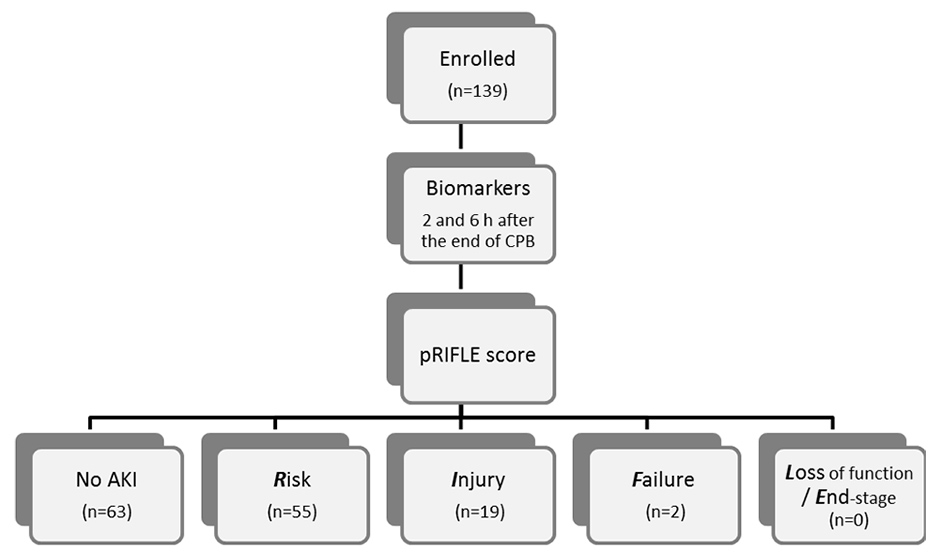
Figure 1
Flow diagram of the study.
DOI: https://doi.org/10.4414/smw.2013.13744
Acute kidney injury (AKI) is a frequent and serious complication encountered in 30–50% of adult patients after cardiac surgery, with 1–5% needing dialysis, AKI is associated with substantial morbidity and mortality [1, 2]. Clinical investigations have identified several risk factors associated with the development of AKI after cardiopulmonary bypass (CPB). The majority related to impaired renal perfusion or decreased renal reserve [3].

Figure 1
Flow diagram of the study.
In current clinical practice AKI is still diagnosed by measuring serum creatinine although serum creatinine is an inadequate marker due to its late rise in AKI pathophysiology [2]. It is a functional marker of glomerular filtration rate rather than injury, only rising once substantial renal injury has already occurred e.g. in a steady state of chronic renal failure and is therefore an unreliable indicator during acute changes in kidney function. Since creatinine formation is proportional to muscle mass and age, serum creatinine may remain within normal range in patients who are elderly, malnourished or chronically ill [4].
Back in 1985 Cystatin C was already investigated as an additional marker for glomerular filtration rate [5]. Cystatin C is a 13 kD protein produced by a housekeeping type gene by all nucleated cells. Cystatin C is freely filtered in the glomerulus and is completely taken up by the proximal tubule [5]. The independence from height, gender, age, and muscle mass is advantageous. Children and the elderly benefit in particular [6]. Numerous studies have already evaluated the use of serum Cystatin C as an endogenous marker of kidney function in populations at risk to develop AKI, showing, that serum Cystatin C has a superior performance in the diagnostic accuracy of serum creatinine levels in the early detection of impaired kidney function [7, 8].
More recently, several novel biomarkers discovered from animal models, have been studied in humans, indicating renal structural damage at an earlier stage to provide potentially effective therapies at a point when damage is still reversible [9–11]. Translational research has identified neutrophil gelatinise-associated lipocalin (NGAL) as one promising early biomarker for diagnosis and prediction of acute renal injury [12, 13]. The first clinical studies in children revealed increased NGAL levels in the urine and plasma by enzyme-linked immunosorbent assay two hours after cardiopulmonary bypass in subjects who subsequently developed AKI [14–17].
