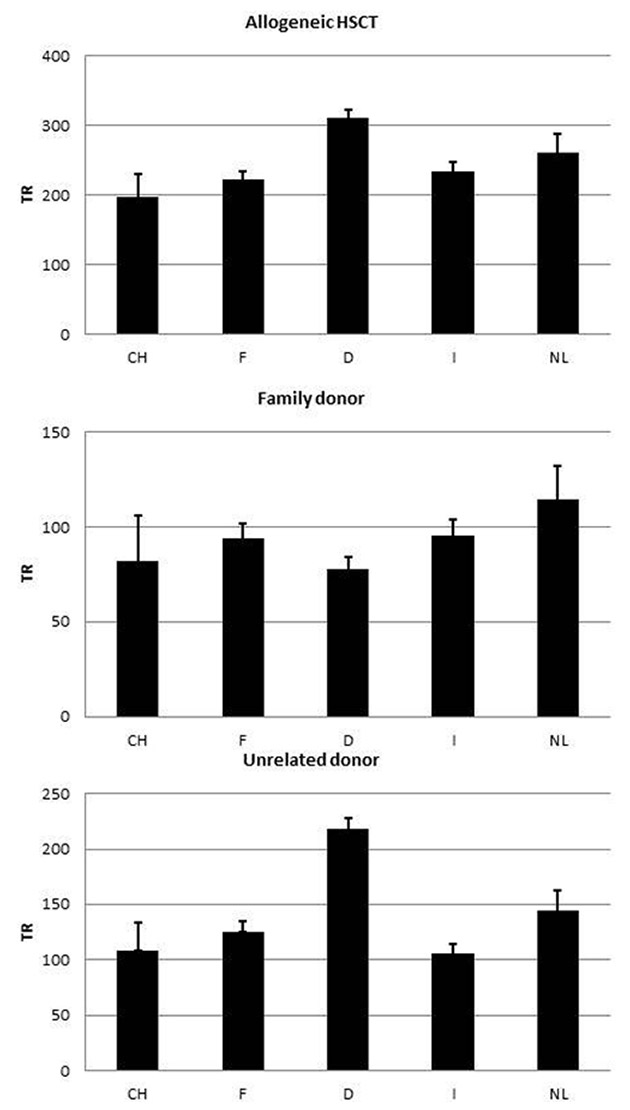
Figure 1
Allogeneic HSCT rates per 10 million inhabitants per year. 1a: all allogeneic HSCT. 1b: allogeneic HSCT from a family donor. 1c: allogeneic HSCT from an unrelated donor (CH: Switzerland, F: France, D: Germany, I: Italy, NL: the Netherlands)
DOI: https://doi.org/10.4414/smw.2013.13757
Haematopoietic stem cell transplantation (HSCT) has become an established treatment option for a wide range of haemato-oncological tumours, some solid tumours, and nonmalignant diseases such as bone marrow failure, primary immunodeficiencies and other congenital and acquired disorders [1–2]. The haematopoietic system is replaced in the form of haematopoietic stem cells from either the patients themselves (autologous HSCT) or other persons (allogeneic HSCT). Autologous HSCT is used to bridge haematopoietic failure during high-dose chemotherapy for the treatment of tumours of the haematopoietic system and some solid tumours that are adequately sensitive to this treatment. Allogeneic HSCT is used to replace the haematopoietic system in patients with acquired or congenital haematopoietic failure, and more commonly to exploit the graft versus tumour effect of allogeneic cells in malignant disease [1–4]. Donors for allogeneic HSCT may come from the patient's family, or may be unrelated and provided by donor registries. Furthermore, cryopreserved cord blood has become an accepted stem cell source [5–7] for certain indications.

