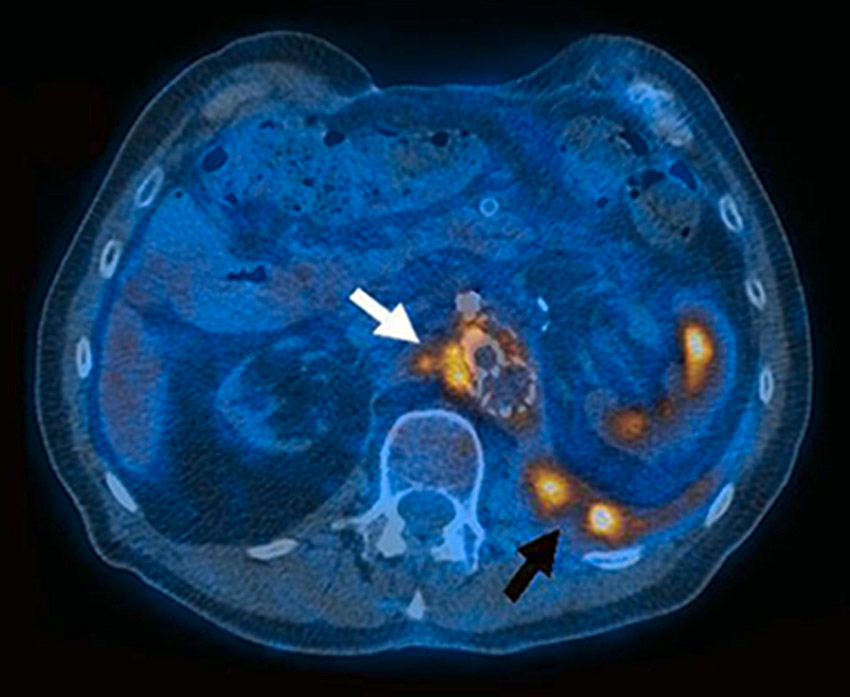
Figure 1
FDG-PET: Distinct, focal metabolic activity (SUV 11.8) adjacent to the Y- and renovisceral grafts (white arrow) with a spatial extension to an abscess in the left psoas region (black arrow).
DOI: https://doi.org/10.4414/smw.2013.13754
Because of the growing prevalence of atherosclerosis and diabetes, vascular grafts are increasingly used, and about 1–6% of vascular procedures are complicated by infection [1]. Morbidity and mortality attributable to prosthetic vascular graft infection (PVGI) are high [2, 3], and are highly dependent on the location of the vascular graft, i.e., peripheral or aortic. Peripheral graft infections occur in about 4% of cases and potentially lead to limb loss and increased mortality; the rate of infection of aortic grafts is lower (1–3%), but the attributable mortality is more than 20% [3–5]. According to the Swiss Federal Office of Statistics, 9/100,000 persons are hospitalised because of PVGI, and mean hospitalisation costs amount to 72,350 Swiss Francs per patient.
Reported risk factors for the development of PVGI include patient-related factors, such as older age, male sex, high body mass index, heart failure, immunodeficiency, diabetes mellitus, renal failure, chronic obstructive pulmonary disease, blood stream infection at the time of graft placement, skin ulcers in the lower extremity and prolonged preoperative hospital stay [6–8]. Surgical risk factors include: arteriography injection site within the operative area; groin incision; prolonged, emergency, or “redo” vascular surgery; bowel injury; extensive lymphatic manipulation; and tissue injury during the dissection [4, 6, 7, 9]. Postoperative risk factors include wound infections, and local complications such as seroma or haematoma owing to pseudoaneurysm or wound-bed bleeding [6, 8]. Other potential risk factors pertain to the type of prosthetic graft. Although the incidence of infection in polyester and polytetrafluoroethylene (PTFE) grafts seems to be comparable, it has been hypothesised that polyester grafts are more resistant than PTFE grafts to eradication of infection [5].
