consequences of chronic exposures. This is fundamental to the adequate risk assessment of nonbiodegradable and non-excreted nanomaterials [231]. New models to study long term outcomes such as, for example, oncogenicity of nanomaterial exposure are still crucially needed.
In conclusion, further research is required in order to understand the potential adverse effects of nanomaterials on the respiratory tract and, via systemic distribution, on the human body in general. In the meantime researchers and workers should take all possible precautions to minimise their exposure to nanomaterials until their specific hazard potential has been clarified.
References
1 Rao C, Cheetham A. Materials Science at the Nanoscale. Nanomaterials Handbook. London, New York: Taylor Francis CRC Press; 2006.
2 Barthlott W, Neinhuis C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta. 1997;202:1-8.
3 Lee J, Mahendra S, Alvarez PJJ. Nanomaterials in the Construction Industry: A Review of Their Applications and Environmental Health and Safety Considerations. ACS Nano. 2010;4(7):3580-90.
4 Liu H, Webster TJ. Nanomedicine for implants: A review of studies and necessary experimental tools. Biomaterials. 2007;28(2):354-69.
5 Yogeswaran U, Chen SM. A review on the electrochemical sensors and biosensors composed of nanowires as sensing material. Sensors-Basel. 2008;8(1):290-313.
6 Choi KJ, Jang HW. One-Dimensional Oxide Nanostructures as Gas-Sensing Materials: Review and Issues. Sensors-Basel. 2010;10(4):4083-99.
7 ISO/TS 80004-1. Nanotechnologies – Vocabulary – Part 1: Core terms. Geneva: International Standards Organization; 2010.
8 Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113(7):823-39.
9 Ruffini Castiglione M, Cremonini R. Nanoparticles and higher plants. Caryologia. 2009;62(2):161-5.
10 Schmid K, Riediker M. Use of nanoparticles in Swiss Industry: a targeted survey. Environ Sci Technol. 2008;42(7):2253-60.
11 Parker PM. The 2011 Report on Nanoparticles in Composites: World Market Segmentation by City. San diego: ICON Group International; 2011.
12 Ultrafine Titanium Dioxide. [22.06.2012]; Available from: http://www.cristalglobal.com/ProductResources/Ultrafine%20Titanium%20Dioxide%20-%20info.pdf.
13 Semmler-Behnke M, Kreyling WG, Lipka J, Fertsch S, Wenk A, Takenaka S, et al. Biodistribution of 1.4- and 18-nm Gold Particles in Rats. Small. 2008;4(12):2108-11.
14 Kreyling WG, Hirn S, Schleh C. Nanoparticles in the lung. Nat Biotech. [10.1038/nbt.1735]. 2010;28(12):1275-6.
15 Stern ST, McNeil SE. Nanotechnology safety concerns revisited. Toxicol Sci. 2008;101(1):4-21.
16 Gehr P, Bachofen M, Weibel ER. The normal human lung: ultrastructure and morphometric estimation of diffusion capacity. Respir Physiol. 1978;32(2):121-40.
17 Ochs M, Weibel ER. Functional design of the human lung for gas exchange. In: Fishman AP, Elias JA, Fishman JA, Grippi MA, Senior RM, Pack A, editors. Fishman’s Pulmonary Diseases and Disorders. 4th edition ed. New York: McGrawHill; 2008.
18 Weibel ER. Stereological methods. Vol.1: Practical Methods for Biological Morphometry. London: Academic Press; 1979.
19 Jachak A, Lai SK, Hida K, Suk JS, Markovic N, Biswal S, et al. Transport of metal oxide nanoparticles and single-walled carbon nanotubes in human mucus. Nanotoxicology. 2012;6(6):614-22.
20 Gehr P. Anatomy and Morphology of the Respiratory Tract. ICRP (eds) Human Respiratory Tract Model for Radiological Protection: Pergamon & Elsevier, ICRP publication 66, Annals of the ICRP; 1994. p. 121-66.
21 Heyder J, Gebhart J, Rudolf G, Schiller CF, Stahlhofen W. Deposition of particles in the human respiratory tract in the size range 0.005–15 μm. J Aerosol Med. 1986;17:811-25.
22 Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6(1):67-74.
23 Nicod LP. Lung defenses: an overview. Eur Respir Rev. 2005;95:45-50.
24 Gil J, Weibel ER. Extracellular lining of bronchioles after perfusion-fixation of rat lungs for electron microscopy. Anat Rec. 1971;169(2):185-99.
25 Schurch S, Gehr P, Im Hof V, Geiser M, Green F. Surfactant displaces particles toward the epithelium in airways and alveoli. Respir Physiol. 1990;80(1):17-32.
26 Kilburn KH. A hypothesis for pulmonary clearance and its implications. Am Rev Respir Dis. 1968;98(3):449-63.
27 Green F, Gehr P, Lee MM, Schürch S. The role of surfactant in disease associated with particle exposure. In: Gehr P, Heyder J, editors. Particle-Lung Interactions. New York: Marcel Dekker; 2000. p. 533-76.
28 Gasser M, Wick P, Clift MJ, Blank F, Diener L, Yan B, et al. Pulmonary surfactant coating of multi-walled carbon nanotubes (MWCNTs) influences their oxidative and pro-inflammatory potential in vitro. Part Fibre Toxicol. 2012;9(1):17.
29 Daniels CB, Orgeig S. Pulmonary Surfactant: The Key to the Evolution of Air Breathing. Physiology. 2003;18(4):151-7.
30 Gehr P, Schurch S, Berthiaume Y, Im Hof V, Geiser M. Particle retention in airways by surfactant. Journal of Aerosol Medicine. 1990;3:27-43.
31 Gehr P, Green FH, Geiser M, Im Hof V, Lee MM, Schurch S. Airway surfactant, a primary defense barrier: mechanical and immunological aspects. J Aerosol Med. 1996;9(2):163-81.
32 Wallace WE, Keane MJ, Murray DK, Chisholm WP, Maynard AD, Ong T-m. Phospholipid lung surfactant and nanoparticle surface toxicity: Lessons from diesel soots and silicate dusts. In: Maynard AD, Pui DYH, editors. Nanotechnology and Occupational Health. Dordrecht: Springer Netherlands; 2007. p. 23-38.
33 Salvador-Morales C, Townsend P, Flahaut E, Vénien-Bryan C, Vlandas A, Green MLH, et al. Binding of pulmonary surfactant proteins to carbon nanotubes; potential for damage to lung immune defense mechanisms. Carbon. 2007;45(3):607-17.
