DOI: https://doi.org/10.4414/smw.2013.13721
Obesity has, in recent years, evolved into a worldwide epidemic [1]. Despite the investigation of meal patterns with varying macronutrient composition, it seems that the most effective diet leading to successful weight loss and maintenance has yet to be established [2]. Low-calorie diets (LCDs), in the form of energy-controlled, micronutrient-fortified liquid meals consumed as meal replacements, have often been prescribed for obese subjects or when rapid weight loss is a medical necessity [3]. Although not intended to act as a substitute for lifestyle modifications, LCDs appear popular, as they generally result in greater weight loss [4] and long-term weight maintenance [5, 6], compared to conventional diets. The success of LCDs is attributed to the production of greater satiety than alternative foods of equal or greater energy content [7]. LCDs, especially when combined with behavioural therapy and active follow-up with nutritional education and physical activity, are likely to lead to a larger long-term weight maintenance [5, 8–12], probably the result of the greater initial weight loss induced by such diets [9].
The efficacy of LCDs has not been investigated in large-scale studies or among people from different regions. DiOGenes (Diet, Obesity and Genes) is an integrated, family-based, randomised controlled dietary intervention study performed in eight European cities, examining dietary means of preventing weight regain after an initial 8-week run-in LCD weight-loss period [13–16]. To date, the majority of published research on LCDs has been undertaken in North America and North or Central European countries [17], including countries participating in DiOGenes (i.e., the Netherlands [18, 19], Denmark [20, 21], UK [22, 23], Germany [24], the Czech Republic [25, 26] and Spain [27, 28]. The aim of the current paper was to investigate the degree of weight loss, as well as changes in anthropometric and body composition measurements, among overweight/obese adults from eight European cities (from Northern, Central and Southern Europe) during the 8-week LCD phase of the DiOGenes study.
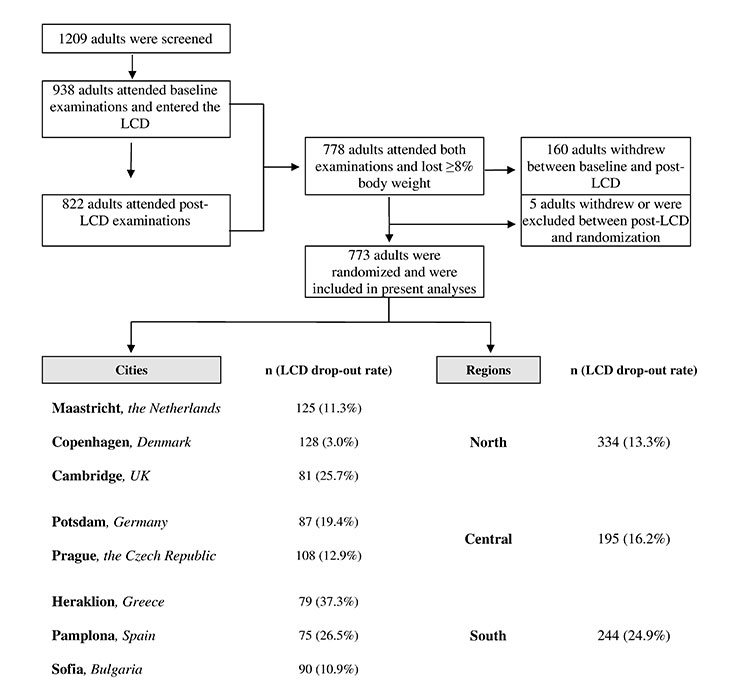
Volunteer families were recruited from eight cities across eight European countries: Maastricht (the Netherlands), Copenhagen (Denmark), Cambridge (United Kingdom), Heraklion (Greece), Potsdam (Germany), Pamplona (Spain), Sofia (Bulgaria) and Prague (Czech Republic). Recruitment commenced in March 2006 and was completed in April 2007. Families attended a screening examination to determine eligibility. Participating adults had to be healthy, overweight/ obese (27≤ body mass index (BMI, kg/m2) <45) and aged <65 y. A detailed description of recruitment strategies, adult exclusion criteria and screening examinations is provided elsewhere [14]. Informed consent was obtained from all participants and the study was approved by the local Medical Ethical Committees in the respective research centers, in accordance with the Helsinki Declaration.