Direct inoculation of bacteria from the patient’s skin at the time of surgery or direct spread of bacteria due to local tissue breakdown may lead to surgical site infections. Most PVGI occurs in the groin as a consequence of a progressive surgical site infection. Factors that have been proved to be associated with PVGI are: (1) prolonged preoperative hospital stay; (2) remote infection before elective vascular surgery; (3) hair removal at the surgical incision site with a razor; (4) simultaneous gastrointestinal procedures during the insertion of an aortic graft; and (5) nasal carriage of Staphylococcus aureus (S. aureus). Several studies have highlighted the importance of preoperative antibiotic prophylaxis, normothermia, control of blood glucose in the setting of surgical procedures and patient surveillance for, and eradication of, nasal S. aureus [3].
PVGI is due to bacterial colonisation of the wound and the underlying prosthetic graft, generally because of direct microbial contamination during the operative procedure by the patient’s skin flora. Haematogenous spread of bacteria may occur during dental manipulation, and urological or endoscopic procedures. Other routes of infection are: (1) contiguous spread from the adjacent or overlying tissues; (2) bacterial colonisation of atherosclerotic plaques surrounding the prosthetic vascular graft; or (3) colonisation of a thrombus in the aneurysmal sac of the vessel wall [9]. Soft tissue oedema and injured skin structures may add to the risk of PVGI [4]. The implantation of a vascular prosthesis contributes to the risk of a vascular surgical site infection through biofilm formation, which may sustain bacterial colonisation, and protect microorganisms from host defences and antimicrobial therapy. Staphylococci (S. aureusand coagulase-negative staphylococci) account for 80% of PVGIs [10], but fungi (mainly Candida), other Gram-positive cocci (enterococci, streptococci), or Gram-negative rods (mainly Eschericha coli, Pseudomonas aeruginosaand Klebsiella spp.), are also reported to cause PVGI [11]. Staphylococci and Pseudomonas spp. are strong biofilm producers. PVGI is often polymicrobial, with involvement of anaerobes, and there are cases where cultures remain negative, often in the context of previous antimicrobial therapy [10, 12].
The diagnosis of PVGI involves several elements, including microbiological and clinical findings and imaging studies, as well as inflammatory markers.

Figure 1
FDG-PET: Distinct, focal metabolic activity (SUV 11.8) adjacent to the Y- and renovisceral grafts (white arrow) with a spatial extension to an abscess in the left psoas region (black arrow).
Microorganisms retrieved from superficial or deep wounds may simply represent colonising flora. Therefore, it is essential to obtain bacterial cultures from the explanted grafts, if available, or from tissue surrounding the graft [12]. For adequate sampling, surgery is often mandatory. Molecular methods such as the 16s rRNA polymerase chain reaction (broad range PCR), and microbiological recovery techniques with broth cultures and sonication of the graft, are used for diagnosis of PVGI [13, 14]. To date, the relevance of cultures obtained from foams used for negative pressure wound therapy (NPWT) is still debated [15].
The clinical presentation of PVGI is very variable and depends upon the location of the vascular graft (aortic or peripheral) and upon the timing of the infection after surgery (early or late).
Early infections develop less than 3 months after surgery. They often present with acute onset of fever, bacteraemia, pain, erythema, swelling, warmth, local bleeding, ulcer formation, graft occlusion or a pulsatile mass (from mycotic pseudoaneurysm) in the groin. Late infections develop more than 3 months after surgery. They may present with more subtle symptoms and signs such as back pain, fistula, graft occlusion, asymptomatic pseudoaneurysm formation and poor incorporation of the graft into the surrounding tissue. Systemic manifestations of infection are often absent (especially in diabetic patients) and blood cultures are often negative. Sometimes overlying soft tissue infections are difficult to distinguish from PVGI. There are two classification systems for vascular graft infections (the Szilagyi [16] and the Samson classifications [17]), which were developed for vascular groin infections in particular (table 1).
The methods most commonly used to evaluate whether vascular grafts are infected include ultrasound, contrast enhanced computed tomographic angiography (CTA), 18F-fluorodeoxyglucose positron-emission tomography (FDG-PET) and fusion PET/computed tomography (CT). Perigraft fluid and inflammation can be rapidly identified with ultrasound, but CT is generally considered the diagnostic test of choice. CTA scans are assessed for the presence of ectopic gas, perigraft fluid (<20 Hounsfield units), perigraft soft tissue enhancement (>20 Hounsfield units), pseudoaneurysm formation, discontinuity of the aneurysmal sac and an increased amount of soft tissue (>5 mm) between the graft and the surrounding aneurysmal wall [18]. CT-guided puncture may yield periprosthetic fluid for Gram stain and culture to establish the microbiological diagnosis.