34 Wiemann M, Erlinghagen C, Bruch J, Rehn B. [Adsorption of lung surfactant by particles studied in an ex vivo model: effects of quartz and amorphous silica]. Materialwissenschaft und Werkstofftechnik. 2010(41):1086-92. German.
35 Ruge CA, Kirch J, Cañadas O, Schneider M, Perez-Gil J, Schaefer UF, et al. Uptake of nanoparticles by alveolar macrophages is triggered by surfactant protein A. Nanomedicine: Nanotechnology, Biology and Medicine. 2011;7(6):690-3.
36 Kendall M, Ding P, Mackay RM, Deb R, McKenzie Z, Kendall K, et al. Surfactant protein D (SP-D) alters cellular uptake of particles and nanoparticles. Nanotoxicology. 2012; Early online 1-11.
37 Ruge CA, Schaefer UF, Herrmann J, Kirch J, Canadas O, Echaide M, et al. The interplay of lung surfactant proteins and lipids assimilates the macrophage clearance of nanoparticles. PLoS One. 2012;7(7):e40775.
38 Konduru NV, Tyurina YY, Feng W, Basova LV, Belikova NA, Bayir H, et al. Phosphatidylserine targets single-walled carbon nanotubes to professional phagocytes in vitro and in vivo. PLoS One. 2009;4(2):e4398.
39 Brain JD. Lung macrophages: how many kinds are there? What do they do? Am Rev Respir Dis. 1988;137(3):507-9.
40 Lehnert BE. Pulmonary and thoracic macrophage subpopulations and clearance of particles from the lung. Environ Health Perspect. 1992;97:17-46.
41 Godfrey RW. Human airway epithelial tight junctions. Microsc Res Tech. 1997;38(5):488-99.
42 Schneeberger EE, Lynch RD. Tight junctions. Their structure, composition, and function. Circ Res. 1984;55(6):723-33.
43 Holt PG, Schon-Hegrad MA. Localization of T cells, macrophages and dendritic cells in rat respiratory tract tissue: implications for immune function studies. Immunology. 1987;62(3):349-56.
44 McWilliam AS, Holt PG, Gehr P. Dendritic cells as sentinels of immune surveillance in the airways. In: Gehr P, Heyder J, editors. Particle-lung interactions. New York, Basel: Marcel Dekker; 2000. p. 473-89.
45 Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13(1):114-9.
46 Holt PG, Stumbles PA. Regulation of immunologic homeostasis in peripheral tissues by dendritic cells: the respiratory tract as a paradigm. J Allergy Clin Immunol. 2000;105(3):421-9.
47 von Garnier C, Nicod LP. Immunology taught by lung dendritic cells. Swiss Med Wkly. 2009;139(13-14):186-92.
48 Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251-62.
49 Blank F, Gerber P, Rothen-Rutishauser B, Sakulkhu U, Salaklang J, De Peyer K, et al. Biomedical nanoparticles modulate specific CD4+ T cell stimulation by inhibition of antigen processing in dendritic cells. Nanotoxicology. 2011;5(4):606-21.
50 Maina JN, West JB. Thin and strong! The bioengineering dilemma in the structural and functional design of the blood-gas barrier. Physiol Rev. 2005;85(3):811-44.
51 Dunsmore SE, Rannels DE. Extracellular matrix biology in the lung. Am J Physiol. 1996;270(1 Pt 1):L3-27.
52 Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91(4):1487-500.
53 Schneeberger EE. Ultrastructure of intercellular junctions in the freeze fractured alveolar-capillary membrane of mouse lung. Chest. 1977;71(2 suppl):299-300.
54 Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am J Respir Crit Care Med. 2001;164(9):1665-8.
55 Takenaka S, Karg E, Roth C, Schulz H, Ziesenis A, Heinzmann U, et al. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ Health Perspect. 2001;109(Suppl 4):547-51.
56 Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105(4):411-4.
57 Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H, et al. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health A. 2002;65(20):1513-30.
58 Mills NL, Amin N, Robinson SD, Anand A, Davies J, Patel D, et al. Do inhaled carbon nanoparticles translocate directly into the circulation in humans? Am J Respir Crit Care Med. 2006;173(4):426-31.
59 Wiebert P, Sanchez-Crespo A, Falk R, Philipson K, Lundin A, Larsson S, et al. No significant translocation of inhaled 35-nm carbon particles to the circulation in humans. Inhal Toxicol. 2006;18(10):741-7.
60 Brown JS, Zeman KL, Bennett WD. Ultrafine particle deposition and clearance in the healthy and obstructed lung. Am J Respir Crit Care Med. 2002;166(9):1240-7.
61 Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16(6-7):437-45.
62 Oberdorster G, Elder A, Rinderknecht A. Nanoparticles and the brain: cause for concern? J Nanosci Nanotechnol. 2009;9(8):4996-5007.
63 Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114(8):1172-8.
64 Bodian D, Howe HA. Experimental studies on intraneural spread of poliomyelitis virus. Bull Johns Hopkins Hosp. 1941;68:248-67.
65 Bodian D, Howe HA. The rate of progression of poliomyelitis virus in nerves. Bull Johns Hopkins Hosp. 1941;69:79-85.
66 DeLorenzo AJD. The olfactory neuron and the blood-brain barrier. In: Wolstenholme GEW, Knight J, editors. Taste and Smell in Vertebrates. London: J&A Churchill; 1970. p. 151-76.
67 Geiser M, Rothen-Rutishauser B, Kapp N, Schurch S, Kreyling W, Schulz H, et al. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect. 2005;113(11):1555-60.
68 Rothen-Rutishauser BM, Schurch S, Haenni B, Kapp N, Gehr P. Interaction of fine particles and nanoparticles with red blood cells visualized with advanced microscopic techniques. Environ Sci Technol. 2006;40(14):4353-9.
69 Rothen-Rutishauser B, Muhlfeld C, Blank F, Musso C, Gehr P. Translocation of particles and inflammatory responses after exposure to fine particles and nanoparticles in an epithelial airway model. Part Fibre Toxicol. 2007;4:9.
70 Kapp N, Kreyling W, Schulz H, Im Hof V, Gehr P, Semmler M, et al. Electron energy loss spectroscopy for analysis of inhaled ultrafine particles in rat lungs. Microsc Res Tech. 2004;63(5):298-305.
71 Muller J, Huaux F, Moreau N, Misson P, Heilier JF, Delos M, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207(3):221-31.