Figure 1
Allogeneic HSCT rates per 10 million inhabitants per year. 1a: all allogeneic HSCT. 1b: allogeneic HSCT from a family donor. 1c: allogeneic HSCT from an unrelated donor (CH: Switzerland, F: France, D: Germany, I: Italy, NL: the Netherlands)
Before HSCT, chemotherapy or chemotherapy in combination with total body irradiation is administered. The goal of these conditioning regimens in autologous HSCT is to administer cytotoxic chemotherapy to the tumour, without any compromises in intensity because of marrow toxicity, but to preserve nonhaematopoietic organ function. HSCT serves to restore bone marrow function and to shorten aplasia. Historically, in allogeneic HSCT marrow-ablative doses of chemotherapy and total-body irradiation were thought to be necessary to eradicate malignancy, to provide immunosuppression in the recipient, and to create space in the stem cell compartment, allowing engraftment of donor haematopoietic cells. A major development over the last 15 years was the introduction of reduced intensity regimens (reduced intensity conditioning; RIC), in which myeloablative treatment is avoided and treatment intensity is just high enough to avoid graft rejection [8]. The goal was to promote engraftment and let the graft versus tumour effect eliminate tumour cells. The reduction in toxicity with RIC results in less morbidity and mortality, and has made allogeneic HSCT available to patients with comorbidities and to older patients (i.e., patients aged 60 to 70 years, the age group that has the highest prevalence of most haematopoietic malignancies).
Health technology assessment is used to identify appropriate treatment and use of medical technology in HSCT. Costs for these technologies are high and indications are therefore scrutinised for their appropriateness in a given patient population. Use of HSCT technology may vary because of socioeconomic conditions determining access, or because of reimbursement issues [9–11], but the number of studies analysing appropriateness of technology use are limited [12, 13]. It was the purpose of this study to compare indications for, and use of, transplant technology in Switzerland with those of neighbouring countries. As these countries are all in a more or less comparable economic situation, and as incidences of haematological malignancies are not expected to differ greatly between them, differences in transplant rates require explanation. No outcome data are provided.
The data for this analysis were taken from the Activity Survey of the European Group for Blood and Marrow Transplantation (EBMT) [14–16]. In brief, teams are requested to report data annually by indication, stem cell source and donor type. Quality control measures include several independent systems: confirmation of validity of the entered data by the reporting team, selective comparison of the survey data with data sets in the EBMT Registry database, cross-checking with the National Registries, and onsite visits of selected teams. In addition, data for Switzerland are validated by the referring teams when they prepare the annual reports for the Federal Office of Public Health under the Swiss Transplant Law.

Figure 2
Number of registered unrelated donors in the national registries of the five countries studied corrected for population size, presented as donors per 10 million inhabitants(CH: Switzerland, F: France, D: Germany, I: Italy, NL: the Netherlands).
For the purpose of this study, data from Switzerland were compared with three neighbouring countries, Germany, France and Italy, and also with the Netherlands, all countries with somewhat differing healthcare systems. Data from 304 centres from the five countries were obtained for the years 2008–2011 and averaged over the 4 years.
Data on registered unrelated donors were obtained from the national registries and crosschecked with bone marrow donors worldwide (BMDW).
Wherever appropriate, patient numbers corresponding to the number of patients receiving a first transplant, and transplant numbers reflecting the total number of transplants performed, are listed. Situations in which a patient could receive more than one transplant include multiple transplants, defined as subsequent transplants within a planned double or triple autologous or allogeneic transplant protocol, and retransplants (autologous or allogeneic) defined as unplanned HSCT for rejection or relapse after a previous HSCT.
Stem cell sources include bone marrow, peripheral blood or cord blood. Myeloablative conditioning usually causes a prolonged state of marrow aplasia and autologous recovery is, if at all, very late and incomplete. Reduced intensity conditioning (RIC) is immunosuppressive to allow engraftment but usually causes only a short period of marrow aplasia or, with some regimens, no aplasia at all.
Transplant rates, defined as numbers of HSCT per 10 million inhabitants, were computed for each country. Population numbers (in 2011) were obtained from the US census bureau database (http://www.census.gov/population/international/data/idb/rank.php).