As we have recently demonstrated, patients with normal baseline serum creatinine levels displayed a significant correlation between plasma NGAL measured subsequently after cardiopulmonary bypass and increase in serum creatinine, supporting the role of NGAL as an early biomarker of acute renal injury [18]. However, this study revealed also the limitations of a single plasma NGAL determination in predicting AKI development [18].
Therefore, we initiated this prospective single centre study of children undergoing cardiac surgery to compare the diagnostic validity of postoperative urinary NGAL and serum Cystatin C as early biomarkers and predictors of acute renal failure.
All patients undergoing CPB for surgical correction or palliation of congenital heart disease from December 2010 until November 2011 were prospectively enrolled. We obtained written informed consent from the legal guardian of every patient before enrolment. The study was approved by the local Ethics Committee and complied with the declaration of Helsinki. Exclusion criteria included preexisting renal insufficiency, and patients with history of potential nephrotoxin use during the preoperative days. Within the context of the routine blood collection, we obtained blood samples within 30 minutes after admission to the Intensive Care Unit (i.e. two to three hours after the end of CPB), and another 4 hours later from an arterial line. Additionally, we obtained spot urine samples 2 and 6 hours after CPB.
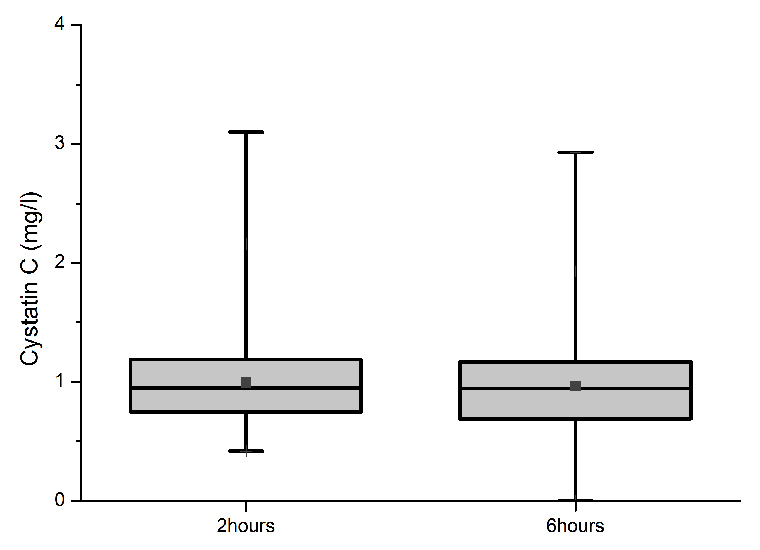
Figure 2
Box and whisker plot of serum Cystatin C concentrations 2 hours and 6 hours after the end of cardiopulmonary bypass. Boxes: 25th percentile (bottom line), median (middle line), and 75th percentile (top line); bars: range; squares: means.

Figure 3
Box and whisker plot of urine neutrophil gelatinase-associated lipocalin (NGAL) values in all patients 2 hours and 6 hours after the end of cardiopulmonary bypass. Boxes: 25th percentile (bottom line), median (middle line), and 75th percentile (top line); bars: range; squares: means. Note the logarithmic scale.
Biomarker levels were compared to perioperative data retrospectively.
A total of 139 patients (76 males and 63 females) were enrolled. Age at surgery was 1 day to 44.3 years (mean age 4.8 years, median age 0.9 years, interquartile range [ICR] 0.3–7.7 years). The overall patients comprised of 24 newborns, 49 infants, 46 children, and 20 adolescent or adult patients. For the underlying congenital heart defects see table 1.
Blood samples were centrifuged and the plasma aliquoted for storage at –80 °C. Cystatin C was measured using a immunonephelometric method (BN ProSpec-II, Siemens Healthcare Diagnostics) [19].
Urine samples were centrifuged and the supernatants stored in tubes at –80 °C. NGAL concentrations were measured by a two-step sandwich assay using chemiluminescent microparticle immunoassay technology (ARCHITECT analyser, Abbott Diagnostics) [17]. High affinitiy antibodies were generated toward distinct epitopes on NGAL and assay standards were prepared using human recombinant NGAL. Urine NGAL concentrations were measured using the standarised manufacturer’s protocol in the range of 0–1,000 ng/ml. Serial dilution was performed where measured values exceeded this range.
