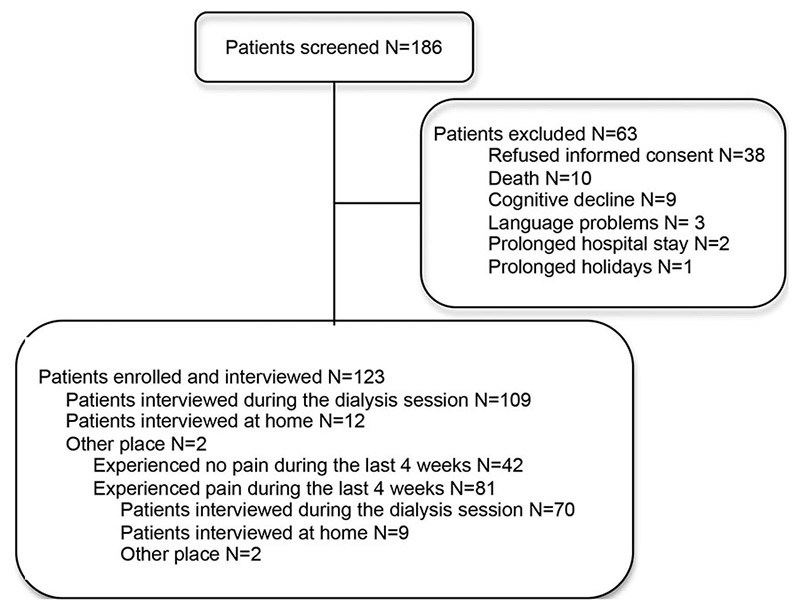
Figure 1
Number of patients screened and interviewed.
DOI: https://doi.org/10.4414/smw.2013.13750
The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” [1, 2]. Pain is a common problem in patients with end-stage renal disease (ESRD) [2, 3], including dialysis patients [4–6], and may be due to their primary disease, concurrent comorbidity or disease following renal failure.
Dialysis therapy is life-saving, but underlying systemic diseases and related painful syndromes such as ischaemic limb, musculoskeletal or neuropathic symptoms persist during treatment. Recent reviews show that 47% of patients with ESRD experience pain [7] and this can be moderate to severe in 82% [5]. Pain often coexists with depression, anxiety and insomnia. Almost two of every five dialysis patients experience troubled sleep, and 38% to 45% suffer some degree of anxiety [7]. Symptom severity in dialysis patients has been reported in some studies to be comparable to that of cancer and HIV patients [4–6].
The complex pharmacokinetics of analgesics in dialysis patients often entails under-treatment of the symptoms [5, 8–11], and may hamper safe and effective use, especially of opioids [12].
Nephrologists often view as priorities dialysis access, management of bone metabolism, anaemia, and quality of dialysis. During the last few years, renal units predominantly focused on dialysis parameters such as blood pressure, anaemia, intact parathyroid hormone (iPTH), Kt/V and on interventions aiming to minimise disease progression, rather than on symptom management. In general, individual pain symptoms are poorly recognised and managed [13–16], although their management should be an integral component of patient care quality. Previous studies have demonstrated that nephrologists commonly underestimate the symptom burden of individual patients [9, 17].
This study aimed to assess the prevalence, severity, cause and management of pain, together with associated symptoms and overall symptom burden, in patients with chronic kidney disease stage 5, on long-term dialysis and living in southern Switzerland.
Inclusion criteria for this cross-sectional, observational, multicentre study were: chronic kidney disease (CKD) stage 5 according to the Kidney Disease Outcome Quality Initiative (K/DOQI) Guidelines [18]; chronic haemodialysis; treatment in one of the five nephrology units in southern Switzerland; age older than 18 years; ability to complete a questionnaire in Italian and to give informed consent. The local ethics committee approved the study and each participant gave written informed consent to participate.

Figure 1
Number of patients screened and interviewed.
Basic demographic data and clinical laboratory data (such as liver and renal function, Kt/V values, electrolytes, complete blood count and C reactive protein) were collected from medical and nursing charts. Two palliative-care nurses interviewed the patients face-to-face at home or during a dialysis session, following a duly prepared grid. Patients were asked to complete the Brief Pain Inventory (BPI), in order to describe pain and associated symptoms relevant to chronic dialysis patients [19]. They were also asked to score the maximum pain experienced during the previous 4 weeks on a 10-cm visual analogue scale (VAS) ranging from “no pain” to “unbearable pain”, in which mild pain ranged from 0–4, moderate from 5–7 and intense from 8–10. Patients were also asked to localise the pain and draw the location on a body diagram. Use of analgesics and drug prescriptions were recorded. Patients were then asked to complete the Edmonton Symptom Assessment System (ESAS) [20–25], used in palliative care as well as for dialysis patients [23], in order to assess the overall symptom burden. The Instrumental Activities of Daily Life (IADL) questionnaire [26] was chosen to quantify restrictions in daily life activities.