In advanced PVGI, the sensitivity of CTA is nearly 100% [19]. However, in the case of low-grade PVGI, the risk of false negative results is high [20]. Therefore, additional imaging modalities are needed. The use of FDG-PET to show the intensity (grade 0–4) and patterns (focal or diffuse) of FDG uptake for the evaluation of infected vascular grafts seems to be promising (figure 1). In a study of 33 consecutive patients with a suspected infected arterial prosthesis, FDG-PET showed a superior sensitivity (91% vs 64%), but a lower specificity (64% vs 84%) compared with CTA. However, when focal uptake was taken as the positive criterion in FDG-uptake [21, 22], the specificity and positive predictive value of FDG-PET for the detection of graft infection improved to 95% [23]. A recently developed fusion technology of FDG-PET and CTA, in a single session, enables the precise localisation of any abnormal FDG uptake, and three studies have confirmed that PET/CTA is a reliable tool for the diagnosis of PVGI [24–27]. However, false-positive FDG uptake because of foreign body reaction has to be taken into account [23].
| Table 1: Classification systems for vascular graft infections. | ||
| Szilagyi Classification [16] | Samson Classfication [17] | |
| Group 1 | Infection involves only the dermis | Infection extends no deeper than the dermis |
| Group 2 | Infection extends into the subcutaneous tissue but does not invade the arterial implant | Infection involves subcutaneous tissues but does not come into grossly observable direct contact with the graft |
| Group 3 | The arterial implant proper is involved in the infection | Infection involves the body of the graft but not at an anastomotic site |
| Group 4 | Infection surrounds an exposted anastomosis, but bacteraemia or anastomotic bleeding has not occurred | |
| Group 5 | Infection involves a graft-to-artery anastomosis and is associated with septicaemia and/ or bleeding at the time of presentation | |
Up to now there are no clear guidelines for management of PVGI, mainly because of the variable clinical presentation and the lack of data from randomised controlled trials. Treatment modalities generally involve both surgical intervention and systemic antibiotics. Antimicrobial therapy alone without surgery is associated with a poor response and high mortality, and is therefore not recommended [2]. However, small case series have shown promising results for long-term suppressive antibiotic therapy in patients who are not fit for surgery because of co-morbidities or an unacceptably high perioperative risk [28–30].
As well as aggressive debridement and the use of antibiotics, many earlier treatment approaches recommended graft excision and revascularisation through a non-infected field in a one-stage procedure. The drawbacks of extra-anatomic bypass revascularisation are long procedure time, low patency, high amputation rates and the risk of rupture along the suture line. These led to various graft-preserving treatment options such as partial graft excision and in situ reconstruction with cryopreserved homografts, fresh arterial allografts, autologous veins, or antibiotic- or silver-bonded prosthetic grafts [3, 31–33] with or without muscle flap coverage. The incidence of infection in arterial and vein grafts is lower than in prosthetic vascular grafts, but complications related to the harvest site should not be ignored [8]. The antibiotics most commonly used to coat vascular grafts are fusidic acid, quinupristin-dalfopristin, vancomycin, teicoplanin, gentamycin and rifampicin. Three randomised controlled trials evaluated the efficacy of rifampicin-bonded grafts in preventing early wound and/or graft infection with promising results [34–36]. However, genuine or acquired rifampicin resistance of microorganisms isolated from PVGI has to be taken into account [33]. Although all these alternative techniques proved to be superior to traditional methods in terms of rates of reinfection, conduit failure and amputation rates [37], overall mortality was still high, underlining the need for novel surgical approaches.
Negative pressure wound therapy (NPWT) has recently provided new options for the treatment of infected vascular wounds [38], although treating a vascular infection by means of a local surgical therapy seems to be counterintuitive. NPWT has been increasingly used for the treatment of vascular surgical site infections either as a bridge to surgical closure or as a primary wound treatment modality [11, 39–41]. Additionally, a combined endovascular and surgical approach using radical debridement and NPWT on top of an endograft has been reported to lower mortality in the treatment of infected arterial ruptures [42].