To calculate transplant rates the numbers of first transplants were used; this reflects the number of patients transplanted rather than the total number of transplants. For certain graphs depicting transplant technology, such as percentage of transplants with RIC, the total numbers of transplants (including double transplants) were used. Where appropriate, transplant rates are given with the 95% confidence interval, represented by an error bar. there was no formal statistical comparison because of the descriptive nature of these observations.
Altogether, 304 teams from the five neighbouring countries reported a total of 57,933 patients receiving their first transplant during 2008–2011: 24,092 (42%) allogeneic and 33,841 (58%) autologous HSCT. The total number of transplants reported was 66,732: 26,161 allogeneic and 40,571 autologous HSCT. This includes patients who received more than one transplant through planned procedures such as double autologous HSCT (e.g., in patients with myeloma) or second transplants after relapse of the original disease.
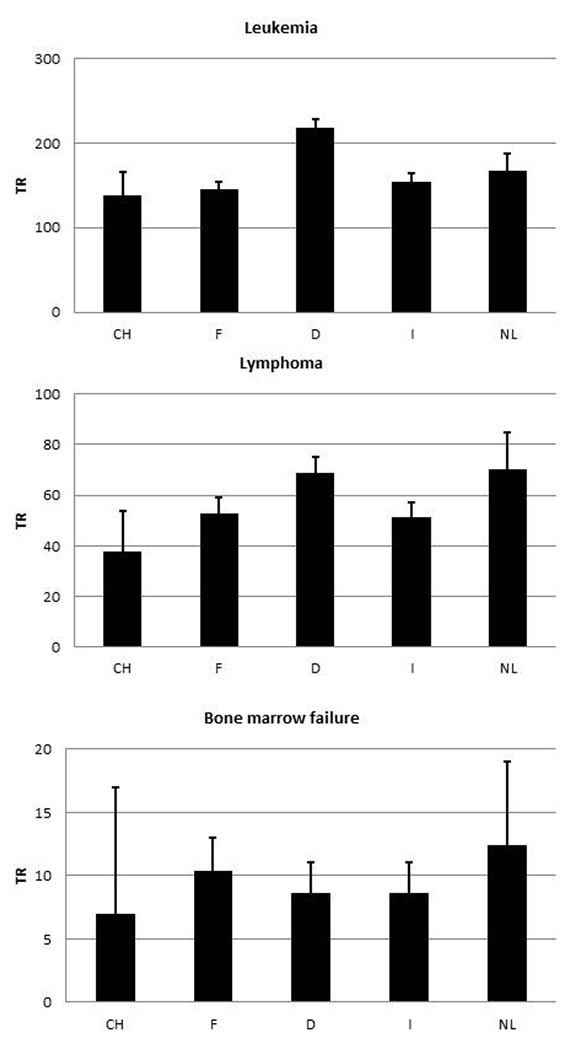
Figure 3
Allogeneic HSCT rates per 10 million inhabitants per year. 1a: leukaemia. 1b: lymphoma. 1c: bone marrow failures syndromes (CH: Switzerland, F: France, D: Germany, I: Italy, NL: the Netherlands).

Figure 4
Allogeneic HSCT rates per 10 million inhabitants and year. 4a: use of cord blood transplants. 4b: use of HLA- mismatched family donors. 4c: use of reduced intensity conditioning as percentage of all allogeneic HSCT (CH: Switzerland, F: France, D: Germany, I: Italy, NL: the Netherlands).
The main indications included acute and chronic leukaemia (16,265 allogeneic; 1,419 autologous), lymphoid malignancies (5,480 allogeneic; 29,031 autologous), solid tumours (187 allogeneic; 3,168 autologous), bone marrow failure syndromes (869 allogeneic), other nonmalignant diseases including many different inherited disorders and autoimmune diseases (1,161 allogeneic; 184 autologous) and other nonspecified diseases (130 allogeneic, 39 autologous). More details of the disease classification are provided in table 1.