72 Gonzalez-Flecha B. Oxidant mechanisms in response to ambient air particles. Mol Aspects Med. 2004;25(1-2):169-82.
73 Vinzents PS, Moller P, Sorensen M, Knudsen LE, Hertel O, Jensen FP, et al. Personal exposure to ultrafine particles and oxidative DNA damage. Environ Health Perspect. 2005;113(11):1485-90.
74 Xiao GG, Wang M, Li N, Loo JA, Nel AE. Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J Biol Chem. 2003;278(50):50781-90.
75 Brown DM, Wilson MR, MacNee W, Stone V, Donaldson K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol Appl Pharmacol. 2001;175(3):191-9.
76 Donaldson K, Tran L, Jimenez LA, Duffin R, Newby DE, Mills N, et al. Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part Fibre Toxicol. 2005;2:10.
77 Rahman I, Macnee W. Oxidative stress and regulation of glutathione in lung infalmmation. Eur Respir J. 2000;16:534-54.
78 Brown DM, Donaldson K, Borm PJ, Schins RP, Dehnhardt M, Gilmour P, et al. Calcium and ROS-mediated activation of transcription factors and TNF-alpha cytokine gene expression in macrophages exposed to ultrafine particles. Am J Physiol Lung Cell Mol Physiol. 2004;286(2):L344-53.
79 Pope CA, 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132-41.
80 Clift MJ, Gehr P, Rothen-Rutishauser B. Nanotoxicology: a perspective and discussion of whether or not in vitro testing is a valid alternative. Arch Toxicol. 2011;85(7):723-31.
81 Krug HF, Wick P. Nanotoxicology: An Interdisciplinary Challenge. Angewandte Chemie International Edition. 2011;50(6):1260-78.
82 Timbrell J. Principles of biochemical toxicology: London, New York: Taylor Francis CRC Press; 1999.
83 Donaldson K, Stone V, Borm PJ, Jimenez LA, Gilmour PS, Schins RP, et al. Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10). Free Radic Biol Med. 2003;34(11):1369-82.
84 Cancer. Fact sheet N° 297: Geneva: World Health Organization; 2009.
85 Donaldson K, Tran CL. An introduction to the short-term toxicology of respirable industrial fibres. Mutat Res. 2004;553(1-2):5-9.
86 Schins RP, Knaapen AM. Genotoxicity of poorly soluble particles. Inhal Toxicol. 2007;19(Suppl 1):189-98.
87 Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3(7):423-8.
88 Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, et al. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci. 2006;92(1):5-22.
89 Van Berlo D, Clift MJ, Albrecht C, Schins RP. Carbon nanotubes: An insight into the mechanisms of their potential genotoxicity. Swiss Med Wkly. 2012;142: w13698.
90 Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422(6927):37-44.
91 Brown BS. Biological membranes. Gull D, editor. London: The Biological Society; 1996.
92 Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593-623.
93 Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8(8):603-12.
94 Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8(3):185-94.
95 Tagawa A, Mezzacasa A, Hayer A, Longatti A, Pelkmans L, Helenius A. Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J Cell Biol. 2005;170(5):769-79.
96 McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12(8):517-33.
97 Brandenberger C, Muhlfeld C, Zulqurnain A, Lenz AG, Schmid O, Parak WJ, et al. Quantitative evaluation of cellular uptake and trafficking of plain and polyethylene glycol-coated gold nanoparticles. Small. 2010;6(15):1669-78.
98 Lesniak W, Bielinska AU, Sun K, Janczak KW, Shi X, Baker JR, Jr., et al. Silver/dendrimer nanocomposites as biomarkers: fabrication, characterization, in vitro toxicity, and intracellular detection. Nano Lett. 2005;5(11):2123-30.
99 Rimai DS, Quesnel DJ, Busnaia AA. The adhesion of dry particles in the nanometer to micrometr size range. Colloids and Surfaces A. 2000;165:3-10.
100 Lehmann AD, Parak WJ, Zhang F, Ali Z, Röcker C, Nienhaus GU, et al. Fluorescent–Magnetic Hybrid Nanoparticles Induce a Dose-Dependent Increase in Proinflammatory Response in Lung Cells in vitro Correlated with Intracellular Localization. Small. 2010;6(6):753-62.
101 Muhlfeld C, Gehr P, Rothen-Rutishauser B. Translocation and cellular entering mechanisms of nanoparticles in the respiratory tract. Swiss Med Wkly. 2008;138(27-28):387-91.
102 Rothen-Rutishauser B, Blank F, Mühlfeld C, Gehr P. Nanoparticle-cell membrane interactions. In: Gehr P, Mühlfeld C, Rothen-Rutishauser B, Blank F, editors. Particle-Lung Interactions. New York: Informa Healthcare; 2009. p. 226-42.
103 Unfried K, Albrecht C, Klowtz LO, von Mikecz A, Grether-Beck S, Schins RP. Cellular responses to nanoparticles: target structures and mechanisms. Nanotoxicology. 2007;1:1-20.
104 Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proceedings of the National Academy of Sciences. 2008 September 23, 2008;105(38):14265-70.
105 Lynch I, Dawson KA. Protein-nanoparticle interactions. Nano Today. 2008;3(1-2):40-7.
106 Mahmoudi M. Cell “Vision”: Complementary Factor of Protein Corona in NanoToxicology. Nanoscale. 2012;4(17):5461-8.
107 Aufderheide M. Direct exposure methods for testing native atmospheres. Exp Toxicol Pathol. 2005;57(Suppl 1):213-26.
108 Sakagami M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv Drug Deliv Rev. 2006;58(9-10):1030-60.
109 Muhlfeld C, Rothen-Rutishauser B, Blank F, Vanhecke D, Ochs M, Gehr P. Interactions of nanoparticles with pulmonary structures and cellular responses. Am J Physiol Lung Cell Mol Physiol. 2008;294(5):L817-29.
110 Rothen-Rutishauser B, Blank F, Muhlfeld C, Gehr P. In vitro models of the human epithelial airway barrier to study the toxic potential of particulate matter. Expert Opin Drug Metab Toxicol. 2008;4(8):1075-89.
111 Gardner DE, Kennedy GL. Methodologies and technology for animal inhalation toxicology studies. In: Gardner DE, Crapo JD, McClellan RO, editors. Toxicology of the lung. 2nd ed. New York: Raven Press; 1993.
112 Pauluhn J. Overview of inhalation exposure techniques: strengths and weaknesses. Exp Toxicol Pathol. 2005;57(Suppl 1):111-28.
113 Pauluhn J, Mohr U. Inhalation studies in laboratory animals – current concepts and alternatives. Toxicol Pathol. 2000;28(5):734-53.