Eligible adults underwent a clinical examination (representing ‘baseline’) following their screening, when anthropometric measures and body composition were assessed. Subsequently participants entered an 8-week LCD period, with the aim of obtaining a minimum weight loss of 8%. A large weight loss in a short time period was chosen, to motivate participants to adhere to the main phase of the study [14] and to select the participants who were likely to show compliance during the randomised phase of the DiOGenes intervention [14]. Participants met the research dieticians approximately every two weeks (a total of approximately 6 visits) during the LCD period for weighing, compliance assessment and dietary instructions. Participants who reported difficulties with adhering to or non-compliance to the LCD were excluded from the study. At the end of the 8-week LCD period, those participants who achieved the target weight loss underwent a second clinical examination (representing ‘post-LCD’) and were subsequently randomised to one of five energy ad libitumdiets for 6–12 months [13, 14, 29]. The present paper reports on these participants. In exceptional circumstances, e.g. when participants were unavailable to attend the post-LCD examination at exactly 8 weeks, less or more time on the LCD was allowed, provided the diet’s duration was between 7–9 weeks.
The energy-restricted LCD (MODIFAST®, Nutrition et Santé, France) consisted of 3.3 MJ/d (800 kcal/d), with a macronutrient composition of 15–20% of total energy from fat, 35–40% from protein and 45–50% from carbohydrates. The MODIFAST®products were available in 55 g sachets and included a range of products in a variety of flavours, namely powder drinks, crèmes and soups. Participants were required to consume a total of four sachets daily, eaten at intervals distributed across the day to replace breakfast, lunch, dinner and one snack-meal. This provided them with a daily intake of 54 g protein, ~5 g essential fatty acids and the daily requirement for vitamins and minerals. In exceptional cases, when lack of satiety was reported, a fifth sachet could be consumed.
Participants were free to add spices, herbs and low-calorie/calorie-free flavourings (i.e., calorie-free juice, coffee or instant coffee) to increase variety and taste of the products. In addition to the four sachets, participants were also permitted without limitation to: drink coffee and tea (if necessary adding a little skimmed milk); drink sufficient quantities of water; drink calorie-free soft drinks; and chew sugar-free chewing gum/pastilles. Further, it was permitted to eat 200 g tomatoes, 125 g cucumber and 50 g lettuce on a daily basis. Thus, participants were provided with 3.3–4.2 MJ/d (800–1000 kcal/d).
Standard operation procedures were produced for all investigations undertaken to ensure standardisation across the cities and the same measurement devices and methods were used in each research centre on every occasion a measurement was provided [14]. Subjects had been fasting for twelve hours before the clinical examinations in the early morning and they were measured in their underwear, with an empty bladder.
Weight was measured on calibrated digital scales to the nearest 0.1 kg on all examinations and dietary counselling sessions, and height was measured at baseline, to the nearest mm with a wall-mounted stadiometer. BMI was calculated as weight divided by height squared (kg/m2). Waist circumference (WC), measured midway between the lower rib and iliac crest) and hip circumference (HC), measured at the widest point between the hips and buttocks) were measured twice, to the nearest 0.5cm, with a tape in a vertical plane and with the subject standing and gently breathing out. Waist-to-hip ratio was then calculated. Sagittal abdominal diameter was measured using an abdominal caliper to the nearest mm, at the highest point of the abdomen during expiration, with subjects supine [14].
Fat mass (kg), body fat percentage and fat-free mass (kg, defined as the sum of lean body mass and bone mineral content) were determined by dual-energy X-ray absorption (DXA) or by bioelectric impedance analysis (BIA). Adults who participated in the determination of body composition were fasting (no intake of foods or liquids for at least 4 hours prior to the examinations, except for a water intake of 350–500 mL).