Of the allogeneic transplants, 9,753 were performed using a family donor and 14,339 an unrelated donor. The family donors were human leukocyte antigen (HLA) identical siblings (n = 8231), twins (n = 72) and HLA mismatched relatives (n = 1,450).
Overall, 5,590 allogeneic transplants were performed using stem cells collected from the bone marrow (2,948 family; 2,642 unrelated), 16,987 with peripheral blood stem cells (6720 family; 10,266 unrelated) and 1515 with cord blood stem cells (84 family; 1,431 unrelated).
A total of 33,841 autologous transplants were performed using peripheral blood stem cells and 234 with bone marrow stem cells. During the study period only two autologous cord blood transplants were reported.
Figure 1 shows the rates of allogeneic HSCT in the five countries, in total (rates of 198–311 per 10 million population per year), and separately for unrelated donor HSCT (106–218 per 10 million population per year) and family donor HSCT. The rates of HSCT from a family donor vary little between the countries and are between 89 and 128 per 10 million inhabitants per year. Of interest is the important difference in the rate of use of unrelated donors for allogeneic HSCT, which in Germany is double the rate in Switzerland (218 vs 109 per 10 million population per year). Figure 2 shows the numbers of donors available in the respective national registries in the five countries, corrected for population size (donor rates 23,026–456,598 per 10 million population per year). The huge differences reflect the major effort undertaken by German registries to recruit stem cell donors. Figure 3 shows rates of allogeneic HSCT for three indications: leukaemias (139–218 per 10 million population), lymphoid malignancies (38–70 per 10 million population per year) and bone marrow failure (7–12 per 10 million population per year). For all three indications Switzerland has the lowest rate of allogeneic HSCT, although some of the differences are small. Figure 4a shows differences in the use of cord blood as a stem cell source; there are striking differences in the use of cord blood for allogeneic HSCT, as high as 32 per 10 million inhabitants per year in France and as low as 3 per 10 million inhabitants per year in Germany. Unrelated cord blood is most commonly used when no HLA matched unrelated donor is available; other options in such a situation include using family donors mismatched at multiple HLA loci (haploidentical donors). Transplant rates with mismatched family donors (3–31 per 10 million population per year) are shown in figure 4b. It appears that there is complementary relationship between use of the two sources: countries that use cord blood rarely use mismatched family donors. Conversely transplantation rates from mismatched family donors are high in Germany (14 per 10 million inhabitants per year) and Italy (33 per 10 million inhabitants per year), where use of cord blood is low. Use of RIC as opposed to standard intensity conditioning is also variable, as shown in figure 4c, where percentages of RIC transplants in the Netherlands (61%) are approximately twice as high as those in, for example, Italy (29%).
Figure 5 shows rates of autologous HSCT in the European countries. Differences appear smaller than for allogeneic HSCT (fig. 5a), especially in the indications that are generally accepted such as non-Hodgkin’s lymphoma (97–143 per 10 million inhabitants per year; fig. 5c) and myeloma (139–160 per 10 million inhabitants per year; fig. 5d), where almost no difference is apparent in transplant rates. This is very different for other indications, however, as exemplified by autologous HSCT for leukaemia (5–34 per 10 million inhabitants per year; fig. 5b) and Hodgkin’s disease (18–53 per 10 million inhabitants per year; fig. 5d).