114 Driscoll KE, Costa DL, Hatch G, Henderson R, Oberdorster G, Salem H, et al. Intratracheal Instillation as an Exposure Technique for the Evaluation of Respiratory Tract Toxicity: Uses and Limitations. Toxicological Sciences. 2000;55(1):24-35.
115 Subcommittee on Manufactured Vitreous Fibers, Committee on Toxicology, Commission on Life Sciences, National Research Council. Review of the U.S. Navy’s Exposure Standard for Manufactured Vitreous Fibers. Washington D.C.: National Academy Press; 2000.
116 Shvedova AA, Kisin E, Murray AR, Johnson VJ, Gorelik O, Arepalli S, et al. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress, and mutagenesis. American Journal of Physiology – Lung Cellular and Molecular Physiology. 2008;295(4):L552-L65.
117 Osier M, Oberdorster G. Intratracheal inhalation vs intratracheal instillation: differences in particle effects. Fundam Appl Toxicol. 1997;40(2):220-7.
118 Warheit DB, Brock WJ, Lee KP, Webb TR, Reed KL. Comparative pulmonary toxicity inhalation and instillation studies with different TiO2 particle formulations: impact of surface treatments on particle toxicity. Toxicol Sci. 2005;88(2):514-24.
119 Steimer A, Haltner E, Lehr CM. Cell culture models of the respiratory tract relevant to pulmonary drug delivery. J Aerosol Med. 2005;18(2):137-82.
120 Nemmar A, Hamoir J, Nemery B, Gustin P. Evaluation of particle translocation across the alveolo-capillary barrier in isolated perfused rabbit lung model. Toxicology. 2005;208(1):105-13.
121 Meiring JJ, Borm PJ, Bagate K, Semmler M, Seitz J, Takenaka S, et al. The influence of hydrogen peroxide and histamine on lung permeability and translocation of iridium nanoparticles in the isolated perfused rat lung. Part Fibre Toxicol. 2005;2:3.
122 Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, et al. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 2002;65(20):1531-43.
123 Nassimi M, Schleh C, Lauenstein HD, Hussein R, Lubbers K, Pohlmann G, et al. Low cytotoxicity of solid lipid nanoparticles in in vitro and ex vivo lung models. Inhal Toxicol. 2009;21 Suppl 1:104-9.
124 Hartung T, Balls M, Bardouille C, Blanck O, Coecke S, Gstraunthaler G, et al. Good Cell Culture Practice. ECVAM Good Cell Culture Practice Task Force Report 1. Altern Lab Anim. 2002;30(4):407-14.
125 Gruber FP, Hartung T. Alternatives to animal experimentation in basic research. ALTEX. 2004;21(Suppl 1):3-31.
126 Rothen-Rutishauser B, Clift MJD, Jud C, Fink A, Wick P. Human epithelial cell in vitro – Are they an advantageous tool to help understand the nanomaterial-biological barrier interaction? EURO NanoTox Letters. 2012;001:0001-0020.
127 Schanker LS, Mitchell EW, Brown RA, Jr. Pulmonary absorption of drugs in the dog: comparison with other species. Pharmacology. 1986;32(3):176-80.
128 Schanker LS, Mitchell EW, Brown RA, Jr. Species comparison of drug absorption from the lung after aerosol inhalation or intratracheal injection. Drug Metab Dispos. 1986;14(1):79-88.
129 Li C, Liu H, Sun Y, Wang H, Guo F, Rao S, et al. PAMAM nanoparticles promote acute lung injury by inducing autophagic cell death through the Akt-TSC2-mTOR signaling pathway. J Mol Cell Biol. 2009;1(1):37-45.
130 Health Risks Of Nantotechnology: How Nanoparticles Can Cause Lung Damage, And How The Damage Can Be Blocked [Internet]. ScienceDaily 2009. Available from: http://www.sciencedaily.com/releases/2009/06/090610192431.htm
131 King LS, Agre P. Man Is not a rodent: aquaporins in the airways. Am J Respir Cell Mol Biol. 2001;24(3):221-3.
132 Gstraunthaler G, Hartung T. Good cell culture practice: good laboratory practice in the cell culture laboratory for the standardization and quality assurance of in vitro studies. In: Lehr CM, editor. Cell culture models of biological barriers In-vitro test systems for drug absorption and delivery. London, New York: Taylor & Francis; 2002. p. 112-20.
133 de Jong PM, van Sterkenburg MA, Kempenaar JA, Dijkman JH, Ponec M. Serial culturing of human bronchial epithelial cells derived from biopsies. In Vitro Cell Dev Biol Anim. 1993;29A(5):379-87.
134 Galietta LJ, Lantero S, Gazzolo A, Sacco O, Romano L, Rossi GA, et al. An improved method to obtain highly differentiated monolayers of human bronchial epithelial cells. In Vitro Cell Dev Biol Anim. 1998;34(6):478-81.
135 Masui T, Wakefield LM, Lechner JF, LaVeck MA, Sporn MB, Harris CC. Type beta transforming growth factor is the primary differentiation-inducing serum factor for normal human bronchial epithelial cells. Proc Natl Acad Sci U S A. 1986;83(8):2438-42.
136 Forrest IA, Murphy DM, Ward C, Jones D, Johnson GE, Archer L, et al. Primary airway epithelial cell culture from lung transplant recipients. Eur Respir J. 2005;26(6):1080-5.
137 Wang J, Edeen K, Manzer R, Chang Y, Wang S, Chen X, et al. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am J Respir Cell Mol Biol. 2007;36(6):661-8.
138 Bingle L, Bull TB, Fox B, Guz A, Richards RJ, Tetley TD. Type II pneumocytes in mixed cell culture of human lung: a light and electron microscopic study. Environ Health Perspect. 1990;85:71-80.
139 Ehrhardt C, Kim KJ, Lehr CM. Isolation and culture of human alveolar epithelial cells. Methods Mol Med. 2005;107:207-16.
140 Elbert KJ, Schafer UF, Schafers HJ, Kim KJ, Lee VH, Lehr CM. Monolayers of human alveolar epithelial cells in primary culture for pulmonary absorption and transport studies. Pharm Res. 1999;16(5):601-8.
141 Fuchs S, Hollins AJ, Laue M, Schaefer UF, Roemer K, Gumbleton M, et al. Differentiation of human alveolar epithelial cells in primary culture: morphological characterization and synthesis of caveolin-1 and surfactant protein-C. Cell Tissue Res. 2003;311(1):31-45.