All analyses were performed using the Statistical Package for the Social Sciences (IBM SPSS for Windows, release 19, 2010, SPSS, Chicago, Illinois). Drop-out from the study during the 8-week LCD phase (non-completers) was determined by assessing the number of participants who entered the LCD phase and the number who successfully completed the LCD and were randomised to the main DiOGenes intervention (completers). For both LCD completers and non-completers the distribution of participants according to gender was compared between the different cities using the Pearson’s chi-square test. Differences in gender distribution and age between completers and non-completers were assessed using Pearson’s chi-square test and analysis of variance respectively. Age of participants and LCD duration (in weeks) were compared between the cities using the Kruskal Wallis rank test. Comparisons of overall changes in anthropometric measurements and body composition during the LCD period between Northern (Maastricht, Copenhagen, Cambridge), Central (Prague, Potsdam) and Southern (Heraklion, Pamplona, Sofia) European regions were performed using analysis of covariance, with age, gender and LCD duration as covariates and pair-wise comparisons, with age, gender, baseline body fat percentage and LCD duration as covariates.
A total of 1 209 adults were registered to attend screening and 938 (77.6%) attended the baseline examination and entered the LCD period. Of these, 773 (82.4%) adults (273 males, 500 females, mean age, 43.1 y) successfully completed the LCD and were included in the present analyses. Overall, 165 adults (17.6% of those who entered the LCD) dropped out or were excluded during the LCD. The distribution of participants across different cities and regions is shown in figure 1. The highest drop-out was observed in Heraklion, Pamplona and Cambridge, and the lowest in Copenhagen. The combined analyses of cities according to region showed that mean drop-out was higher in Southern (24.9%) than in Central (16.2%) or Northern European (13.3%) cities (fig. 1). Among non-completers of the LCD period, there was no difference between the cities in the distribution of genders (P= 0.869, data not shown). Non-completers were younger (41.2 vs. 43.1y, P<0.001) than LCD completers.

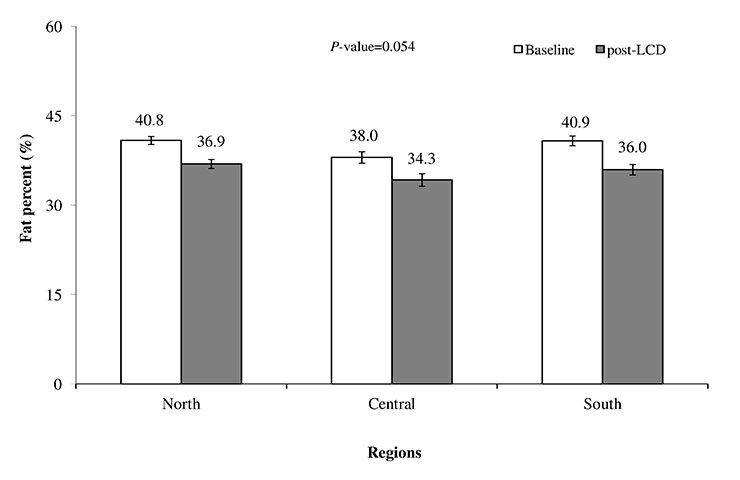
Figure 2
Body fat percentage over the LCD period in the DiOGenes participating regions, 2006–2007. LCD = low-calorie diet; bars (I) indicate 95% confidence intervals; comparisons in body fat percentage changes between regions were performed using analysis of covariance, with age, gender, baseline body fat percentage and LCD duration (weeks) as covariates.
Among cities, gender distribution differed significantly (P= 0.033). Participants in Heraklion were significantly younger (P<0.001) and followed the LCD for a longer period (P<0.001), compared to other cities (table 1).
The mean and percental changes in anthropometric and body composition measurements during the LCD period in the three different European regions and the total sample are shown in table 2. Overall, the LCD induced favuorable changes in all measured outcomes and an 11.1% (11.1 kg) and 11.0% reduction in body weight and fat percentage, respectively. Changes in all outcomes, except body weight and BMI, differed significantly between the regions. In general, participants in the Northern and Central European regions achieved higher reductions in most anthropometric measurements assessed, compared to the Southern European region (table 2). However, participants from the Southern European cities reduced their fat mass significantly more (–21.5%, P<0.01) and their fat-free mass significantly less (–3.6%, P<0.01), compared to participants from the Northern (–19.3% and -5.7%, respectively) and Central (–19.6% and –5.7%, respectively) European regions. Body fat percentage changes during the LCD period tended to differ between the groups of cities (P= 0.054) (fig. 2). Pair-wise comparisons revealed that participants in the Southern region reduced their body fat significantly more than participants in the Northern European region (P= 0.017) (fig. 2).