Figures 6 and 7 show team densities (the number of transplant teams per 10 million inhabitants) for allogeneic HSCT (3.8–10.6) and autologous HSCT (8.7–15.1), and the number of transplants per team averaged over the 4 years (22–51 allogeneic HSCT per team, 26–41 autologous HSCT per team). There is an inverse correlation, as the country with the lowest team density has the highest number of procedures per team, as exemplified by team density figures for allogeneic HSCT (3.8 teams per 10 million population) and rates of allogeneic HSCT in Switzerland (51 transplants per team). The fact that Switzerland also has the lowest overall rate of allogeneic HSCT demonstrates that the higher number of procedures per team only partially compensates for the lower team density.
| Table 1: Absolute numbers of patients transplanted by disease indication and transplant type. | |||
| Indication stage | Allogeneic HSCT | Autologous HSCT | Total |
| Leukaemias | 16265 | 1419 | 17684 |
| Acute myeloid leukaemia | 7896 | 1133 | 9029 |
| Acute lymphoblastic leukaemia | 3582 | 217 | 3799 |
| Chronic myeloid leukaemia | 688 | 5 | 693 |
| Myelodysplastic syndrome / myeloproliferative neoplasm | 4099 | 64 | 4163 |
| Lymphoid malignancies | 5480 | 29031 | 34511 |
| Chronic lymphatic leukaemia | 871 | 140 | 1011 |
| Plasma cell disorder | 1618 | 14449 | 16067 |
| Hodgkin’s lymphoma | 622 | 2943 | 3565 |
| Non-Hodgkin’s lymphoma | 2369 | 11499 | 13868 |
| Solid tumours | 187 | 3168 | 3355 |
| Neuroblastoma | 82 | 699 | 781 |
| Soft tissue | 27 | 102 | 129 |
| Germ cell tumours | 2 | 785 | 787 |
| Breast Cancer | 11 | 257 | 268 |
| Ewing sarcoma | 30 | 422 | 452 |
| All other solid tumours | 35 | 903 | 938 |
| Nonmalignant disorders | 2030 | 184 | 2214 |
| Bone marrow failure | 869 | 869 | |
| Haemoglobinopathy | 449 | 449 | |
| Primary Immune deficiency | 510 | 13 | 523 |
| Inherited metabolic disease | 173 | 8 | 181 |
| Autoimmune disease | 29 | 163 | 192 |
| Others | 130 | 39 | 169 |
| Total first HSCT | 24092 | 33841 | 57933 |
This study by the Swiss Blood Stem Cell Transplantation Group (SBST) describes the use of haematopoietic stem cell transplantation technology in Switzerland, making use of the database used to report transplants to the federal government and comparing rates and indications with neighbouring countries. This analysis is based on the accepted activity surveys of the EBMT, confirming and extending previous findings. In Switzerland, all transplant centres are accredited by JACIE (the joint accreditation committee of ISCT and EBMT; http://www.jacie.org) and through this mechanism all transplants are reported. Within the JACIE accreditation programme, in Switzerland 100% of centres, in the Netherlands 93%, in France 58%, in Germany 43% and in Italy 34% of centres, have either registered an application for accreditation, are already accredited or are in the process of being accredited (JACIE annual report 2011; http://www.jacie.org/about/annual-report).
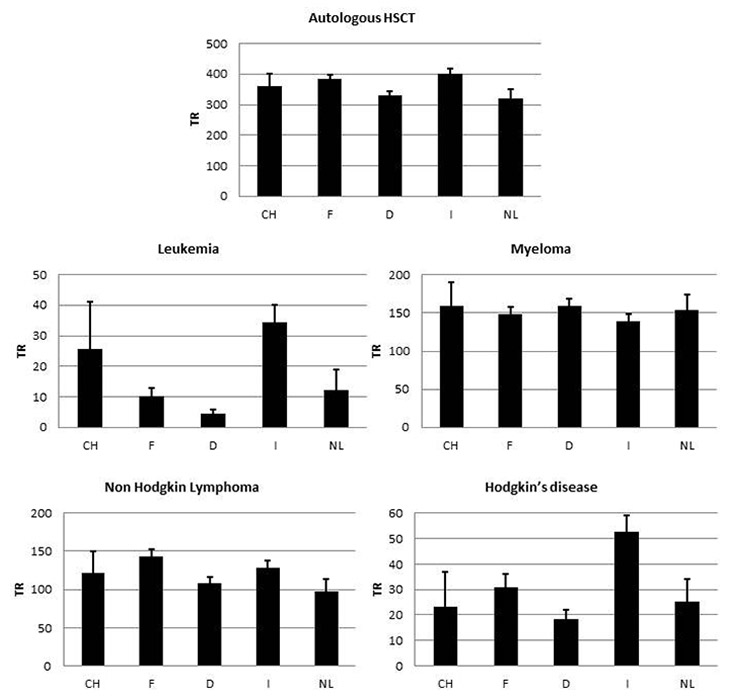
Figure 5
Autologous HSCT rates per 10 million inhabitants per year. 5a: autologous HSCT. 5b: leukaemia. 5c: myeloma. 5d: non-Hodgkin’s lymphoma. 5e: Hodgkin‘s disease (CH: Switzerland, F: France, D: Germany, I: Italy, NL: the Netherlands).