Serum creatinine was measured routinely at the time of preoperative evaluation and shortly after surgery (at the same time as the first postoperative Cystatin C measurements). Table 2 gives the normal values of serum creatinine in our laboratory. Estimated creatinine clearance (eCrCl) was calculated using the Schwartz formula: eCrCl (ml/min/1.73 m2) = height (cm) x k/SCr (with k = 0.45 in the first year of life, k = 0.55 in infants and children, k = 0.7 in male patients >12 years).
The primary outcome variable was the development of AKI according to the RIFLE criteria. RIFLE is an acronym for Risk for renal injury, Injury to the kidney, Failure of kidney function, Loss of kidney function, and End-stage renal disease. All patients were classified using the paediatric modified criteria of RIFLE [20]. This scheme stages renal function from risk through to end-stage renal failure based on serum creatinine/glomerular filtration rate or urine output. In practice, a decrease of estimated creatinine clearance of at least 25%, 50% or 75% leads to pRIFLE scores of R, I or F, respectively. Persistent renal failure for more than four weeks or three months indicates classification L or E, respectively [20]. The patients were classified according to the pRIFLE score once a day during the stay in the intensive care unit.
All laboratory measures were interpreted blind to the pRIFLE score.
Other variables included: age, gender, cardiopulmonary bypass time, aortic cross clamping time, length of mechanical ventilation and length of stay in the intensive care unit.
The associations of urine NGAL and serum Cystatin C to continuous variables were assessed by univariate regression analysis. The association of urine NGAL and Cystatin C to the pRIFLE scores was assessed by Spearman’s correlation coefficient. Statistical differences between the two sexes, and between pRIFLE grades were evaluated using the rank score test on unpaired data (Wilcoxon rank-sum test). P-values were two-tailed, with a value <0.05 considered to be significant and <0.01 considered to be highly significant.
Receiver operating characteristic (ROC) curves were computed to assess whether Cystatin C and urine NGAL could predict the risk of developing different pRIFLE scores. The sensitivity and specifity for the biomarkers at varying cut-off levels were calculated by calculating the area under the ROC curve. The best cut-off level was defined as the value that is closest to the point on the ROC curve where sensitivity and specifity approach unity.
All data was analysed with SPSS 18.0 for Windows.
| Table 1: Cardiac diagnosis of the patient group. | |
| Heart defect | n |
| Functional univentricular heart of all stages Ventricular septal defect Atrial septal defect Transposition of the great arteries Pulmonary atresia with VSD Tetralogy of Fallot Aortic stenosis (subaortic, valvular, supravalvular) Atrioventricular septal defect Coarctation/Interruption of the aortic arch Double outlet right ventricle and (complex forms) Truncus arteriosus communis Pulmonary artery stenosis Vascular ring Mitral valve insufficiency Total anoumalous pulmonary venous drainage Pulmonary atresia with intact ventricle septum Ebstein anomaly | 19 18 17 12 11 11 11 9 9 4 4 3 3 3 2 2 1 |
| Table 2: Normal values of serum creatinine. | |
| Age | Upper limit of the normal range (mg/dl) |
| <60 days 60–360 days 1–2 years 3–4 years 5–6 years 7–8 years 9–10 years 11–12 years 13–14 years >15 years (female) >15 years (male) | 0.88 0.39 0.35 0.42 0.47 0.53 0.64 0.68 0.77 0.95 1.17 |
Figure 1 summarises the design of the study. Perioperative data of the 139 patients included in the study are given in table 3.