142 Fogh J, Trempe G. New human tumor cell lines. In: Fogh J, editor. Human Tumor Cells In Vitro. New York: Plenum Press; 1975. p. 115-59.
143 Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, et al. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10(1):38-47.
144 Reddel RR, Ke Y, Gerwin BI, McMenamin MG, Lechner JF, Su RT, et al. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988;48(7):1904-9.
145 Ehrhardt C, Forbes B, Kim K-J. In Vitro Models of the Tracheo-Bronchial Epithelium. In: Ehrhardt C, Kim K-J, editors. Drug Absorption Studies: Springer US; 2008. p. 235-57.
146 Blank F, Rothen-Rutishauser B, Gehr P. Dendritic cells and macrophages form a transepithelial network against foreign particulate antigens. Am J Respir Cell Mol Biol. 2007;36(6):669-77.
147 Penn A, Murphy G, Barker S, Henk W, Penn L. Combustion-derived ultrafine particles transport organic toxicants to target respiratory cells. Environ Health Perspect. 2005;113(8):956-63.
148 Gurr JR, Wang AS, Chen CH, Jan KY. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology. 2005;213(1-2):66-73.
149 Herzog E, Casey A, Lyng FM, Chambers G, Byrne HJ, Davoren M. A new approach to the toxicity testing of carbon-based nanomaterials--the clonogenic assay. Toxicol Lett. 2007;174(1-3):49-60.
150 Veranth JM, Kaser EG, Veranth MM, Koch M, Yost GS. Cytokine responses of human lung cells (BEAS-2B) treated with micron-sized and nanoparticles of metal oxides compared to soil dusts. Part Fibre Toxicol. 2007;4:2.
151 Zabner J, Karp P, Seiler M, Phillips SL, Mitchell CJ, Saavedra M, et al. Development of cystic fibrosis and noncystic fibrosis airway cell lines. Am J Physiol Lung Cell Mol Physiol. 2003;284(5):L844-54.
152 Forbes II. Human airway epithelial cell lines for in vitro drug transport and metabolism studies. Pharm Sci Technolo Today. 2000;3(1):18-27.
153 Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976;17(1):62-70.
154 Foster KA, Oster CG, Mayer MM, Avery ML, Audus KL. Characterization of the A549 cell line as a type II pulmonary epithelial cell model for drug metabolism. Exp Cell Res. 1998;243(2):359-66.
155 Shapiro DL, Nardone LL, Rooney SA, Motoyama EK, Munoz JL. Phospholipid biosynthesis and secretion by a cell line (A549) which resembles type II aleveolar epithelial cells. Biochim Biophys Acta. 1978;530(2):197-207.
156 Ehrhardt C, Laue M, Kim K-J. In Vitro Models of the Alveolar Epithelial Barrier. In: Ehrhardt C, Kim K-J, editors. Drug Absorption Studies: Springer US; 2008. p. 258-82.
157 Shlyonsky V, Goolaerts A, Van Beneden R, Sariban-Sohraby S. Differentiation of epithelial Na+ channel function. An in vitro model. J Biol Chem. 2005;280(25):24181-7.
158 Woollhead AM, Baines DL. Forskolin-induced cell shrinkage and apical translocation of functional enhanced green fluorescent protein-human alphaENaC in H441 lung epithelial cell monolayers. J Biol Chem. 2006;281(8):5158-68.
159 Duncan JE, Whitsett JA, Horowitz AD. Pulmonary surfactant inhibits cationic liposome-mediated gene delivery to respiratory epithelial cells in vitro. Hum Gene Ther. 1997;8(4):431-8.
160 Rehan VK, Torday JS, Peleg S, Gennaro L, Vouros P, Padbury J, et al. 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha,25-dihydroxy vitamin D3: production and biological activity studies in pulmonary alveolar type II cells. Mol Genet Metab. 2002;76(1):46-56.
161 Newton DA, Rao KM, Dluhy RA, Baatz JE. Hemoglobin is expressed by alveolar epithelial cells. J Biol Chem. 2006;281(9):5668-76.
162 Zhang L, Whitsett JA, Stripp BR. Regulation of Clara cell secretory protein gene transcription by thyroid transcription factor-1. Biochim Biophys Acta. 1997;1350(3):359-67.
163 Kemp SJ, Thorley AJ, Gorelik J, Seckl MJ, O’Hare MJ, Arcaro A, et al. Immortalization of human alveolar epithelial cells to investigate nanoparticle uptake. Am J Respir Cell Mol Biol. 2008;39(5):591-7.
164 Freshney RI. Culture of animal cells: a munaul of basic techniques. 4th ed. New York: Wiley-Liss; 2000.
165 Roggen EL, Soni NK, Verheyen GR. Respiratory immunotoxicity: an in vitro assessment. Toxicol In Vitro. 2006;20(8):1249-64.
166 Carterson AJ, Honer zu Bentrup K, Ott CM, Clarke MS, Pierson DL, Vanderburg CR, et al. A549 lung epithelial cells grown as three-dimensional aggregates: alternative tissue culture model for Pseudomonas aeruginosa pathogenesis. Infect Immun. 2005;73(2):1129-40.
167 Vertrees RA, Zwischenberger JB, Boor PJ, Popov V, McCarthy M, Solley TN, et al. Cellular differentiation in three-dimensional lung cell cultures. Cancer Biol Ther. 2008;7(3):404-12.
168 Steele RE, Preston AS, Johnson JP, Handler JS. Porous-bottom dishes for culture of polarized cells. Am J Physiol. 1986;251(1 Pt 1):C136-9.
169 Lehmann AD, Blank F, Baum O, Gehr P, Rothen-Rutishauser BM. Diesel exhaust particles modulate the tight junction protein occludin in lung cells in vitro. Part Fibre Toxicol. 2009;6:26.
170 Muller L, Riediker M, Wick P, Mohr M, Gehr P, Rothen-Rutishauser B. Oxidative stress and inflammation response after nanoparticle exposure: differences between human lung cell monocultures and an advanced three-dimensional model of the human epithelial airways. J R Soc Interface. 2009;7(Suppl 1):S27-40.
171 Bur M, Huwer H, Lehr CM, Hagen N, Guldbrandt M, Kim KJ, et al. Assessment of transport rates of proteins and peptides across primary human alveolar epithelial cell monolayers. Eur J Pharm Sci. 2006;28(3):196-203.
172 Rothen-Rutishauser BM, Kiama SG, Gehr P. A three-dimensional cellular model of the human respiratory tract to study the interaction with particles. Am J Respir Cell Mol Biol. 2005;32(4):281-9.