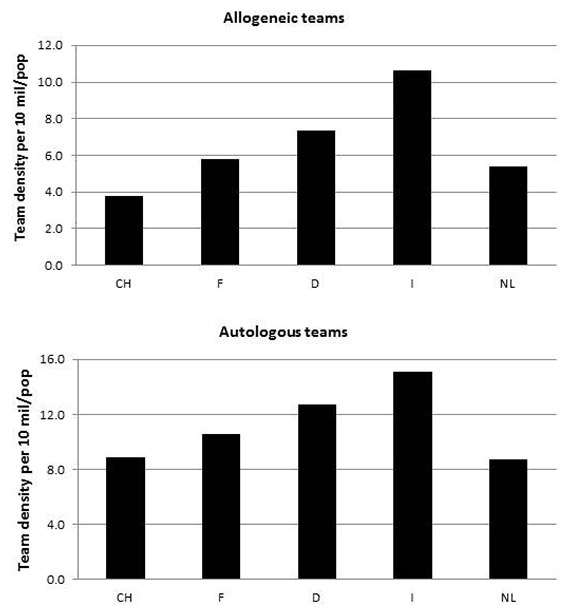
Figure 6
Transplant team density (number of teams per 10 million inhabitants). 6a: allogeneic HSCT. 6b: autologous HSCT (CH: Switzerland, F: France, D: Germany, I: Italy, NL: the Netherlands).

Figure 7
Average annual number of allogeneic and autologous HSCT per transplanting team. 7a: allogeneic HSCT. 7b: autologous HSCT (CH: Switzerland, F: France, D: Germany, I: Italy, NL: the Netherlands).
The activity survey of the EBMT was used for data on the countries other than Switzerland. Main findings include lower rates of allogeneic HSCT in Switzerland than in neighbouring countries. Most of this difference is due to a higher rate of HSCT from unrelated donors, and the most striking difference is with Germany where the rate is double that in Switzerland.
There is published data to explain some of the differences in HSCT rates. Adoption of HSCT is primarily influenced by availability of resources, governmental support and access to a transplant centre [17]. Use of HSCT is also influenced by the economic situation, medical evidence, external regulations and expectations [7]. Use of unrelated donors correlates with resources, availability of an unrelated donor registry and number of donors in the registry of that country. Given the wide variation between countries in the size of unrelated donor registries, it is obvious that additional efforts in education and recruitment have to be undertaken in countries such as Switzerland. It is particularly disturbing that the country with the highest GDP in the series examined has a particularly low rate of donor availability.
As family size does not vary greatly in Europe and the likelihood of a given pair of siblings being HLA identical is 25% by the law of Mendelian inheritance, it is not surprising that rates for allogeneic HSCT using family donors do not differ greatly among European countries.
A possible explanation for the huge difference in unrelated donor transplant rates among the five countries (almost twice as many are performed in Germany than in the other countries) lies in donor availability. German registries have made major efforts to recruit unrelated donors, and although donor searches are conducted internationally it is conceivable that HLA matched donors are more easily identified in populations that share the same gene pool as the patient [18]. Some of the differences in rates of unrelated donor HSCT are, however, unexplained as donors are mutually accessible across registries worldwide.