The statistical analyses were performed using SPSS (ver. 17; SPSS Inc., Chicago, Illinois, USA). Prevalence and severity of symptoms were described using proportions, means or medians, as appropriate. The one-sample Kolmogorov-Smirnov test was used to check for normal distribution of the data. Categorical data were compared using the chi-square or Fisher’s exact test. Spearman’s rho was used to compute univariate correlations. Multiple linear regression was used to study the multivariate relationship between pain and its predictors. Variables are expressed as mean ± standard deviation (SD), if not specified otherwise. The significance level was set to α = 0.05, two-tailed.
| Table 1: Population and dialysis characteristics, haematology parameters (N = 123). | ||
| Mean age (years; mean ± SD) (range) | 71 ± 12.5 (36–90) | |
| Mean BMI (kg/m2; mean ± SD) (range) | 27 ± 5.5 (16–47.5) | |
| Gender: male/female (n and %) | 75/48 | 61%/39% |
| Household situation (n and %): Living alone Living with others | 36 85 | 29% 71% |
| Years of treatment (n and %): <1 1–5 5–10 >10 | 9 96 13 5 | 7% 78% 10% 5% |
| Modality of treatment (n and %): Haemodialysis Haemodiafiltration Not available | 69 50 4 | 56% 41% 3% |
| Filters (n and %): High flux Low Flux Not available | 97 20 6 | 79% 16% 5% |
| Comorbidities in >30% of the patients (n and %): Hypertension Coronary heart disease Diabetes mellitus Peripheral vascular disease Gastrointestinal and liver disease | 91 56 41 39 38 | 74% 45% 33% 32% 31% |
| Haematology parameters | Mean (range) | SD |
| Haemoglobin (g/dl) | 11.80 (8.5–15) | 1.7 |
| Haematocrit (%) | 36 (26–49) | 3.8 |
| Ca++ (mmol/l) | 2.21 (1.14–2.62) | 0.2 |
| PO4-- (mmol/l) | 1.5 (0.53–3.28) | 0.5 |
| iPTH (pmol/l) | 23 (1–127) | 19.3 |
| Albumin (g/l) | 34.5 (20–45) | 3.8 |
| Ferritin (µg/l) | 306 (11–1054) | 220 |
| PCR (mg/l) | 14.5 (0.8–191) | 24.7 |
| Total cholesterol (mmol/l) | 4.3 (1.9–7.4) | 1.2 |
| Triglycerides (mmol/l) | 2.1 (0.4–8.0) | 1.3 |
| Dialyse-quality parameters | Mean (range) | SD |
| Kt/V (n = 112) | 1.41 (0.94–2.29) | 0.27 |
| nPCR (n = 51) | 1.13 (0.67–1.73) | 0.28 |
| Abbreviations: SD, standard deviation; BMI, body mass index; iPTH, intact parathyroid hormone; PCR, protein/creatinine ratio; nPCR, normalised protein catabolic rate. | ||
| Table 2: Patients reporting concomitant symptoms (N = 123). | |||
| Number | % | ||
| Asthenia/fatigue | No | 69 | 56.1% |
| Yes | 54 | 43.9% | |
| Sleep disturbances | No | 98 | 79.7% |
| Yes | 25 | 20.3% | |
| Constipation | No | 102 | 82.9% |
| Yes | 21 | 17.1% | |
| Nausea/vomiting | No | 105 | 85.4% |
| Yes | 18 | 14.6% | |
| Loss of appetite | No | 106 | 86.2% |
| Yes | 17 | 13.8% | |
| Dyspnoea | No | 107 | 87.0% |
| Yes | 16 | 13.0% | |
| Anxiety | No | 110 | 89.4% |
| Yes | 13 | 10.6% | |
| Depression | No | 110 | 89.4% |
| Yes | 13 | 10.6% | |
| Nightmares | No | 123 | 100.0% |
| Yes | 0.0% | ||
| Hallucinations | No | 123 | 100.0% |
| Yes | 0.0% | ||
Between September 2008 and March 2009, 123 consecutive patients were enrolled and 109 (88%) have been interviewed during dialysis (fig. 1). Population and dialysis characteristics are displayed in table 1 and are similar to those generally described for CKD patients. Hypertension was the most frequent comorbidity. The mean time on dialysis was 3.5 years (range 1–22 years): 78% of the patients were on dialysis for 1–5 years, 11% for 5–10 years, 7% for less than 1 year and 4% for more than 10 years. The most recent laboratory Kt/V value (1.41 ± 0.27, range: 0.94–2.29, n = 99) and normalised protein catabolic rate (nPCR) value (1.13 ± 0.28, range: 0.67–1.73, n = 51) showed that dialysis was effective in all patients. Sixty-five percent of the patients had excellent acceptance of dialysis, 20% satisfactory acceptance and 13% bad acceptance, as judged by dialysis nursing staff.