173 Brandenberger C, Rothen-Rutishauser B, Muhlfeld C, Schmid O, Ferron GA, Maier KL, et al. Effects and uptake of gold nanoparticles deposited at the air-liquid interface of a human epithelial airway model. Toxicol Appl Pharmacol. 2010;242(1):56-65.
174 Raemy DO, Limbach LK, Rothen-Rutishauser B, Grass RN, Gehr P, Birbaum K, et al. Cerium oxide nanoparticle uptake kinetics from the gas-phase into lung cells in vitro is transport limited. Eur J Pharm Biopharm. 2011;77(3):368-75.
175 Alfaro-Moreno E, Nawrot TS, Vanaudenaerde BM, Hoylaerts MF, Vanoirbeek JA, Nemery B, et al. Co-cultures of multiple cell types mimic pulmonary cell communication in response to urban PM10. Eur Respir J. 2008;32(5):1184-94.
176 Hoang ATN, Chen P, Juarez J, Sachamitr P, Billing B, Bosnjak L, et al. Dendritic cell functional properties in a three-dimensional tissue model of human lung mucosa. American Journal of Physiology – Lung Cellular and Molecular Physiology. 2012 January 15, 2012;302(2):L226-L37.
177 Nalayanda DD, Wang Q, Fulton WB, Wang TH, Abdullah F. Engineering an artificial alveolar-capillary membrane: a novel continuously perfused model within microchannels. J Pediatr Surg. 2010;45(1):45-51.
178 Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328(5986):1662-8.
179 Choe MM, Sporn PH, Swartz MA. Extracellular matrix remodeling by dynamic strain in a three-dimensional tissue-engineered human airway wall model. Am J Respir Cell Mol Biol. 2006;35(3):306-13.
180 Tomei AA, Choe MM, Swartz MA. Effects of dynamic compression on lentiviral transduction in an in vitro airway wall model. Am J Physiol Lung Cell Mol Physiol. 2008;294(1):L79-86.
181 Duffin R, Tran L, Brown D, Stone V, Donaldson K. Proinflammogenic effects of low-toxicity and metal nanoparticles in vivo and in vitro: highlighting the role of particle surface area and surface reactivity. Inhal Toxicol. 2007;19(10):849-56.
182 Limbach LK, Li Y, Grass RN, Brunner TJ, Hintermann MA, Muller M, et al. Oxide nanoparticle uptake in human lung fibroblasts: effects of particle size, agglomeration, and diffusion at low concentrations. Environ Sci Technol. 2005;39(23):9370-6.
183 Stearns RC, Paulauskis JD, Godleski JJ. Endocytosis of ultrafine particles by A549 cells. Am J Respir Cell Mol Biol. 2001;24(2):108-15.
184 Voisin C, Aerts C, Jakubczak E, Houdret JL, Tonnel TB. Effects of nitrogen dioxide on alveolar macrophages surviving in the gas phase. A new experimental model for the study of in vitro cytotoxicity of toxic gases (author’s transl). Bull Eur Physiopathol Respir. 1977;13(1):137-44.
185 Voisin C, Aerts C, Jakubczk E, Tonnel AB. La culture cellulaire en phase gazeuse. Un nouveau modele experimental d’etude in vitro des activites des macrophages alveolaires. Bull Eur Physiopathol Respir. 1977;13(1):69-82. French.
186 Blank F, Rothen-Rutishauser BM, Schurch S, Gehr P. An optimized in vitro model of the respiratory tract wall to study particle cell interactions. J Aerosol Med. 2006;19(3):392-405.
187 Ehrhardt C, Kneuer C, Fiegel J, Hanes J, Schaefer UF, Kim KJ, et al. Influence of apical fluid volume on the development of functional intercellular junctions in the human epithelial cell line 16HBE14o-: implications for the use of this cell line as an in vitro model for bronchial drug absorption studies. Cell Tissue Res. 2002;308(3):391-400.
188 Radyuk SN, Mericko PA, Popova TG, Grene E, Alibek K. In vitro-generated respiratory mucosa: a new tool to study inhalational anthrax. Biochem Biophys Res Commun. 2003;305(3):624-32.
189 Rothen-Rutishauser B, Grass RN, Blank F, Limbach LK, Muhlfeld C, Brandenberger C, et al. Direct combination of nanoparticle fabrication and exposure to lung cell cultures in a closed setup as a method to simulate accidental nanoparticle exposure of humans. Environ Sci Technol. 2009;43(7):2634-40.
190 Seagrave J, Dunaway S, McDonald JD, Mauderly JL, Hayden P, Stidley C. Responses of differentiated primary human lung epithelial cells to exposure to diesel exhaust at an air-liquid interface. Exp Lung Res. 2007;33(1):27-51.
191 Lenz AG, Karg E, Lentner B, Dittrich V, Brandenberger C, Rothen-Rutishauser B, et al. A dose-controlled system for air-liquid interface cell exposure and application to zinc oxide nanoparticles. Part Fibre Toxicol. 2009;6:32.
192 Muller L, Comte P, Czerwinski J, Kasper M, Mayer AC, Gehr P, et al. New exposure system to evaluate the toxicity of (scooter) exhaust emissions in lung cells in vitro. Environ Sci Technol. 2010;44(7):2632-8.
193 Abe S, Takizawa H, Sugawara I, Kudoh S. Diesel exhaust (DE)-induced cytokine expression in human bronchial epithelial cells: a study with a new cell exposure system to freshly generated DE in vitro. Am J Respir Cell Mol Biol. 2000;22(3):296-303.
194 Holder AL, Lucas D, Goth-Goldstein R, Koshland CP. Cellular response to diesel exhaust particles strongly depends on the exposure method. Toxicol Sci. 2008;103(1):108-15.
195 Aufderheide M, Mohr U. CULTEX — an alternative technique for cultivation and exposure of cells of the respiratory tract to airborne pollutants at the air/liquid interface. Experimental and Toxicologic Pathology. 2000;52(3):265-70.
196 Tippe A, Heinzmann U, Roth C. Deposition of fine and ultrafine aerosol particles during exposure at the air/cell interface. Journal of Aerosol Science. 2002;33(2):207-18.
197 Kim SC, Chen D-R, Qi C, Gelein RM, Finkelstein JN, Elder A, et al. A nanoparticle dispersion method for in vitro and in vivo nanotoxicity study. Nanotoxicology. 2009;4(1):42-51.
198 Muller L, Gasser M, Raemy DO, Herzog F, Brandenberger C, Schmid O, et al. Realistic exposure methods for investigating the interaction of nanoparticles with the lung at the air-liquid interface in vitro. Insciences Journal. 2011;1(1):30-64.