Indications for allogeneic HSCT do not vary greatly (see fig. 3), that is, leukaemia, lymphoproliferative neoplasia and marrow failure syndromes. Areas of uncertainty, however, include the use of HSCT technology in patients without a matched donor. This is exemplified by the wide variation in use of cord blood or mismatched family donors (see fig. 4). The complementarity indicates that, for example, in France cord blood donors are used rather than mismatched family donors, whereas in Germany this is reversed. Such differences are attributed to the lack of clear guidelines, and local preference in the absence of clear data [16]. Another area of uncertainty is the use of RIC as opposed to standard conditioning. This is evident by the percentage of allogeneic HSCT using RIC, which is, for example, in Italy half the fraction seen in the Netherlands (fig. 4c).
Similar observations can be made for autologous HSCT, where overall rates are similar across countries. When analysing by indication it becomes evident that well-established indications such as non-Hodgkin's lymphoma and myeloma show comparable rates, whereas wide variation is seen in autologous HSCT for acute leukaemia and for Hodgkin’s lymphoma.
As well as donor availability and physician preference, use of technology may also be determined by factors such as access to care, which may be measured as team density (i.e., the number of teams performing transplants per 10 million inhabitants). It is of interest to see that the country with the lowest rate of allogeneic HSCT (Switzerland) is also the country with the lowest team density in this field. This is, however, partially compensated by the higher number of transplants per team, as shown in figure 7. Access to care is an important topic in healthcare decision making, and in several countries concentration of highly specialised technology in few centres is being discussed. Arguments used are economic, as one large centre will be less expensive than two smaller ones, but also concern treatment quality, as the assumption is that centres performing more procedures will have greater clinical experience and, therefore, better results. For a number of medical procedures it has been shown that a high caseload is beneficial to outcome although, for reasons not easily explained, this has not been confirmed for HSCT [19]. A study has shown, however, that accreditation by JACIE is associated with better outcome [20], and accreditation is dependent on a minimum caseload. Our data show that for allogeneic and autologous HSCT, fewer centres, while being associated with a higher caseload per centre, also lead to fewer procedures. It would be of interest to compare outcome for particular indications, looking at the relationship between team experience, and treatment quality and outcome [21].
Given the nature of the data presented here, it is not possible to determine the appropriate rate of allogeneic HSCT. Overuse and underuse of technology cannot be determined because of the lack of a clearly defined benchmark. Overall, these data show that standardisation in the field of HSCT is needed, because the wide variations shown here are mainly unexplained.
It was the task, therefore, of this analysis to describe differences and to make them available to the scientific community for discussion. Quality management systems, and mandatory data reporting and analysis of data, are to be promoted and should be considered an integral part of patient treatment.
Acknowledgments: The cooperation of the European Group for Blood and Marrow Transplantation (EBMT) Transplant Activity Survey office in Basel, Switzerland, the French Registry (SFGM) (N. Milpied, N. Raus), the German Registry (DRST) (H. Ottinger, K. Fuchs, C. Müller, H. Neidlinger, F. Strehle), the Italian Registry (GITMO) (A. Rambaldi, R. Oneto, B. Bruno, A. Camboni), the Dutch Registry (A. Schattenberg, R. van der Holt, M. Groenendijk) and the Swiss Registry (SBST) (U. Schanz, H. Baldomero), is greatly appreciated.
1 Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26.
2 Ljungman P, Bregni M, Brune M, Cornelissen J, deWitte T, Dini G, et al. European Group for Blood and Marrow. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009. Bone Marrow Transplantation. 2010;45:219–34.
3 Gyurkocza B, Rezvani A, Storb RF. Allogeneic hematopoietic cell transplantation: the state of the art. Expert Rev Hematol. 2010;3(3):285–99. Review.
4 Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med. 2007;357:1472–5.
5 Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007; 369(9577):1947–54.