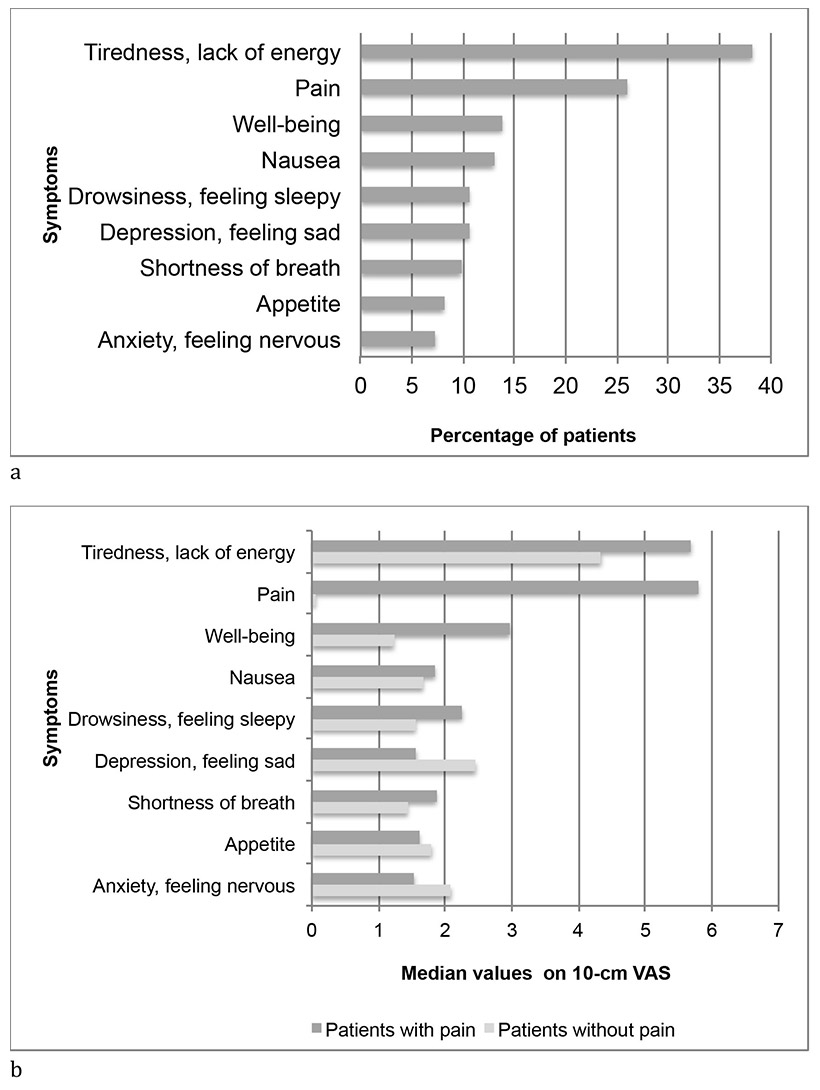
Figure 2
Outcome of ESAS: a. Percentage of patients with score >4 (n = 123). b. Median values of items on 10-cm VAS for patients with (n = 81) and without pain during the last 4 weeks (n = 39; missing 3). High values indicate high burden.
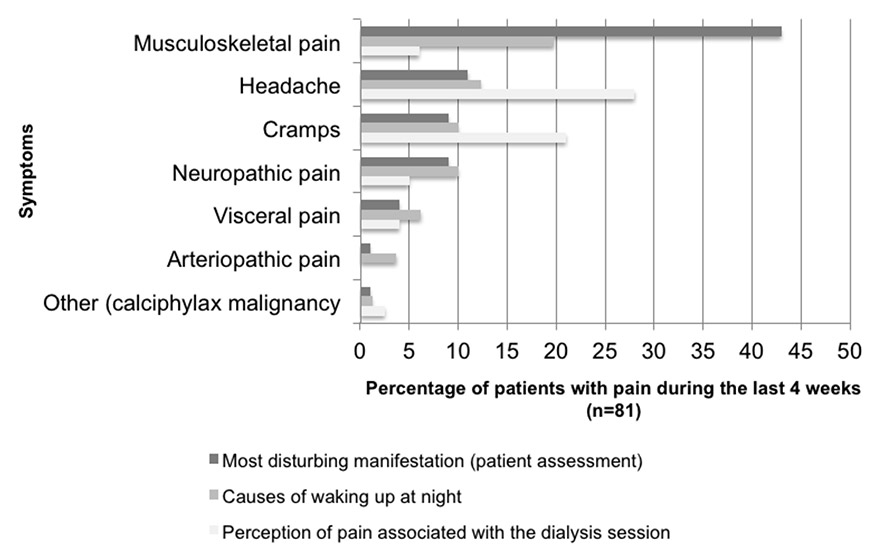
Figure 3
Important pain symptoms in different situations (n = 81).
In the whole population (n = 123), tiredness and pain were the symptoms most commonly perceived as a burden (ESAS score >4) (fig. 2), and were reported to be clinically relevant by the 81 patients complaining of pain during the previous 4 weeks (score >5). Median values for depression/feeling sad and anxiety/feeling nervous scored higher in patients without pain than in those with pain during the previous 4 weeks (fig. 2).
Asthenia and fatigue were the most common concomitant symptoms, being present in 54 (44%) of the patients. In addition, 25 (20%) said they suffered from sleep disturbance and 21 (17%) reported constipation. Anxiety and depression were each present in 13 (10.5%) patients (table 2).