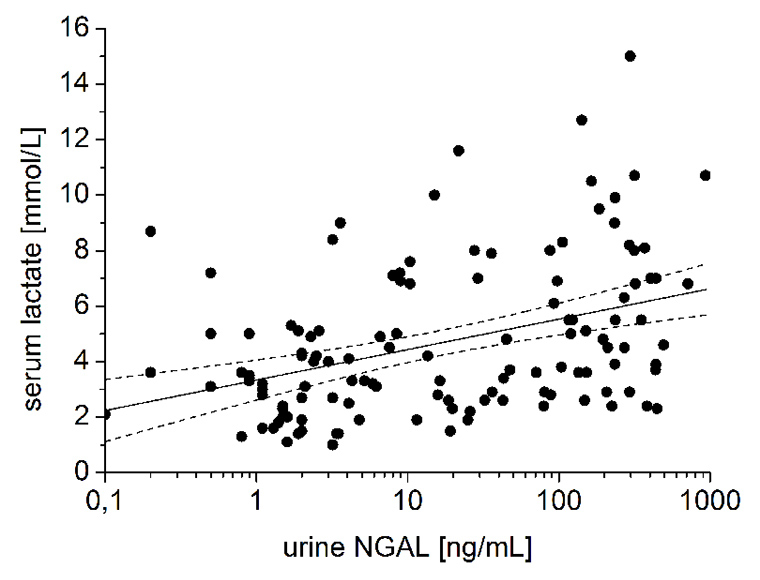
Figure 4
Correlations between urine NGAL 2 hours after the end of cardiopulmonary bypass (CPB) and (a) duration of CPB (P <0.01), and (b) serum lactate concentration (P <0.01). Solid and dotted lines represent the regression line and the 95% confidence interval, respectively.
According to the pRIFLE criteria 63/139 (45%) patients developed no evidence of acute kidney injury. However, 55/139 (40%) patients and 19/139 (14%) patients reached a pRIFLE max of R and I, respectively. A total of 2 patients (1.4%) were in the “Failure“ category and underwent dialysis. No patient reached a pRIFLE max of L or E.
All patients had normal serum creatinine levels according to sex and age before surgery between 0.16 and 0.89 mg/dl (mean 0.38 mg/dl, median 0.33 mg/dl, interquartile range [IQR] 0.26–0.46 mg/dl).
Serum creatinine increased in all patients within 72 hours to peak levels between 0.22 and 2.01 mg/dl (mean 0.58, median 0.49, IQR 0.37–0.73 mg/dl), the serum creatinine increase from baseline to peak value was between 0 and 1.12 mg/dl (mean 0.20, median 0.13, IQR 0.06–0.22 mg/dl). 22/139 patients (15%) had an increase of >50%.
The preoperative estimated creatinine clearance was between 19 and 202 ml/min/1.73 m² (mean 114, median 116, IQR 84–141 ml/min/1.73 m²). The postoperative creatinine clearance was between 13 and 153 ml/min/1.73 m² (mean 80, median 78, IQR 48–110 ml/min/1.73 m²). The estimated creatinine clearance decreased from 114 ± 44 to 80 ± 36 ml/min/1.73 m² within the first 18 hours after surgery.
Serum Cystatin C concentration in the first (fig. 2) and second sample was between 0.42 and 3.25 mg/l (mean 0.99, median 0.95, IQR 0.75–1.19 mg/l; n = 139) and between 0.42 and 3.2 mg/l (mean 0.96, median 0.94, IQR 0.69–1.17 mg/l; n = 126), respectively.
Serum Cystatin C levels began to increase 2 hours after the end of CPB and became only marginally higher after 6 hours after the end of CBP. Serum concentrations 2 hours and 6 hours after the end of CPB showed highly significant correlation with peak postoperative serum creatinine, absolute and relative increase in serum creatinine, estimated creatinine clearance postoperatively and the pRIFLE score (P <0.01). In addition, serum Cystatin C concentrations 2 hours and 6 hours after the end of CPB strongly correlated with length of hospital stay and mechanical ventilation (P <0.01).
To determine the ability of serum Cystatin C to predict AKI the ROC curves were generated for each time-point after CPB (2 and 6 hours). AUC were 0.741 (95% CI: 0.606 to 0.875) for the 2-hour Cystatin C (P = 0.003) and 0.707 (95% CI: 0.543 to 0.871) for the 6-hour Cystatin C (P = 0.009). Further analysing the ROC curves demonstrate the superiority of 2 hour serum Cystatin C for the detection of AKI with an optimal sensitivity and specificity of using a cut-off value of 0.995 mg/l (fig. 5, table 4).