| Table 1: Distribution of gender, participant age and LCD duration in the DiOGenes participating cities (n 773), 2006–2007. | ||||||||
| Gender | Age (y) | LCD duration (weeks) | ||||||
| Males | Females | |||||||
| n | % | Pa | Mean (SD) | Pb | Mean (SD) | Pb | ||
| Maastricht | 125 | 39.2 | 60.8 | 0.033 | 43.8 (5.9) | <0.001 | 8.2 (1.4) | <0.001 |
| Copenhagen | 128 | 40.6 | 59.4 | 43.5 (5.4) | 8.3 (1.4) | |||
| Cambridge | 81 | 25.9 | 74.1 | 43.9 (7.2) | 8.0 (1.2) | |||
| Heraklion | 79 | 36.7 | 63.3 | 40.1 (5.8) | 9.0 (1.2) | |||
| Potsdam | 87 | 39.1 | 60.9 | 42.6 (6.1) | 8.7 (1.1) | |||
| Pamplona | 75 | 45.3 | 54.7 | 44.3 (5.6) | 7.8 (1.5) | |||
| Sofia | 90 | 28.9 | 71.1 | 44.0 (7.5) | 7.9 (0.9) | |||
| Prague | 108 | 25.9 | 74.1 | 42.3 (6.6) | 7.9 (1.3) | |||
| Total | 773 | 35.3 | 64.7 | 43.1 (6.3) | 8.2 (1.3) | |||
| LCD = low-calorie diet; SD = standard deviation; a the distribution of gender at the different centres is compared using Pearson chi-square test; b age and LCD duration at the different centres are compared using Kruskal Wallis rank test. | ||||||||
| Table 2: Mean (SD) and percental changes in anthropometric and body composition measures over the LCD period in the DiOGenes participating regions (n 773), 2006–2007. | |||||
| Regions | |||||
| Measurement | North | Central | South | Total | |
| Weight (kg) | Baseline | 99.1 (16.0) | 100.0 (16.0) | 101.5 (16.0) | 100.0 (16.1) |
| (n = 773) | Mean change | –11.3 (3.3) | –11.1 (3.3) | –10.8 (3.3) | –11.1 (3.3) |
| % change | –11.4 | –11.1 | –10.6 | –11.1 | |
| BMI (kg/m2) | Baseline | 33.7 (4.9) | 33.7 (4.9) | 36.1 (4.8) | 34.6 (5.0) |
| (n = 771) | Mean change | –3.8 (1.1) | –3.7 (1.1) | –3.6 (1.1) | –3.8 (1.1) |
| % change | –11.3 | –11.0 | –10.0 | –11.0 | |
| WC (cm) b | Baseline | 107.4 (11.9) | 105.7 (11.9) | 109.8 (11.9) | 107.9 (11.9) |
| (n = 756) | Mean change | –10.2 (4.7) | –10.2 (4.7) | –8.9 (4.7) | –9.7 (4.8) |
| % change | –9.5 | –9.6 | –8.1 | –9.0 | |
| HC (cm) b | Baseline | 114.8 (10.3 | 116.6 (10.3) | 119.3 (10.3) | 117.1 (10.3) |
| (n = 756) | Mean change | –6.9 (4.1) | –7.9 (4.0) | –7.1 (4.0) | –7.2 (3.9) |
| % change | –6.0 | –6.8 | –6.0 | –6.1 | |
| Waist-to-hip ratio b | Baseline | 0.937 (0.073) | 0.908 (0.069) | 0.921 (0.061) | 0.923 (0.055) |
| (n = 756) | Mean change | –0.036 (0.055) | –0.029 (0.042) | –0.022 (0.045) | –0.029 (0.055) |
| % change | –3.8 | –3.2 | –2.4 | –3.1 | |
| Sagittal height (cm) a | Baseline | 24.4 (3.6) | 26.2 (3.5) | 25.8 (3.5) | 25.4 (3.4) |
| (n = 727) | Mean change | –3.6 (1.7) | –3.4 (1.7) | –2.7 (1.7) | –3.2 (1.7) |
| % change | –14.8 | –13.0 | –10.5 | –12.6 | |
| FM (kg) b | Baseline | 40.9 (10.9) | 37.7 (10.9) | 42.4 (10.9) | 40.3 (10.9) |
| (n = 563) | Mean change | –7.9 (4.4) | –7.4 (4.4) | –9.1 (4.4) | –8.3 (4.4) |
| % change | –19.3 | –19.6 | –21.5 | –20.6 | |
| FFM (kg) b | Baseline | 59.2 (8.4) | 61.6 (8.4) | 60.9 (8.4) | 60.1 (8.1) |
| (n = 563) | Mean change | –3.4 (4.0) | –3.5 (4.0) | –2.2 (4.0) | –2.8 (4.0) |
| % change | –5.7 | –5.7 | –3.6 | –4.7 | |