Univariate analyses showed that pain perceived during the previous 4 weeks was correlated with dyspnoea, asthenia/fatigue, loss of appetite, nausea/vomiting, constipation, anxiety and sleep disturbances. Only dyspnoea and fatigue were correlated with pain in the multivariate model. Pain perceived at the interview was correlated with asthenia/fatigue, loss of appetite, anxiety and sleep disturbances using the univariate model. Multivariate analyses did not show any correlation with concomitant symptoms. Other factors, such as Kt/V value, ferritin value, gender and acceptance of dialysis therapy were not correlated with pain during the last 4 weeks. However, recent Kt/V value (univariate model), female gender (univariate and multivariate model) and bad acceptance of dialysis therapy, as judged by the healthcare team (multivariate model) were correlated with pain at the interview. Ferritin values were not correlated to pain (table 3).
In our population, 81 (66%) of the 123 patients reported pain during the previous 4 weeks. Of these, 38 (47%) had experienced pain for 1–5 years and 23 (28%) for >5 years. Intense pain (VAS score 8–10) was reported by 49 (60.5%), moderate pain (VAS score 5–7) by 17 (21%), and mild pain (VAS score <4) by 17 (17.3%). At the time of the interview, 55 (68%) patients said they did not experience pain. Episodic pain was present in 63 (59%) patients and continuous pain in16 (12%); 26 (32%) patients had pain during the dialysis session and 11 (14%) during movement. Musculoskeletal pain was the most prevalent (52 patients, 64%), headache and cramps, respectively, were reported by 25 (31%) and 20 (25%) patients (table 4). Musculoskeletal pain was perceived as the most disturbing symptom and being the major cause of night awakening, whereas during the dialysis session headache and cramps predominated (fig. 3).
As analgesic therapy, the use of non-steroidal anti-inflammatory drugs (NSAIDs) or similar agents predominated (65 patients, 80%), whereas 13 (16%) patients were treated with weak opioids and 4 (5%) with strong opioids. Only 35 (43%) patients used laxatives (table 5). Ten (12%) patients received treatment specifically for their musculoskeletal pain.
When asked specifically, 29 (36%) patients said they woke up at night because of pain several times a week. In 16 patients the cause was musculoskeletal pain, in 10 headache, and cramps and neuropathic pain in 8 each (fig. 3). Changing position helped in 9 cases, movement in 8 and drug use in 5, 7 patients reported that they lacked any strategy for pain control in such a situation. Twenty-two (75%) patients said that they were being treated with nonopioid analgesics, 14 (48%) with laxatives, 6 (21%) with analgesics for neuropathic pain, 5 (17%) with opioids for moderate pain, 3 (10%) with opioids for severe pain, 3 (10%) with muscle relaxants.
Sixty-one (75%) patients reported that pain completely or partly limited their daily activities. Housekeeping was the activity most often limited, as indicated by 47 (58%) patients, followed by grocery shopping in 30 (38%) and cooking in 25 (31%). Only 12 (15%) felt that pain limited their own therapy.
| Table 3: Factors correlated with pain. | |||||||||
| Pain during previous 4 weeks (N = 123) | Pain at the interview (N = 123) | ||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||
| Concomitant symptoms | |||||||||
| Dyspnoea | Pearson correlation | 0.402** | Model coefficient | 4.2** | Pearson correlation | 0.099 | Model coefficient | 0.08 | |
| p-value | 0.000 | p-value | 0.002 | p-value | 0.278 | p-value | 0.913 | ||
| Asthenia/fatigue | Pearson correlation | 0.404** | Model coefficient | 3.0** | Pearson correlation | 0.195* | Model coefficient | 0.43 | |
| p-value | 0.000 | p-value | 0.002 | p-value | 0.031 | p-value | 0.432 | ||
| Loss of appetite | Pearson correlation | 0.259** | Model coefficient | 1.5 | Pearson correlation | 0.178* | Model coefficient | 0.98 | |
| p-value | 0.004 | p-value | 0.226 | p-value | 0.049 | p-value | 0.18 | ||
| Nausea/vomiting | Pearson correlation | 0.181* | Model coefficient | 0.6 | Pearson correlation | 0.072 | Model coefficient | -0.73 | |
| p-value | 0.045 | p-value | 0.600 | p-value | 0.429 | p-value | 0.284 | ||
| Constipation | Pearson correlation | 0.307** | Model coefficient | 2.5* | Pearson correlation | 0.168 | Model coefficient | 0.65 | |
| p-value | 0.001 | p-value | 0.032 | p-value | 0.064 | p-value | 0.344 | ||
| Anxiety | Pearson correlation | 0.264** | Model coefficient | -0.8 | Pearson correlation | 0.256** | Model coefficient | 1.4 | |
| p-value | 0.003 | p-value | 0.573 | p-value | 0.004 | p-value | 0.105 | ||
| Depression | Pearson correlation | 0.161 | Model coefficient | -.1.7 | Pearson correlation | 0.101 | Model coefficient | 0.015 | |