199 Paur H-R, Cassee FR, Teeguarden J, Fissan H, Diabate S, Aufderheide M, et al. In-vitro cell exposure studies for the assessment of nanoparticle toxicity in the lung – A dialog between aerosol science and biology. Journal of Aerosol Science. 2011;42(10):668-92.
200 Fall AB, Lindström SB, Sundman O, Ödberg L, Wågberg L. Colloidal Stability of Aqueous Nanofibrillated Cellulose Dispersions. Langmuir. 2011 2011/09/20;27(18):11332-8.
201 Ghosh S, Jiang W, McClements JD, Xing B. Colloidal Stability of Magnetic Iron Oxide Nanoparticles: Influence of Natural Organic Matter and Synthetic Polyelectrolytes. Langmuir. 2011;27(13):8036-43.
202 Gebauer JS, Malissek M, Simon S, Knauer SK, Maskos M, Stauber RH, et al. Impact of the Nanoparticle–Protein Corona on Colloidal Stability and Protein Structure. Langmuir. 2012;28(25):9673-9.
203 Bouwmeester H, Lynch I, Marvin HJ, Dawson KA, Berges M, Braguer D, et al. Minimal analytical characterization of engineered nanomaterials needed for hazard assessment in biological matrices. Nanotoxicology. 2011;5(1):1-11.
204 Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6(4):662-8.
206 Petri-Fink A, Rothen-Rutishauser B. Nanoparticles and cells: an interdisciplinary approach. Chimia (Aarau). 2012;66(3):104-9.
207 Vallhov H, Qin J, Johansson SM, Ahlborg N, Muhammed MA, Scheynius A, et al. The Importance of an Endotoxin-Free Environment during the Production of Nanoparticles Used in Medical Applications. Nano Letters. 2006;6(8):1682-6.
208 Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622-7.
209 Stone V, Donaldson K. Nanotoxicology: signs of stress. Nat Nanotechnol. 2006;1(1):23-4.
210 Han X, Gelein R, Corson N, Wade-Mercer P, Jiang J, Biswas P, et al. Validation of an LDH assay for assessing nanoparticle toxicity. Toxicology. 2011;287(1-3):99-104.
211 Stone V, Johnston H, Schins RP. Development of in vitro systems for nanotoxicology: methodological considerations. Crit Rev Toxicol. 2009;39(7):613-26.
212 Jiang W, KimBetty YS, Rutka JT, ChanWarren CW. Nanoparticle-mediated cellular response is size-dependent. Nat Nano. [10.1038/nnano.2008.30]. 2008;3(3):145-50.
213 Steiner S, Müller L, Raemy DO, Popovicheva OB, Czerwinski J, Comte P, et al. Cerium dioxide nanoparticles can interfere with the associated cellular mechanistic response to diesel exhaust exposure. Toxicology Letters. 2012;214:218-25.
214 Yoo SI, Yang M, Brender JR, Subramanian V, Sun K, Joo NE, et al. Inside Cover: Inhibition of Amyloid Peptide Fibrillation by Inorganic Nanoparticles: Functional Similarities with Proteins. Angewandte Chemie International Edition. 2011;50(22):5110-5.
215 Radovic-Moreno AF, Lu TK, Puscasu VA, Yoon CJ, Langer R, Farokhzad OC. Surface Charge-Switching Polymeric Nanoparticles for Bacterial Cell Wall-Targeted Delivery of Antibiotics. ACS Nano. 2012;6(5):4279-87.
216 Salata O. Applications of nanoparticles in biology and medicine. J Nanobiotechnology. 2004;2(1):3.
217 de Haar C, Hassing I, Bol M, Bleumink R, Pieters R. Ultrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in mice. Clin Exp Allergy. 2006;36(11):1469-79.
218 Semmler-Behnke M, Takenaka S, Fertsch S, Wenk A, Seitz J, Mayer P, et al. Efficient elimination of inhaled nanoparticles from the alveolar region: evidence for interstitial uptake and subsequent reentrainment onto airways epithelium. Environ Health Perspect. 2007;115(5):728-33.
219 Stephens MS. The significance of alternative techniques in biomedical research: an analysis of Nobel prize awards. In: Fox MW, Mickley LD, editors. Advances in Animal Welfare Science. Boston: Milhoff; 1987.
220 Gornati R, Papis E, Di Cioacchino M, Sabbioni E, Dalle-Donne I, Milzani A, et al. In Vivo and In Vitro Models for Nanotoxicology Testing. In: Sahu S, Casciano D, editors. Nanotoxicity. From In Vivo and In Vitro Models to Health Risks. Chichester: Wiley; 2009.
221 Dockery DW, Pope CA, 3rd, Xu X, Spengler JD, Ware JH, Fay ME, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329(24):1753-9.
222 Pope CA, 3rd, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med. 2009;360(4):376-86.
223 Bremner SA, Anderson HR, Atkinson RW, McMichael AJ, Strachan DP, Bland JM, et al. Short-term associations between outdoor air pollution and mortality in London 1992-4. Occup Environ Med. 1999;56(4):237-44.
224 Choudhury AH, Gordian ME, Morris SS. Associations between respiratory illness and PM10 air pollution. Arch Environ Health. 1997;52(2):113-7.
225 Aga E, Samoli E, Touloumi G, Anderson HR, Cadum E, Forsberg B, et al. Short-term effects of ambient particles on mortality in the elderly: results from 28 cities in the APHEA2 project. Eur Respir J Suppl. 2003;40:28s-33s.
226 Schwartz J. The effects of particulate air pollution on daily deaths: a multi-city case crossover analysis. Occup Environ Med. 2004;61(12):956-61.
227 Zanobetti A, Schwartz J, Samoli E, Gryparis A, Touloumi G, Peacock J, et al. The temporal pattern of respiratory and heart disease mortality in response to air pollution. Environ Health Perspect. 2003;111(9):1188-93.
228 Abbey DE, Nishino N, McDonnell WF, Burchette RJ, Knutsen SF, Lawrence Beeson W, et al. Long-term inhalable particles and other air pollutants related to mortality in nonsmokers. Am J Respir Crit Care Med. 1999;159(2):373-82.
229 Peters A, Wichmann HE, Tuch T, Heinrich J, Heyder J. Respiratory effects are associated with the number of ultrafine particles. Am J Respir Crit Care Med. 1997;155(4):1376-83.