There is no consensus on which classes of antimicrobial agents are adequate for empirical treatment of PVGI. The British Society for Antimicrobial Chemotherapy (BSAC) Steering Group on the treatment of hospital infections recommends combination treatment with cefuroxime and metronidazole [43], but since staphylococciand Gram-negative rods are likely to be isolated, empirical treatment might also include a penicillinase-resistant beta-lactam or a glycopeptide, plus an aminoglycoside for Gram-negative coverage and synergistic treatment in case of staphylococcal infection [44]. In a French study involving 37 patients admitted to the Intensive Care Unit for suspected or proven PVGI, combination therapy including aminoglycosides was associated with a lower 30-day mortality [45]. Empirical antifungal therapy is probably not necessary, but empirical combination treatment might include rifampicin because of its good penetration into biofilms. When the microorganisms involved are isolated, their susceptibility should be tested in order to optimise bacterial coverage and to narrow the antimicrobial spectrum.
For oral therapy, quinolones, trimethoprim-sulfamethoxazole, tetracyclines and rifampicin should preferably be used because of their high oral bioavailability. An increasing prevalence of multidrug-resistant organisms isolated from PVGI has been observed in recent years [4, 46, 47]. Whether multidrug-resistant organisms must be considered in the prophylaxis or empirical treatment of PVGI depends on the local patterns of resistance in individual hospitals.
Details of the type and duration of antimicrobial therapy are unfortunately often missing in reports of studies of PVGI. A superficial infection might be treated with a 1-week course of oral antibiotics, whereas a deeper infection involving the subcutaneous tissue should presumably be treated longer [39, 48, 49]. The duration of treatment for Szilagyi grade 3 or Samson grade 3–5 PVGI remains uncertain, and is highly dependent on the extent of infection, the location (peripheral or aortic), and the type of graft material (synthetic, biological or vein). Presumably, antimicrobial therapy should be prolonged, as is the case with prosthetic valve endocarditis [50]. After surgical debridement of the surrounding tissue (i.e., removal of necrosis, infected tissues and biofilm) and the infected graft (i.e., removal of biofilm), intravenous antimicrobial therapy for at least 4–6 weeks is warranted. Calligaro et al. and Legout et al. even suggest intravenous antibiotic treatment for 6 weeks followed by oral antiinfective treatment for a minimum of 6‒12 months [3, 44]. In a small case series of nine Japanese patients with infected aortic grafts, surviving individuals received intravenous antimicrobial treatment followed by oral therapy for up to 3–6 months [51]. In a retrospective study of 68 infected autogenous vein grafts, the duration of intravenous treatment was 3 weeks without additional oral treatment [44]. However, in a recent study, 20% of patients with Szilagyi grade 3 PVGI treated with NPWT and graft preservation without administration of any antibiotic showed good long-term results [11]. Future studies should focus therefore not only on the various forms of surgical treatment but especially on the need for antibiotic treatment.
Few studies have compared the outcomes of different surgical strategies for treatment of vascular surgical site infection. Ohta et al. compared different graft-removal techniques with either extra-anatomic or in situ revascularisation, immediate vs staged timing of graft excision, and different graft materials (prosthetic, autogenous or allograft). The authors concluded that in situ techniques were superior to extra-anatomic reconstructions in preventing new graft failure and early mortality [51]. In another study of 85 patients, age >70 years and aortic graft infection were independent risk factors for in-patient mortality [44]. Graft preserving techniques are increasingly used [3], and clinical cure rates of up to 100% have been reported in small case series if the graft was patent, and if there was no evidence of systemic sepsis, local bleeding or formation of a pseudoaneurysm [51]. For the largest series (44 patients), Mayer et al. recently reported an excellent outcome with a graft preserving approach including NPWT as an adjunct [11]. All the patients survived 30 days. One-year mortality was 16% (7/44), and long-term mortality after a mean follow-up of 43 months was 41% (18/44).