| p-value | 0.076 | p-value | 0.237 | p-value | 0.267 | p-value | 0.985 | ||
| Sleep disturbances | Pearson correlation | 0.400** | Model coefficient | 1.9 | Pearson correlation | 0.245** | Model coefficient | 1.1 | |
| p-value | 0.000 | p-value | 0.092 | p-value | 0.006 | p-value | 0.085 | ||
| Other factors | |||||||||
| Recent Kt/V value | Pearson correlation | 0.094 | Model coefficient | 0.18 | Pearson correlation | 0.242* | Model coefficient | 0.74 | |
| p-value | 0.326 | p-value | 0.909 | p-value | 0.01 | p-value | 0.424 | ||
| Recent ferritin value | Pearson correlation | -0.110 | Model coefficient | 0.002 | Pearson correlation | -0.013 | Model coefficient | 0.0 | |
| p-value | 0.231 | p-value | 0.335 | p-value | 0.89 | p-value | 0.853 | ||
| Female gender | Pearson correlation | 0.015 | Model coefficient | 0.5 | Pearson correlation | 0.253** | Model coefficient | 1.6** | |
| p-value | 0.868 | p-value | 0.562 | p-value | 0.005 | p-value | 0.003 | ||
| Acceptance of dialysis therapy | Pearson correlation | 0.041 | Model coefficient | 0.045 | Pearson correlation | -0.168 | Model coefficient | -0.17* | |
| p-value | 0.659 | p-value | 0.729 | p-value | 0.066 | p-value | 0.033* | ||
| ** Correlation significant at p <0.01 level, 2 tailed * Correlation significant at p <0.05 level, 2 tailed | |||||||||
| Table 4: Pain duration, intensity, perception and localisation in the patients reporting pain during the 4 weeks before the interview (N = 81). | |
| N (%)* | |
| Duration: | |
| <6 months | 16 (20%) |
| 6 months to 1 year | 7 (9%) |
| 1–5 years | 38 (47%) |
| >5 years | 23 (28%) |
| Intensity during the previous 4 weeks: | |
| Intense (VAS score 8–10) | 49 (60.5%) |
| Moderate (VAS score 5–7) | 17 (21%) |
| Mild (VAS score <4) | 14 (17.3%) |
| No pain | |
| Intensity at the time of interview: | |
| Intense (VAS score 8–10) | 5 (6%) |
| Moderate (VAS score 5–7) | 7 (8.5%) |
| Mild (VAS score <4) | 13 (16%) |
| No pain | 55 (68%) |
| Perception: | |
| Episodic pain | 63 (59%) |
| Continuous pain | 16 (12% |
| Localisation by the patient: | |
| Lower limbs | 65 (80.5%) |
| Upper limbs | 40 (50%) |
| Thorax | 36 (44%) |
| Head | 26 (32%) |
| Back, vertebral column, lumbar region | 22 (27%) |
| Abdominal region | 11 (13.5) |
| Chest | 3 (3.5%) |
| Localisation by the physician: | |
| Musculoskeletal pain | 52 (64%) |
| Headache | 25 (31%) |
| Cramps | 20 (25%) |
| Neuropathic pain | 14 (17%) |
| Visceral pain | 10 (12%) |
| Arteriopathic pain | 7 (9%) |
| Other (calciphylaxis, malignancy) | 2 (2%) |
| * Missing values have not been included in the totals; figures, therefore, may not always add up to 100. | |
| Table 5: Pharmacotherapy (N = 81). | ||
| Pain specific pharmacotherapy: | N | % |
| Non-steroidal anti-inflammatory drugs or similar | 65 | 80 |
| Weak opioids | 13 | 16 |
| Strong opioids | 4 | 5 |
| Non-opioid topical analgesics | 3 | 4 |
| Drugs for neuropathic pain | 9 | 11 |
| Steroids | 19 | 15 |
| Antidepressants | 8 | 10 |
| Other pharmacotherapy: | ||
| Laxatives | 35 | 43 |
| Other | 18 | 21 |
| Antiemetics | 15 | 19 |
| Anti-Parkinson treatment | 3 | 4 |
| Myorelaxants | 2 | 3 |
The interviewed patients had chronic kidney disease (CKD) stage 5 and were on long-term dialysis. They experienced multiple and severe symptoms, which interfered with daily living.
The prevalence of pain in our population was similar to or larger than that observed in other studies conducted in the same population of haemodialysis patients [4, 5, 27].
Musculoskeletal pain prevailed in our cohort and was experienced as more bothersome than other pain types; this was consistent with the perception of some patients that movement was a pain trigger.
Musculoskeletal pain is one of the leading causes of chronic health problems in people over 65 years of age. Studies suggest a high proportion of older adults suffering from musculoskeletal pain (65% to 80%) and back pain (36% to 40%) [28]. It is very difficult to conclude from our results if, and to what extent, dialysis influenced musculoskeletal pain in our population, because there are many factors implicated in the aetiopathology of bone pain in dialysed patients.
In our population, musculoskeletal pain was a leading cause of sleep disturbances and waking up at night, and it was treated with opioids in only a very few patients. Sleep disturbances include a variety of disorders in ESDR patients: difficulties in falling asleep and awakening, interrupted sleep, nightmares, restless legs syndrome, sleep apnoea syndrome and others [29]. The occurrence of sleep disturbances in our cohort is confirmed by other published data [2, 3]. The major aetiological factors for sleep disorders in the uraemic patient are still controversial. Pain is rarely identified as a trigger.