Urine NGAL concentration in the first (fig. 3a) and second sample (fig. 1b) was between 0 and 940 ng/ml (mean 100, median 16, IQR 2–149 ng/ml; n = 125), and between 0 and 795 ng/ml (mean 74, median 6, IQR 2–127 ng/ml; n = 114), respectively. There were no differences in urine NGAL levels between females and males, and there were no correlations between urine NGAL and age, body weight, or body height.
However, highly significant correlations between the first urine NGAL sample and the duration of cardiopulmonary bypass (P <0.01; fig. 4a), the time of aortic cross clamping (P <0.01), serum lactate concentration (P <0.01; fig. 4b) and estimated creatinine clearance postoperatively (P <0.01) were displayed. Furthermore, the first and second urine sample were both correlated to length of hospital stay (P <0.05) and time of mechanical ventilation (P <0.05).
No correlations were demonstrated between urine NGAL and peak postoperative serum creatinine, absolute and relative increase in serum creatinine, and the pRIFLE score.
| Table 3: Baseline data of the patients (n = 139; males/females = 1.2). | |
| Median [IQR] | |
| Age, years | 0.9 [0.3–7.7] |
| Height, cm | 72 [57–118] |
| Weight, kg | 8 [5–22] |
| Cardiopulmonary bypass, min | 138 [87–195] |
| Aortic cross clamping, min | 59 [24–110] |
| Serum lactate, mmol/L | 3.6 [2.6–6.3] |
| Mechanical ventilation, hours | 28 [11–153] |
| ICU, days | 4.5 [2.3–10.0] |
| Table 4: Sensitivity, specifity and accuracy for the patient cohort at the optimal serum Cystatin C and urine NGAL concentration. | ||||
| Cutt-off-value | Sensitivity | 1-Specifity | Accuracy | |
| Cystatin C, 2 hour level | 0.995 mg/l | 0.800 | 0.342 | 0.65 |
| Cystatin C, 6 hour level | 0.945 mg/l | 0.800 | 0.459 | 0.58 |
| Urine NGAL, 2 hour level | 27.6 ng/ml | 0.545 | 0.426 | 0.55 |
| Urine NGAL, 6 hour level | 10.4 ng/ml | 0.636 | 0.351 | 0.64 |
The aetiology of AKI after CPB is multifactorial and incompletely understood. Possible aetiologies include ischaemia-reperfusion injury, oxidative stress, and the systemic inflammatory response. All these factors may contribute to endothelial dysfunction, resulting in capillary leak and vasomotor instability [14].
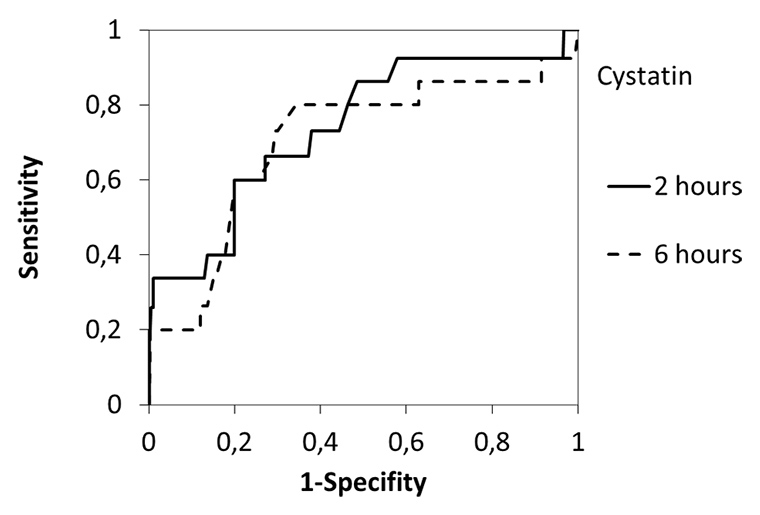
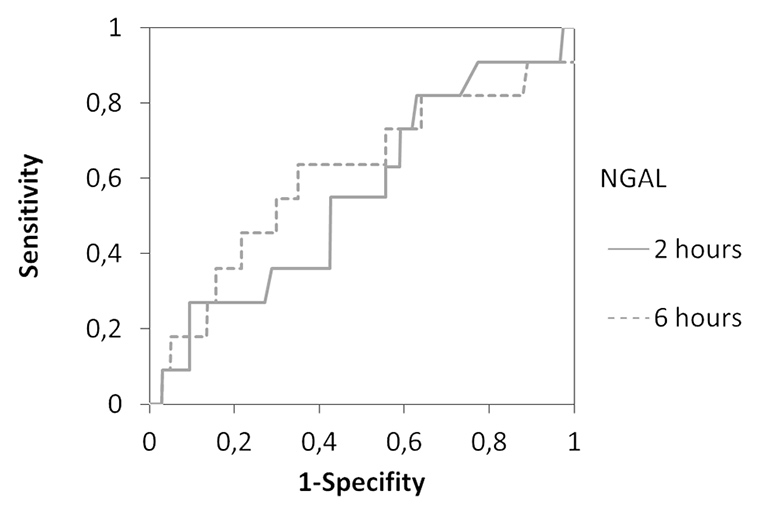
Figure 5