| Fat percent (%) b | Baseline | 40.8 (5.6) | 38.0 (5.6) | 40.9 (5.6) | 40.0 (5.5) |
| (n = 563) | Mean change | –3.9 (3.9) | –3.7 (3.9) | –4.8 (3.9) | –4.4 (3.9) |
| % change | –9.5 | –9.7 | –11.8 | –11.0 | |
| LCD = low-calorie diet; SD = standard deviation; BMI = body mass index; WC = waist circumference; HC = hip circumference; FM = fat mass; FFM = fat-free mass. Comparisons of overall mean changes between regions were performed using analysis of covariance, with age, gender and LCD duration (weeks) as covariates. a P<0.001, b P<0.01. | |||||
LCDs have frequently been scientificall evaluated in the last decades, as they result in rapid weight loss, which renders this method popular among obese subjects who consider it acceptable [6, 30] and are likely to help achieve long-term weight loss maintenance [6, 9, 24], compared to conventional diets. In the present study, overweight/obese adult volunteers from 8 European cities participating in the DiOGenes study followed an LCD for a mean of 8.2 weeks and lost an average of 11.1% body weight. Such a reduction in body weight has been associated with significant improvements in obesity-related disorders and complications [11, 24]. In addition, the LCD produced favorable changes in a variety of anthropometric and body composition measurements across different European regions, indicating its efficacy in a large-scale study where a large weight loss in a short time period was required.
Participants in the present study lost an average of 2.8 kg (4.7%) of fat-free mass, corresponding to 25.2% of the weight lost, which is comparable to earlier research and considered acceptable in weight loss efforts [18, 31–34]. Although earlier studies have been inconsistent with regard to the role of initial BMI and body composition in the loss of fat-free mass and body weight respectively [18, 33], we accounted for any potential effect of baseline values by examining absolute and percentage changes in these parameters. Nevertheless, weight reduction in the present study was mainly attributed to reduction in body fat. It should be noted however, that comparisons with earlier studies are hindered, due to differences in diet type and energy content (LCD vs. very-low-calorie diets), LCD definition and duration and baseline characteristics of participants, such as initial BMI. DiOGenes is, to the best of our knowledge, the first study to provide an opportunity for a comparison of LCD efficacy between different European regions. All eight research centres successfully implemented the LCD, although changes in the majority of outcome measures differed significantly between regions, despite standardisation of protocols and procedures. In general, participants in the Northern and Central European regions achieved higher percentage reductions in most anthropometric measurements assessed, compared to participants from Southern Europe. Nonetheless, participants in the Southern European region generally achieved significantly higher reductions in body fat percentage.