| Table 2: Diagnosis and management of Prosthetic Vascular Graft Infection (PVGI). | ||
| Diagnostic or management modalities | Remarks | |
| Imaging | ||
| Extremities or neck | Ultrasound | Detection of pseudoaneurysms Can be performed at the bedside |
| Thorax, abdomen or pelvis | Combined FDG-PET/CTA | Precise localisation of abnormal FDG uptake Imaging-guided aspiration possible |
| MRI | Good soft tissue resolution | |
| Microbiology | ||
| Blood cultures | Identification of microorganism and antimicrobial resistance testing | |
| Tissue cultures | Identification of microorganism and antimicrobial resistance testing | |
| Broad range PCR | Identification of microorganism if cultures are negative >72 h | |
| Surgery | ||
| Graft and anastomosis intact | Graft preservation Debridement Negative pressure wound therapy | Plus antimicrobial therapy |
| Graft and anastomosis at risk for disruption | Surgical removal of parts at risk Local in situ reconstruction Debridement Negative pressure wound therapy | Plus antimicrobial therapy |
| Antimicrobial therapy | ||
| Empirical therapy | Systemic manifestations, SIRS or surgical intervention delayed (>24 h): Start empirical therapy immediately No systemic manifestations, no SIRS and prompt surgical intervention (<24 h): Hold antimicrobials until microbiological results are available | |
| Targeted therapy | In accordance with blood cultures, surgical deep tissue cultures or broad range PCR results | |
| Suppressive therapy | Long term suppressive antimicrobial therapy in the case of inoperable situation | |
| Abbreviations: FDG-PET, 18F-fluorodeoxyglucose positron-emission tomography; CTA, computed tomographic angiography; MRI, Magnetic resonance imaging; Broad range PCR, 16s rRNA Gene Polymerase Chain Reaction; SIRS; Systemic inflammatory response syndrome | ||
Prosthetic vascular graft infections are catastrophic events with a high morbidity and mortality. To date, there is no consensus about the best surgical treatment algorithm or antimicrobial management of PVGI. Recently, however, more targeted and conservative approaches (graft preserving techniques), especially when supported by NPWT, have shown excellent long-term results in nonrandomised case series. In order to address these uncertainties, we plan to establish a prospective, observational cohort study of a multidisciplinary approach to the management of PVGI at the University Hospital of Zurich (VASGRA-Cohort). Until further results are available, graft infection should be treated according to current knowledge; this includes repeated radical local debridement, graft preservation (if possible), NPWT and targeted antimicrobial therapy (table 2).
1 Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422–9.
2 Saleem BR, Meerwaldt R, Tielliu IF, Verhoeven EL, van den Dungen JJ, Zeebregts CJ. Conservative treatment of vascular prosthetic graft infection is associated with high mortality. Am J Surg. 2010;200(1):47‒52
3 Calligaro KD, Veith FJ, Yuan JG, Gargiulo NJ, Dougherty MJ. Intra-abdominal aortic graft infection: complete or partial graft preservation in patients at very high risk. J Vasc Surg. 2003;38:1199–205.
4 Bandyk DF. Vascular surgical site infection: risk factors and preventive measures. Semin Vasc Surg. 2008;21:119–23.
5 Swain TW, 3rd, Calligaro KD, Dougherty MD. Management of infected aortic prosthetic grafts. Vasc Endovascular Surg. 2004;38:75–82.
6 Antonios VS, Noel AA, Steckelberg JM, Wilson WR, Mandrekar JN, Harmsen WS, et al. Prosthetic vascular graft infection: a risk factor analysis using a case-control study. J Infect. 2006;53:49–55.
7 Turtiainen J, Saimanen E, Partio T, Karkkainen J, Kiviniemi V, Makinen K, et al. Surgical wound infections after vascular surgery: prospective multicenter observational study. Scand J Surg. 2010;99:167–72.
8 Nagpal A, Sohail MR. Prosthetic vascular graft infections: a contemporary approach to diagnosis and management. Curr Infect Dis Rep. 2011;13:317–23.
9 Seeger JM. Management of patients with prosthetic vascular graft infection. Am Surg. 2000;66:166–77.
10 Stone PA, Back MR, Armstrong PA, Brumberg RS, Flaherty SK, Johnson BL, et al. Evolving microbiology and treatment of extracavitary prosthetic graft infections. Vasc Endovascular Surg. 2008;42:537–44.
11 Mayer D, Hasse B, Koelliker J, Enzler M, Veith FJ, Rancic Z, et al. Long-term results of vascular graft and artery preserving treatment with negative pressure wound therapy in Szilagyi grade III infections justify a paradigm shift. Ann Surg. 2011;254:754–9; discussion 760.