Receiver operating characteristic (ROC) curve analysis using 2h and 6h postoperative serum Cystatin C (a) and urine NGAL (b) concentration in the patient cohort. The area under the ROC curve for Cystatin C was 0.74 (2h; p = 0.003) and 0.71 (6h; p = 0.009), respectively. The area under the ROC curve for urine NGAL was 0.56 (2h; n.s.) and 0.60 (6h; n.s.). Optimal sensitivity and specifity occurred at a value of 0.995 mg/l for 2h Cystatin C.
Serum creatinine is a well established parameter for kidney function despite its generally recognised limitations as an ideal marker for GFR especially in children and elderly. Recently, experimental research has proposed several novel promising renal biomarkers indicating renal tubular injury [9, 10, 21, 22]. However, none of these markers has yet been successfully implemented in routine clinical practice to replace serum creatinine.
Cystatin C has already been recognised to be a superior marker to serum creatinine in the assessment of impaired kidney function in numerous studies. These findings revealed that a rise in Cystatin C precedes the increase in serum creatinine in a select population at high risk for development of AKI [6–8, 23]. Cystatin C is mainly a marker for GFR, serum concentration might only rise after the rate begins to fall. Particularly in children, the independence from: height, gender, age and skeletal muscles, is advantageous [6].
Many studies have focused on the diagnostic predictive value of serum Cystatin C levels for AKI. These studies demonstrated the superiority of serum Cystatin C compared to serum creatinine as a useful biomarker for the early prediction AKI. These findings were confirmed by a systemic review and meta-analysis of diagnostic test studies on the predictive value of Cystatin C in populations at risk for AKI [24].
Most of these studies on Cystatin C were obtained on adult patients [7, 23–25], only two studies investigated paediatric patients after cardiac surgery [19, 26]. The first clinical study in children indicated a significant increase of serum Cystatin C after CPB. Maximal sensitivity and specificity for prediction of AKI were described 12 hours after CPB. The 12 hours Cystatin C also correlated with severity and duration of AKI as well as length of hospital stay [19]. The second multicentre study of 288 children undergoing cardiac surgery evaluated the predictive value of Cystatin C compared to serum creatinine [26]. This study revealed that Cystatin C levels obtained within the first 6 hours of surgery strongly predicts stage 1 and 2 AKI development in the subsequent days as well as longer duration of mechanical ventilation and longer hospital stay [26].