Drop-out in the present study was slightly higher than withdrawal rates reported in some earlier studies [35, 36], but lower compared to the only other multi-centre study we could identify, which comprised a national intervention in the USA [37], and to attrition reported in the review by Tsai & Wadden [2006]. In DiOGenes, most subjects who did not complete the LCD period withdrew during the first half of the LCD period. No severe adverse effects were reported and participants were excluded if they reported non-compliance with the treatment or voluntarily withdrew. It is usual in weight loss studies that patients who do not achieve the intended outcomes are more likely to drop out [11] and it has recently been suggested that early weight loss is a predictor of final weight loss during an 8-week LCD [38]. In the present study, many of the participants who withdrew reported that, although initially driven to participate due to the rapid weight loss to be induced by the LCD, they realized that this treatment method did not agree with their lifestyle, such as outdoor eating obligations.
The marked differences in drop-out between different research centres, e.g. 3% in Copenhagen and ~37% in Heraklion, and regions, require further investigation. However, participants in Heraklion who completed the LCD achieved higher percentage changes in anthropometric measurements, compared to the other Southern European cities, and the biggest changes in body composition measurements among all participating cities (data not shown). One might argue that these changes are the result of the longer LCD period in this research centre, thus allowing more time to achieve favourable results. However, it has been suggested that increases in LCD duration of more than 8 weeks result in adherence difficulties and reduced compliance, due to lack of dietary variation [34]. In addition, LCD duration was taken into account in the present analyses. As participants in Heraklion also had one of the highest percentage body weight reductions among all participating cities (data not shown), it may be that the weight loss achieved encouraged higher degrees of compliance among participants not withdrawing from the LCD period early on in the procedure. Issues of palatability/familiarity with meal replacement products should also be considered when attempting to clarify the present drop-out rates. For example, soups provided in the current study were asparagus and potato and leek soups, which are not part of the usual Greek diet and might have discouraged some participants from Heraklion from continuing on the LCD phase. Therefore, future studies utilizing meal replacement products to promote weight loss should ideally provide products with flavours similar to local cuisines. Nevertheless, future studies should include objective measures of palatability/acceptability of LCDs, instead of relying on self-reports of non-compliance with the treatment, in order to better understand differences in compliance, drop-out and effects among different regions.
In the present study, no severe adverse effects of the LCD were reported. Nonetheless, it should be noted that such diets are not intended to substitute long-term behavioural modification for weight loss/control. Indeed, the superiority of LCDs to conventional diets has been questioned in the past [9, 11], whereas the combined treatment of LCDs and behavioural therapy appears to be more effective, compared to LCD alone, especially with regard to long-term weight maintenance [5, 11].
To the best of our knowledge, this is the first study to investigate the effect of LCDs on anthropometric measurements and body composition in such a large and diverse sample of overweight/obese individuals. The current findings might therefore prove helpful to scientists intending to use this treatment method at a national level in the future. However, it should be noted that our sample was not representative of the general population in either the respective cities or countries, and thus our findings cannot be generalised. A limitation of the current study is that physical activity levels during the LCD were not assessed. Thus, we cannot rule out that the observed favourable changes in obesity measures were not the combined result of the LCD and increased physical activity. However, and also for reasons of standardisation assurance across the research centres, subjects were particularly requested not to change their usual lifestyle habits, including physical activity, during the LCD period. Nevertheless, it is noteworthy that studies specifically designed to examine the effect of physical exercise revealed no additional weight loss when physical exercise was added to the LCD treatment [39]. In addition, our database did not provide information on more specific acceptability measures, such as satiety produced by the diet or the taste of the LCD products. Such measurements should, if possible, be assessed during treatment to provide an additional estimate of product acceptability and explain potential drop-out differences. Furthermore, the LCD in this study was not compared to any other treatment, so recommendations regarding its efficacy might seem inappropriate. Nevertheless, this study provides additional evidence on the effect of LCDs in a free-living situation and shows this treatment’s potential for use in different regions.
In conclusion, the LCD significantly improved anthropometric and body composition measurements in participants in all cities participating in the DiOGenes study. Future studies should examine objective acceptability measures of this treatment in order to further explain regional differences in the effect of this diet on obesity.
1 World Health Organisation. Diet, nutrition and the prevention of chronic diseases. Geneva:Joint FAO/WHO; 2002.
2 Franz MJ, Bantle JP, Beebe CA, Brunzell JD, Chiasson JL, Garg A, et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2002;25:148–98.