230 Li XY, Gilmour PS, Donaldson K, MacNee W. In vivo and in vitro proinflammatory effects of particulate air pollution (PM10). Environ Health Perspect. 1997;105(Suppl 5):1279-83.
231 Simko M, Mattsson MO. Risks from accidental exposures to engineered nanoparticles and neurological health effects: a critical review. Part Fibre Toxicol. 2010;7:42.
232 Bivas-Benita M, Romeijn S, Junginger HE, Borchard G. PLGA-PEI nanoparticles for gene delivery to pulmonary epithelium. Eur J Pharm Biopharm. 2004;58(1):1-6.
233 Grenha A, Grainger CI, Dailey LA, Seijo B, Martin GP, Remunan-Lopez C, et al. Chitosan nanoparticles are compatible with respiratory epithelial cells in vitro. Eur J Pharm Sci. 2007;31(2):73-84.
234 Rotoli BM, Bussolati O, Bianchi MG, Barilli A, Balasubramanian C, Bellucci S, et al. Non-functionalized multi-walled carbon nanotubes alter the paracellular permeability of human airway epithelial cells. Toxicol Lett. 2008;178(2):95-102.
235 Grainger CI, Greenwell LL, Lockley DJ, Martin GP, Forbes B. Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharm Res. 2006;23(7):1482-90.
236 Florea BI, Cassara ML, Junginger HE, Borchard G. Drug transport and metabolism characteristics of the human airway epithelial cell line Calu-3. J Control Release. 2003;87(1-3):131-8.
237 Fiegel J, Ehrhardt C, Schaefer UF, Lehr CM, Hanes J. Large porous particle impingement on lung epithelial cell monolayers – toward improved particle characterization in the lung. Pharm Res. 2003;20(5):788-96.
238 Cooney D, Kazantseva M, Hickey AJ. Development of a size-dependent aerosol deposition model utilising human airway epithelial cells for evaluating aerosol drug delivery. Altern Lab Anim. 2004;32(6):581-90.
239 Amidi M, Romeijn SG, Borchard G, Junginger HE, Hennink WE, Jiskoot W. Preparation and characterization of protein-loaded N-trimethyl chitosan nanoparticles as nasal delivery system. J Control Release. 2006;111(1-2):107-16.
240 Brzoska M, Langer K, Coester C, Loitsch S, Wagner TO, Mallinckrodt C. Incorporation of biodegradable nanoparticles into human airway epithelium cells-in vitro study of the suitability as a vehicle for drug or gene delivery in pulmonary diseases. Biochem Biophys Res Commun. 2004;318(2):562-70.
241 Ehrhardt C, Kneuer C, Laue M, Schaefer UF, Kim KJ, Lehr CM. 16HBE14o- human bronchial epithelial cell layers express P-glycoprotein, lung resistance-related protein, and caveolin-1. Pharm Res. 2003;20(4):545-51.
242 Jang M, Ghio AJ, Cao G. Exposure of BEAS-2B cells to secondary organic aerosol coated on magnetic nanoparticles. Chem Res Toxicol. 2006;19(8):1044-50.
243 Park S, Lee YK, Jung M, Kim KH, Chung N, Ahn EK, et al. Cellular toxicity of various inhalable metal nanoparticles on human alveolar epithelial cells. Inhal Toxicol. 2007;19(Suppl 1):59-65.
244 Steerenberg PA, Zonnenberg JA, Dormans JA, Joon PN, Wouters IM, van Bree L, et al. Diesel exhaust particles induced release of interleukin 6 and 8 by (primed) human bronchial epithelial cells (BEAS 2B) in vitro. Exp Lung Res. 1998;24(1):85-100.
245 Sun W, Wu R, Last JA. Effects of exposure to environmental tobacco smoke on a human tracheobronchial epithelial cell line. Toxicology. 1995;100(1-3):163-74.
246 Chemuturi NV, Hayden P, Klausner M, Donovan MD. Comparison of human tracheal/bronchial epithelial cell culture and bovine nasal respiratory explants for nasal drug transport studies. J Pharm Sci. 2005;94(9):1976-85.
247 Forbes B, Ehrhardt C. Human respiratory epithelial cell culture for drug delivery applications. Eur J Pharm Biopharm. 2005;60(2):193-205.
248 Thurnherr T, Brandenberger C, Fischer K, Diener L, Manser P, Maeder-Althaus X, et al. A comparison of acute and long-term effects of industrial multiwalled carbon nanotubes on human lung and immune cells in vitro. Toxicol Lett. 2011;200(3):176-86.
249 Kim KJ, Borok Z, Crandall ED. A useful in vitro model for transport studies of alveolar epithelial barrier. Pharm Res. 2001;18(3):253-5.
250 Bermudez LE, Sangari FJ, Kolonoski P, Petrofsky M, Goodman J. The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells. Infect Immun. 2002;70(1):140-6.
251 Birkness KA, Swisher BL, White EH, Long EG, Ewing EP, Jr., Quinn FD. A tissue culture bilayer model to study the passage of Neisseria meningitidis. Infect Immun. 1995;63(2):402-9.
252 Hermanns MI, Kasper J, Dubruel P, Pohl C, Uboldi C, Vermeersch V, et al. An impaired alveolar-capillary barrier in vitro: effect of proinflammatory cytokines and consequences on nanocarrier interaction. J R Soc Interface. 2010;7(Suppl 1):S41-54.
253 Hermanns MI, Fuchs S, Bock M, Wenzel K, Mayer E, Kehe K, et al. Primary human coculture model of alveolo-capillary unit to study mechanisms of injury to peripheral lung. Cell Tissue Res. 2009;336(1):91-105.
254 Choe MM, Tomei AA, Swartz MA. Physiological 3D tissue model of the airway wall and mucosa. Nat Protoc. 2006;1(1):357-62.
255 Rothen-Rutishauser B, Mueller L, Blank F, Brandenberger C, Muehlfeld C, Gehr P. A newly developed in vitro model of the human epithelial airway barrier to study the toxic potential of nanoparticles. ALTEX. 2008;25(3):191-6.
256 Lehmann AD, Daum N, Bur M, Lehr CM, Gehr P, Rothen-Rutishauser BM. An in vitro triple cell co-culture model with primary cells mimicking the human alveolar epithelial barrier. Eur J Pharm Biopharm. 2011;77(3):398-406.
257 Blank F, Gehr P, Rothen-Rutishauser B. In vitro human lung cell culture models to study the toxic potential of nanoparticles. In: Sahu S, editor. Nanotoxicity: From in vitro, in vivo models to health risks. Chichester, England: Wiley; 2009. p. 379-95.