12 FitzGerald SF, Kelly C, Humphreys H. Diagnosis and treatment of prosthetic aortic graft infections: confusion and inconsistency in the absence of evidence or consensus. J Antimicrob Chemother. 2005;56:996–9.
13 Bergamini TM, Bandyk DF, Govostis D, Vetsch R, Towne JB. Identification of Staphylococcus epidermidis vascular graft infections: a comparison of culture techniques. J Vasc Surg. 1989;9:665–70.
14 Kanemitsu S, Shimono T, Nakamura A, Yamamoto K, Wada H, Shimpo H. Molecular diagnosis of nonaneurysmal infectious aortitis. J Vasc Surg. 2011;53:472–4.
15 Anagnostakos K, Mosser P. Bacteria identification on NPWT foams: clinical relevance or contamination? J Wound Care. 2012;21:333–4, 336–9.
16 Szilagyi DE, Smith RF, Elliott JP, Vrandecic MP. Infection in arterial reconstruction with synthetic grafts. Ann Surg. 1972;176:321–33.
17 Samson RH, Veith FJ, Janko GS, Gupta SK, Scher LA. A modified classification and approach to the management of infections involving peripheral arterial prosthetic grafts. J Vasc Surg. 1988;8:147–53.
18 Low RN, Wall SD, Jeffrey RB, Jr., Sollitto RA, Reilly LM, Tierney LM, Jr. Aortoenteric fistula and perigraft infection: evaluation with CT. Radiology. 1990;175:157–62.
19 Mark A, Moss AA, Lusby R, Kaiser JA. CT evaluation of complications of abdominal aortic surgery. Radiology. 1982;145:409–14.
20 Fiorani P, Speziale F, Rizzo L, De Santis F, Massimi GJ, Taurino M, et al. Detection of aortic graft infection with leukocytes labeled with technetium 99m-hexametazime. J Vasc Surg. 1993;17:87–95; discussion 95–86.
21 Stumpe KD, Dazzi H, Schaffner A, von Schulthess GK. Infection imaging using whole-body FDG-PET. Eur J Nucl Med. 2000;27:822–32.
22 von Schulthess GK, Meier N, Stumpe KD. Joint accumulations of FDG in whole body PET scans. Nuklearmedizin. 2001;40:193–7.
23 Fukuchi K, Ishida Y, Higashi M, Tsunekawa T, Ogino H, Minatoya K, et al. Detection of aortic graft infection by fluorodeoxyglucose positron emission tomography: comparison with computed tomographic findings. J Vasc Surg. 2005;42:919–25.
24 Keidar Z, Engel A, Hoffman A, Israel O, Nitecki S. Prosthetic vascular graft infection: the role of 18F-FDG PET/CT. J Nucl Med. 2007;48:1230–6.
25 Bruggink JL, Glaudemans AW, Saleem BR, Meerwaldt R, Alkefaji H, Prins TR, et al. Accuracy of FDG-PET-CT in the diagnostic work-up of vascular prosthetic graft infection. Eur J Vasc Endovasc Surg. 2010;40:348–54.
26 Stadler P, Bilohlavek O, Spacek M, Michalek P. Diagnosis of vascular prosthesis infection with FDG-PET/CT. J Vasc Surg. 2004;40:1246–7.
27 Spacek M, Belohlavek O, Votrubova J, Sebesta P, Stadler P. Diagnostics of “non-acute” vascular prosthesis infection using 18F-FDG PET/CT: our experience with 96 prostheses. Eur J Nucl Med Mol Imaging. 2009;36:850–8.
28 Coselli JS, Koksoy C, LeMaire SA. Management of thoracic aortic graft infections. Ann Thorac Surg. 1999;67:1990–3; discussion 1997–1998.
29 Roy D, Grove DI. Efficacy of long-term antibiotic suppressive therapy in proven or suspected infected abdominal aortic grafts. J Infect. 2000;40:184–204.
30 Baddour LM. Long-term suppressive antimicrobial therapy for intravascular device-related infections. Am J Med Sci. 2001;322:209–12.
31 Cherry KJ, Jr., Roland CF, Pairolero PC, Hallett JW, Jr., Meland NB, Naessens JM, et al. Infected femorodistal bypass: is graft removal mandatory? J Vasc Surg. 1992;15:295–303; discussion 303–295.