In our single centre study of children undergoing cardiac surgery, the results are consistent with the findings of these two former paediatric studies [19, 26]. We also found that serum Cystatin C performs well as a marker to detect early AKI following paediatric CPB. However, at first glance our results seem to be contradictory to the findings of the Cincinnati study group regarding the optimal time of Cystatin C measurement. According to their data the Cincinnati study group suggest the 12 hour value of Cystatin C as the optimal threshold and a cut-off value of 1.16 mg/l [19]. In contrast, our data revealed an optimal time point for the prediction of AKI at a Cystatin C concentration of 0.995 mg/l, already 2 hours after the end of CPB with adequate certainty. The 2 hour Cystatin C also strongly correlated with severity and duration of AKI as well as length of hospital stay, and mechanical ventilation. Looking in detail at the study design of the two reports, the results are less conflicting. The Cincinnati study group collected their serum samples 2, 12, and 24 hours after the initiation of CPB, whereas we timed the sample collection after the termination of CPB.
Moreover, only a few studies on NGAL measurements in children after CPB have been performed so far, most of them were attained by the Cincinnati study group. The results of these studies have provided evidence, that plasma NGAL and urine NGAL concentrations rise substantially in children after CPB and furthermore proofed to be significant predictive biomarkers for AKI and its clinical outcome [14–16]. Recently, we evaluated the diagnostic value of plasma NGAL in routine clinical practice for the detection of AKI [18]. Our results identified significant correlations between plasma NGAL and an increase in postoperative serum creatinine; however, a marked overlap between the patient groups and only a marginal predictive value depicted the limitations of a single NGAL determination in the effective determination of AKI [18].
In our present study we focused solely on the measurement of urine NGAL. Correspondingly, we found significant correlations between the increase of urine NGAL two hours after CPB and the duration of CPB, the time of aortic cross clamping, serum lactate concentration and estimated creatinine clearance postoperatively. Our findings are consistent with previous clinical studies on NGAL measurements in children after CPB where significant correlations have been found between acute renal ischemia-reperfusion injury and both serum and urine NGAL [14–16, 18]. However, while former studies have detected predictive values, the regression coefficient of the positive correlation in our patients was small, and there was a spread of individual values. Therefore, it was not possible to estimate conclusively the subsequent increase in serum creatinine from one single postoperative urine NGAL level. Correspondingly, we were not able to generate a reasonable optimal cut-off value for urine NGAL. Moreover, no significant correlation was detected between urine NGAL and the severity of the pRIFLE score. These findings albeit less distinctive than our previous data imply that neither urine nor plasma NGAL were accurate predictive biomarkers for AKI. Nevertheless, the significant correlations in our study emphasise that NGAL is indeed an effective early marker of acute ischaemic renal injury in accordance with studies on NGAL in early post-ischaemic mouse kidneys in which an up regulation of NGAL messenger RNA in the thick ascending limb of Henle’s loop and the collecting ducts resulted in the synthesis of NGAL protein levels in the distal nephron and the secretion in the urine have been demonstrated [12, 27]. Furthermore, a marked amelioration of ischaemic acute renal injury by intravenous administration of recombinant NGAL has been discovered [28].
In summary, our findings indicate, that urine NGAL is a valuable immediate biomarker for acute renal injury in paediatric patients undergoing cardiac surgery, preceding the increase in serum creatinine by 1–3 days. The determination of serum Cystatin C provides an efficient diagnostic device to detect the development and characterise the severity of an early deterioration of kidney function initiated by the ischaemic injury. The diagnosis of AKI can be assumed at a Cystatin C concentration of 0.995 mg/l, 2 hours after the end of CPB. In routine clinical practice both biomarkers each have a limited predictive value due to their spread distribution, but Cystatin C determination seems to have a major impact for the individual patient by predicting AKI. Additional measurement of urine NGAL, however, helps to assess kidney injury.
1 McIllroy DR, Wagner G, Lee TH. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clin J Am Soc Nephrol. 2010;5:211–9.
2 Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–24.
3 Thakar CV, Arrigain S, Worley S, Yared JP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–8.
4 Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: physiological principles. Intensive Care Med. 2004;30:33–7.
5 Simonsen O, Grubb A, Thysell H. The blood serum concentration of Cystatin C (gamma-trace) as a measure of the glomerular filtration rate. Scand J Clin Lab Invest. 1985;45:97–101.