Poor sleep quality, with a prevalence of 49%, was observed in the haemodialysis patients included in the Dialysis Outcomes and Practice Patterns Study (DOPPS) population. It was independently associated with a higher degree of physical pain, higher medication use and mortality [23, 30, 31]. Diabetic haemodialysis patients also have an increased risk of insomnia [32], increased body pain and reduced quality of life [31].
Our population identified pain as an important factor related to frequent awakenings.
Patients identified the dialysis session itself as a trigger for headache and cramps. These painful syndromes are typical for CKD and are often dialysis-related, because of the disequilibrium of electrolytes that occurs during the dialysis session. Worsening of pain during haemodialysis is described but pathophysiologically-based treatments are matter of hypothesis [2, 3].
In our population, 81% of the patients with pain recorded values of 8–10 in the VAS scale. The patients described the dialysis session asa pain trigger. Up to one-third of the patients describing other symptoms, such as nausea and vomiting, or dyspnoea, indicated VAS values higher than 5. This indicates a high level of global distress.
In the literature, the severity of symptoms in dialysis patients has been reported to be comparable to or even worse than in patients with CKD stage 5 managed without dialysis [2, 3]. On the other hand, the proportion of patients in our cohort with asthenia and fatigue, poor appetite, or dyspnoea was smaller than that of patients with stage 5 CKD managed without dialysis. In fact, previous studies report a prevalence of lack of energy and fatigue in as many as 75% of patients, poor appetite in 58% and dyspnoea in 49%, as compared with 58%, 19% and 19%, respectively, in our study population [2, 3].
The benefit of a high-quality dialysis session in terms of lower fatigue or symptom burden and pain related to the session itself should be balanced by corresponding pain treatment. Pain assessment and treatment during the dialysis sessions, and the assessment of overall symptoms by means of the ESAS, could help in developing early specific pain-relief protocols.
Data provide evidence that dialysis patients with chronic pain suffer more from insomnia and depression than those without pain, and that these symptoms are not adequately treated [33, 34]. In our study, 13% of the patients had significant levels of depression, as assessed using the ESAS. The size of our population does not allow further analysis, but it can be hypothesised that high levels of pain could be related with depression and affects daily living.
As pain therapy, opioids were prescribed to only 21% of our patients; nonopioid analgesics (mainly NSAIDs) were taken by 80% of the patients. A recent systematic review of the use of opioids in ESDR patients confirmed that the prevalence of opioid use is highly variable, ranging from 5% to 36% [35], suggesting a substantial under treatment of pain. Clear guidelines for pain management in dialysis patients are warranted to avoid under-prescription of analgesics, and also to consider their prescription in ESDR [12].
Improvement of sleep quality could lead to less fatigue, since in our study fatigue was not associated with haemoglobin levels. Management of sleep quality, however, requires adequate pain relief, which should focus primarily on musculoskeletal pain, as in our study it emerged as the most bothersome pain. Future pain management has also to consider the lack of correlation, as seen in our study, between hypertension and severity of pain, as well as hyperparathyroidism and musculoskeletal pain [27], although musculoskeletal pain was prevalent in our study.
The high burden of physical and psychological symptoms is known to be multifactorial, and in our study it was associated with impaired daily activity and a considerable impact on quality of life and independence [21].
With an estimated prevalence of 14% to 30%, major depression is the most common psychiatric problem in patients with stage 5 chronic kidney disease [36].
The dialysis patient’s perception of symptom burden may be more important than objective clinical parameters in determining quality of life in this patient population, because quality of life is an important outcome in the treatment of end-stage renal disease [23].
The increasing number of patients with ESRD calls for the development of appropriate care models for these patients and their families, involving dialysis providers, doctors and nurses, and primary care and palliative care providers. A first step in the routine care in renal units could be a regular, comprehensive symptom assessment, especially in dialysis patients with advanced stages of CKD. In our study, ESAS has proved to be a simple, easily understandable tool to evaluate symptom burden in haemodialysis patients. Renal units should pay more attention to the K/DOQI Clinical Practice Guidelines, which recommend regular assessment of quality of life for all patients with CKD [18, 23]. This should also include the recognition and treatment of spiritual and emotional suffering in these patients.
Our study focused only on global symptom prevalence in a relatively small number of patients and does not allow firm correlations. The 4-week recall period might have biased the scoring of pain intensity and burden, as well as the questions on impact on daily life activities. The results rely on the patients’ subjective perception. However, the outcome is in line with published literature and thus bias can be considered as minimal. Our observations provide further evidence of the need to include pain relief protocols in the treatment of dialysis patients, together with a comprehensive palliative care approach in our community.