32 Gutowski P. Aortoiliac graft infection as a diagnostic and treatment problem. Ann Acad Med Stetin. 1998;Suppl 41:1–72.
33 Topel I, Audebert F, Betz T, Steinbauer MG. Microbial Spectrum and Primary Resistance to Rifampicin in Infectious Complications in Vascular Surgery: Limits to the Use of Rifampicin-Bonded Prosthetic Grafts. Angiology. 2010;61:423‒6.
34 D’Addato M, Curti T, Freyrie A. Prophylaxis of graft infection with rifampicin-bonded Gelseal graft: 2-year follow-up of a prospective clinical trial. Italian Investigators Group. Cardiovasc Surg. 1996;4:200–4.
35 Braithwaite BD, Davies B, Heather BP, Earnshaw JJ. Early results of a randomized trial of rifampicin-bonded Dacron grafts for extra-anatomic vascular reconstruction. Joint Vascular Research Group. Br J Surg. 1998;85:1378–81.
36 Earnshaw JJ, Whitman B, Heather BP. Two-year results of a randomized controlled trial of rifampicin-bonded extra-anatomic dacron grafts. Br J Surg. 2000;87:758–9.
37 O’Connor S, Andrew P, Batt M, Becquemin JP. A systematic review and meta-analysis of treatments for aortic graft infection. J Vasc Surg. 2006;44:38–45.
38 Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553–62.
39 Dosluoglu HH, Loghmanee C, Lall P, Cherr GS, Harris LM, Dryjski ML. Management of early (<30 day) vascular groin infections using vacuum-assisted closure alone without muscle flap coverage in a consecutive patient series. J Vasc Surg. 2010;51:1160‒6
40 Dosluoglu HH, Schimpf DK, Schultz R, Cherr GS. Preservation of infected and exposed vascular grafts using vacuum assisted closure without muscle flap coverage. J Vasc Surg. 2005;42:989–92.
41 Nordmyr J, Svensson S, Bjorck M, Acosta S. Vacuum assisted wound closure in patients with lower extremity arterial disease. The experience from two tertiary referral-centres. Int Angiol. 2009;28:26–31.
42 Kragsterman B, Bjorck M, Wanhainen A. EndoVAC, a novel hybrid technique to treat infected vascular reconstructions with an endograft and vacuum-assisted wound closure. J Endovasc Ther. 2011;18:666–73.
43 BSAC – The British Society for Antimicrobial Chemotherapy. Treatment of hospital infections prosthetic vascular graft infections. http://www.bsac.org.uk http://bsac.org.uk/ (accessed 14 december 2011)
44 Legout L, Sarraz-Bournet B, D’Elia PV, Devos P, Pasquet A, Caillaux M, et al. Characteristics and prognosis in patients with prosthetic vascular graft infection: a prospective observational cohort study. Clin Microbiol Infect. 2012;18:352‒6.
45 Szczot M, Meybeck A, Legout L, Pasquet A, Van Grunderbeeck N, Langlois J, et al. Vascular graft infections in the intensive care unit: clinical spectrum and prognostic factors. J Infect. 2011;62:204–11.
46 Pounds LL, Montes-Walters M, Mayhall CG, Falk PS, Sanderson E, Hunter GC, et al. A changing pattern of infection after major vascular reconstructions. Vasc Endovascular Surg. 2005;39:511–7.
47 Cowie SE, Ma I, Lee SK, Smith RM, Hsiang YN. Nosocomial MRSA infection in vascular surgery patients: impact on patient outcome. Vasc Endovascular Surg. 2005;39:327–34.
48 Kotsis T, Lioupis C. Use of vacuum assisted closure in vascular graft infection confined to the groin. Acta Chir Belg. 2007;107:37–44.
49 Pinocy J, Albes JM, Wicke C, Ruck P, Ziemer G. Treatment of periprosthetic soft tissue infection of the groin following vascular surgical procedures by means of a polyvinyl alcohol-vacuum sponge system. Wound Repair Regen. 2003;11:104–9.
50 Baddour LM, Wilson WR, Bayer AS, Fowler VG, Jr., Bolger AF, Levison ME, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:e394–434.
51 Ohta T, Hosaka M, Ishibashi H, Sugimoto I, Takeuchi N, Kazui H, et al. Treatment for aortic graft infection. Surg Today. 2001;31:18–26.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.