6 Filler G, Bökenkamp A, Hoffmann W, Le Bricon T, Martinez-Bru C, Grubb A. Cystatin C as a marker of GFR- indications, and future research. Clin Biochem. 2005;38:1–8.
7 Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, et al. Early detection of acute renal failure by serum Cystatin C. Kidney Int. 2004;66:1115–22.
8 Coll E, Botey A, Alvarez L, Poch E, Quintó L, Saurina A, et al. Serum Cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36:29–34.
9 Bellomo R, Ronco C, Kellum JA, Metha R L, Palevsky P, ADQI workgroup. Acute renal failure-definition, outcome measures, animal models, fluid therapy and the information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12.
10 Al-Ismaili Z, Palijan A, Zappitelli M. Biomarkers of acute kidney injury in children: discovery, evaluation, and clinical application. Pediatr Nephrol. 2011;26:29–40.
11 Cruz DN, Goh CY, Haase-Fielitz A, Ronco C, Haase M. Early biomarkers of renal injury. Congest Heart Fail. 2010;16(Suppl 1):25–31.
12 Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinise-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–43.
13 Bolignano D, Donato V, Coppolino G, Campo S, Buemi A, Lacquaniti A, et al. Neutrophil gelatinise-associated lipocalin (NGAL) as a biomarker for acute kidney damage. Am J Kidney Dis. 2008;52:595–605.
14 Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes M, Barasch J, et al. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric surgery: a prospective uncontrolled cohort study. Crit Care. 2007;11:R127.
15 Krawczeski CD, Woo JG, Wang Y, Bennett MR, Ma Q, Devarajan P. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr. 2011;158:1009–15.
16 Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Quing M, Kelly C, et al. Neutrophil gelatinise-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8.
17 Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, et al. Urine-NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–73.
18 Koch AM, Dittrich S, Cesnjevar R, Rüffer A, Breuer C, Glöckler M. Plasma neutrophil gelatinase-associated lipocalin measured in consecutive patients after congenital heart surgery using point-of-care technology. Interact Cardiovascular Thorac Surg. 2011;13:133–6.
19 Krawczeski CD, Vandevoorde RG, Kathman T, Bennett MR, Woo JG, Wang Y, et al. Serum Cystatin C is an early predictive biomarker of acute kidney injury after pediatric cardiopulmonary bypass. Clin J Am Soc Nephrol. 2010;5:1552–7.
20 Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71:963–4.
21 Haase M , Bellomo R, Devarajan P, Ma Q, Bennett MR, Möckel M, et al. Novel biomarkers early predict the severity of acute kidney injury after cardiac surgery in adults. Ann Thorac Surgery. 2009;88:124–30.
22 Che M, Xie B, Xue S, Dai H, Qian J, Ni Z, et al. Clinical usefulness of novel biomarkers for the detection of acute kidney injury following cardiac surgery. Nephron Clin Pract. 2010;115:66–72.
23 Roos JF, Doust J, Tett SE, Kirkpatrick CM. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children a meta-analysis. Clin Biochem. 2007;40:383–91.
24 Zhang Z, Lu B, Sheng X, Jin N. Cystatin C in prediction of Acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis. 2011;58:356–65.
25 Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, Dragun D, et al. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery-a prospective cohort study. Crit Care Med. 2009;37:553–60.
26 Zappitelli M, Krawczeski CD, Devarajan P, Wang Z, Sint K, Thiessen-Philbrook H, et al. Early postoperative serum cystatin C predicts severe kidney injury following pediatric cardiac surgery. Kidney Int. 2011;80:655–62.
27 Devarajan P, Mishra J, Supavekin S, Patterson LT, Potter SS. Gene expression in early ischemic renal injury:clues towards pathogenesis, biomarker discovery and novel therapeutics. Mol Genet Metab. 2003;80:365–76.
28 Mishra J, Mori K, Ma Q, Dent C, Kelly C, Yang J, et al. Amelioration of ischemic acute injury by neutrophile gelatinase associated lipocalin. J Am Nephrol. 2004;15:3073–82.
Funding / potential competing interests: Reagents for measuring urine NGAL were provided free of cost by Abbott Diagnostics.