Acknowledgement: The authors are grateful to the participating patients for giving us the opportunity to understand better their suffering, to Sistiana Nava-Santinelli and Silvia Walther (IOSI) for interviewing the patients, to Dr. med. Hans Neuenschwander (Palliative Care Service, Istituto Oncologico della Svizzera Italiana, Bellinzona, Switzerland) for his guidance during the study, to PD Dr. med. Michael Dickenmann (Clinic for Transplantation Immunology and Nephrology, University Hospital Basel, Switzerland) for his contribution during editing of the manuscript, to Mr. C. Limoni (Riva San Vitale, Switzerland) for reviewing the statistical analyses, to Liliane Petrini (Ph.D, Breganzona, Switzerland) for her help to finalizing the manuscript.
1 Merskey H, Bogduk N. Classification of chronic pain. Seattle, WA: International Association for the Study of Pain Press; 1994. 240 p.
2 Murtagh FE, Addington-Hall JM, Edmonds PM, Donohoe P, Carey I, Jenkins K, et al. Symptoms in advanced renal disease: a cross-sectional survey of symptom prevalence in stage 5 chronic kidney disease managed without dialysis. J Palliat Med. 2007;10(6):1266–76. PubMed PMID: 18095805. Epub 2007/12/22.
3 Murphy EL, Murtagh FE, Carey I, Sheerin NS. Understanding symptoms in patients with advanced chronic kidney disease managed without dialysis: use of a short patient-completed assessment tool. Nephron Clin Pract. 2009;111(1):c74–80. PubMed PMID: 19088482. Epub 2008/12/18.
4 Bouattar T, Skalli Z, Rhou H, Ezzaitouni F, Ouzeddoun N, Bayahia R, et al. [The evaluation and analysis of chronic pain in chronic hemodialysis patients.] Nephrol Ther. 2009;5(7):637–41. PubMed PMID: 19625232. Epub 2009/07/25French.
5 Davison SN. Pain in hemodialysis patients: prevalence, cause, severity, and management. Am J Kidney Dis. 2003;42(6):1239–47. PubMed PMID: 14655196. Epub 2003/12/05.
6 Ferro C, Chambers J, Davison S. Management of pain in renal failure. In: Chambers JE GM, Brown E editor. Supportive care for the renal patient: Oxford University Press; 2005. p. 105–53.
7 Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Advances in chronic kidney disease. 2007;14(1):82–99. PubMed PMID: 17200048. Epub 2007/01/04.
8 Bailie GR, Mason NA, Bragg-Gresham JL, Gillespie BW, Young EW. Analgesic prescription patterns among hemodialysis patients in the DOPPS: potential for underprescription. Kidney Int. 2004;65(6):2419–25. PubMed PMID: 15149355. Epub 2004/05/20.
9 Claxton RN, Blackhall L, Weisbord SD, Holley JL. Undertreatment of symptoms in patients on maintenance hemodialysis. J Pain Symptom Manage. 2010;39(2):211–8. PubMed PMID: 19963337. Epub 2009/12/08.
10 Fortina F, Agliata S, Regazzoni E, Sacco A, Cardillo V, Ravaglini S, et al. Chronic pain during dialysis: Pharmacologic therapy and its costs. Minerva Urol Nefrol. 1999;51:85–7.
11 Meyer KB, Espindle DM, DeGiacomo JM, Jenuleson CS, Kurtin PS, Davies AR. Monitoring dialysis patients’ health status. Am J Kidney Dis. 1994;24(2):267–79. PubMed PMID: 8048434. Epub 1994/08/01.
12 King S, Forbes K, Hanks GW, Ferro CJ, Chambers EJ. A systematic review of the use of opioid medication for those with moderate to severe cancer pain and renal impairment: a European Palliative Care Research Collaborative opioid guidelines project. Palliat Med. 2011;25(5):525–52. PubMed PMID: 21708859. Epub 2011/06/29.
13 Andreucci VE, Fissell RB, Bragg-Gresham JL, Ethier J, Greenwood R, Pauly M, et al. Dialysis Outcomes and Practice Patterns Study (DOPPS) data on medications in hemodialysis patients. Am J Kidney Dis. 2004;44(5 Suppl 2):61–7. PubMed PMID: 15486876. Epub 2004/10/16.
14 Barakzoy AS, Moss AH. Efficacy of the world health organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol. 2006;17(11):3198–203. PubMed PMID: 16988057. Epub 2006/09/22.
15 Saini T, Murtagh FE, Dupont PJ, McKinnon PM, Hatfield P, Saunders Y. Comparative pilot study of symptoms and quality of life in cancer patients and patients with end stage renal disease. Palliat Med. 2006;20(6):631–6. PubMed PMID: 17060257. Epub 2006/10/25.
16 Weisbord SD, Carmody SS, Bruns FJ, Rotondi AJ, Cohen LM, Zeidel ML, et al. Symptom burden, quality of life, advance care planning and the potential value of palliative care in severely ill haemodialysis patients. Nephrol Dial Transplant. 2003;18(7):1345–52. PubMed PMID: 12808172. Epub 2003/06/17.
17 Weisbord SD, Fried LF, Mor MK, Resnick AL, Unruh ML, Palevsky PM, et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2007;2(5):960–7. PubMed PMID: 17702730. Epub 2007/08/19.
18 Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–47. PubMed PMID: 12859163. Epub 2003/07/16.