6 Gluckman E. Ten years of cord blood transplantation: from bench to bedside Br J Haematol. 2009;147(2):192–9.
7 Navarrete C, Contreras M. Cord blood banking: a historical perspective. Br J Haematol. 2009;147(2):236–45.
8 Gyurkocza B, Storb R, Storer BE, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;28(17):2859–67.
9 Gratwohl A, Schwendener A, Baldomero H, Gratwohl M, Apperley J Niederwieser D, et al. Changes in use of hematopoietic stem cell transplantation; a model for diffusion of medical technology. Haematologica. 2010;95:637–43.
10 Blommestein HM, Verelst SG, Huijgens PC, Blijlevens NM, Cornelissen JJ, Uyl-de Groot CA. Real-world costs of autologous and allogeneic stem cell transplantations for haematological diseases: a multicentre study. Ann Hematol. 2012;91(12):1945–52.
11 Leelahavarong P, Chaikledkaew U, Hongeng S, Kasemsup V, Lubell Y, Teerawattananon Y. A cost-utility and budget impact analysis of allogeneic hematopoietic stem cell transplantation for severe thalassemic patients in Thailand. BMC Health Serv Res. 2010;10:209.
12 Gliklich RE, Dreyer NA, eds. Registries for Evaluating Patient Outcomes: A User’s Guide. 2nd ed. (Prepared by Outcome DEcIDE Center [Outcome Sciences, Inc. d/b/a Outcome] under Contract No. HHSA290200500351 TO3.) AHRQ Publication No. 10-EHC049. Rockville, MD: Agency for Healthcare Research and Quality. September 2010.
13 Ashfaq K, Yahaya I, Hyde C, Andronis L, Barton P, Bayliss S, Chen Y-F. Clinical effectiveness and cost-effectiveness of stem cell transplantation in the management of acute leukaemia: a systematic review. Health Technology Assessment. 2010;54:1.141.
14 Gratwohl A, Baldomero H, Schwendener A, Gratwohl M, Apperley J, Frauendorfer K, et al. The EBMT activity survey 2008 impact of team size, team density and new trends. Bone Marrow Transplant. 2011;46(2):174–91.
15 Gratwohl A, Baldomero H, Horisberger B, Schmid C, Passweg J, Urbano-Ispizua A. Accreditation Committee of the European Group for Blood and Marrow Transplantation (EBMT). Current trends in haematopoietic stem cell transplantation in Europe. Blood. 2002;100:2374–86.
16 Passweg JR, Baldomero H, Gratwohl A, Bregni M, Cesaro S, Dreger P, et al. European Group for Blood and Marrow Transplantation (EBMT). The EBMT activity survey: 1990-2010. Bone Marrow Transplant. 2012;47(7):906–23.
17 Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, Szer J, et al. Worldwide Network of Blood and Marrow Transplantation. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303(16):1617–24.
18 Buhler S, Nunes JM, Nicoloso G, Tiercy JM, Sanchez-Mazas A. The Heterogeneous HLA Genetic Makeup of the Swiss Population. PLoS One. 2012;7(7):e41400. Epub 2012 Jul 25.
19 Loberiza FR Jr, Serna DS, Horowitz MM, Rizzo JD. Transplant center characteristics and clinical outcomes after hematopoietic stem cell transplantation: what do we know? Bone Marrow Transplant. 2003;31(6):417–21.
20 Gratwohl A, Brand R, Niederwieser D, Baldomero H, Chabannon C, Cornelissen J, et al. Introduction of a Quality Management System and Outcome After Hematopoietic Stem-Cell Transplantation. J Clin Oncol. 2011;29(15):1980–6.
21 Fausto R. Loberiza, Jr, Mei-Jie Zhang, Stephanie J. Lee, John P. Klein, Charles F. LeMaistre, Derek S. Serna, et al. Association of transplant center and physician factors on mortality after hematopoietic stem cell transplantation in the United States Blood. 2005;105:2979–87.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.