19 Cleeland C. Research in cancer pain. What we know and what we need to know. Cancer. 1991;67(3 Suppl):823–7. PubMed PMID: 1986852. Epub 1991/02/01.
20 Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6–9. PubMed PMID: 1714502. Epub 1991/01/01.
21 Bruera E, MacMillan K, Hanson J, MacDonald RN. The Edmonton staging system for cancer pain: preliminary report. Pain. 1989;37(2):203–9. PubMed PMID: 2748193. Epub 1989/05/01.
22 Caraceni A, Cherny N, Fainsinger R, Kaasa S, Poulain P, Radbruch L, et al. Pain measurement tools and methods in clinical research in palliative care: recommendations of an Expert Working Group of the European Association of Palliative Care. J Pain Symptom Manage. 2002;23(3):239–55. PubMed PMID: 11888722. Epub 2002/03/13.
23 Davison SN, Jhangri GS, Johnson JA. Longitudinal validation of a modified Edmonton symptom assessment system (ESAS) in haemodialysis patients. Nephrol Dial Transplant. 2006;21(11):3189–95. PubMed PMID: 16957010. Epub 2006/09/08.
24 Watanabe SM, Nekolaichuk C, Beaumont C, Johnson L, Myers J, Strasser F. A multicenter study comparing two numerical versions of the Edmonton Symptom Assessment System in palliative care patients. J Pain Symptom Manage. 2011;41(2):456–68. PubMed PMID: 20832987. Epub 2010/09/14.
25 Zech DF, Grond S, Lynch J, Hertel D, Lehmann KA. Validation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective study. Pain. 1995;63(1):65–76. PubMed PMID: 8577492. Epub 1995/10/01.
26 Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86. PubMed PMID: 5349366. Epub 1969/01/01.
27 Poux J, Lagarde C, Peyronez P, Boudet R, Gontier Y, Benevent D. La douleur en hémodialyse: résultats d’une enquête prospective chez 172 patients utilisant un questionnaire multidimension d’autoévaluation. Nephr Ther. 2005;1:S77–S137. French
28 D’Astolfo CJ, Humphreys BK. A record review of reported musculoskeletal pain in an Ontario long term care facility. BMC Geriatr. 2006;6:5. PubMed PMID: 16556306. Pubmed Central PMCID: 1435899. Epub 2006/03/25.
29 Gusbeth-Tatomir P, Boisteanu D, Seica A, Buga C, Covic A. Sleep disorders: a systematic review of an emerging major clinical issue in renal patients. Int Urol Nephrol. 2007;39(4):1217–26. PubMed PMID: 17914660. Epub 2007/10/05.
30 Elder SJ, Pisoni RL, Akizawa T, Fissell R, Andreucci VE, Fukuhara S, et al. Sleep quality predicts quality of life and mortality risk in haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2008;23(3):998–1004. PubMed PMID: 17911092. Epub 2007/10/04.
31 Sanner BM, Tepel M, Esser M, Klewer J, Hoehmann-Riese B, Zidek W, et al. Sleep-related breathing disorders impair quality of life in haemodialysis recipients. Nephrol Dial Transplant. 2002;17(7):1260–5. PubMed PMID: 12105250. Epub 2002/07/10.
32 Han SY, Yoon JW, Jo SK, Shin JH, Shin C, Lee JB, et al. Insomnia in diabetic hemodialysis patients. Prevalence and risk factors by a multicenter study. Nephron. 2002;92(1):127–32. PubMed PMID: 12187095. Epub 2002/08/21.
33 Davison SN, Jhangri GS. The impact of chronic pain on depression, sleep, and the desire to withdraw from dialysis in hemodialysis patients. J Pain Symptom Manage. 2005;30(5):465–73. PubMed PMID: 16310620. Epub 2005/11/29.
34 Parker KP. Sleep disturbances in dialysis patients. Sleep Med Rev. 2003;7(2):131–43. PubMed PMID: 12628214. Epub 2003/03/12.
35 Wyne A, Rai R, Cuerden M, Clark WF, Suri RS. Opioid and benzodiazepine use in end-stage renal disease: a systematic review. Clin J Am Soc Nephrol. 2011;6(2):326–33. PubMed PMID: 21071517. Pubmed Central PMCID: 3052223. Epub 2010/11/13.
36 Nagler EV, Webster AC, Vanholder R, Zoccali C. Antidepressants for depression in stage 3–5 chronic kidney disease: a systematic review of pharmacokinetics, efficacy and safety with recommendations by European Renal Best Practice (ERBP). Nephrol Dial Transplant. 2012;27(10):3736–45. PubMed PMID: 22859791. Epub 2012/08/04.
Funding / potential competing interests: The “Alfred und Erika Bär-Spycher Stiftung”, Auf der Wacht 41, 4104 Oberwil BL, Switzerland, provided financial support for interviewing the patients, evaluating the data and using medical writing services by Dr. Liliane Petrini, PhD, for manuscript finalisation and submission. The “Alfred und Erika Bär-Spycher Stiftung” financial support is gratefully acknowledged. This was an investigator-initiated study. All authors declare that they have no conflicting relationships with companies or individuals concerning the information contained in this manuscript.