3 National Task Force on the Prevention and Treatment of Obesity. Very-low-caloric-diets. JAMA. 1993;270:967–74.
4 Donnelly JE, Jakicic J, Gunderson S. Diet and body composition. Effect of very low calorie diets and exercise. Sports Med. 1991;12:237–49.
5 Saris WHM. Very-low-calorie diets and sustained weight loss. Obes Res. 2001;9:295S–301S.
6 Jebb SA, Goldberg GR. Efficacy of very low-energy diets and meal replacements in the treatment of obesity. J Hum Nutr Diet. 1998;11:219–25.
7 Kovacs EMR, Cheatham BR, Meullenet JF, Mela DJ. Comparing satiety profiles of different foods: The example of meal replacement drinks. Appetite. 2008;50:561.
8 Torgerson JS, Lissner L, Lindroos AK, Kruijer H, Sjöström L. VLCD plus dietary and behavioural support versus support alone in the treatment of severe obesity. A randomised two-year clinical trial. Int J Obes Relat Metab Disord. 1997;21:987–94.
9 Astrup A, Rössner S. Lessons from obesity management programmes: greater initial weight loss improves long-term maintenance. Obes Rev. 2000;1:17–9.
10 Miura J, Arai K, Tsukahara S, Ohno M, Ikeda Y. The long term effectiveness of combined therapy by behavior modification and very low calorie diet: 2 years follow-up. Int J Obes. 1989;13:73–7.
11 Ayyad C, Andersen T. Long-term efficacy of dietary treatment of obesity: a systematic review of studies published between 1931 and 1999. Obes Rev. 2000;1:113–99.
12 Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74:579–84.
13 Moore CS, Lindroos AK, Kreutzer M, Larsen TM, Astrup A, van Baak M, et al. Strategy to manipulate ad libitum macronutrient intake, and glycaemic index, across eight European countries in the DIOGENES study. Obes Rev. 2010;11:67–75.
14 Larsen TM, Dalskov S, van Baak M, Jebb S, Kafatos A, Pfeiffer A, et al. The Diet, Obesity and Genes (Diogenes) dietary study in eight European countries – a comprehensive design for long term intervention. Obes Rev. 2010;11:76–91.
15 Saris WH, Harper A. DiOGenes: a multidisciplinary offensive focused on the obesity epidemic. Obes Rev. 2005;6:175–6.
16 Papadaki A, Linardakis M, Larsen TM, van Baak M, Lindroos AK, Pfeiffer AFH, et al. The effect of protein and glycemic index on children’s body composition: The Diogenes randomized study. Pediatrics. 2010;126:e1143–52.
17 Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and meta-analysis. Obesity. 2006;14:1283–93.
18 Claessens M, van Baak MA, Monsheimer S, Saris WHM. The effect of a low-fat, high protein or high-carbohydrate ad libitum diet on weight loss maintenance and metabolic risk factors. Int J Obes. 2009;33:296–304.
19 Westerterp-Plantenga MS, Kempen KP, Saris WH. Determinants of weight maintenance in women after diet-induced weight reduction. Int J Obes Relat Metab Disord. 1998;22:1–6.
20 Gasteyger C, Larsen TM, Vercruysse F, Pedersen D, Toubro S, Astrup A. Visceral fat loss induced by a low-calorie diet: a direct comparison between women and men. Diabetes Obes Metab. 2009;11:596–602.
21 Gasteyger C LT, Vercruysse F, Astrup A. Effect of a dietary-induced weight loss on liver enzymes in obese subjects. Am J Clin Nutr. 2008;87:1141–7.
22 Kreitzman SN, Beeson V. Obese patients in UK general practices lose 16 tonnes. VLCD Update. 1996;3:5–8.
23 Packianathan I, Sheikh M, Boniface D, Finer N. Predictors of programme adherence and weight loss in women in an obesity programme using meal replacements. Diabetes Obes Metab. 2005;7:439–47.
24 Ditschyneit HH, Flechtner-Mors M. Value of structured meals for weight management: risk factors and long-term weight maintenance. Obes Res. 2001;9 284S–9S.
25 Anderlová K, Kremen J, Dolezalová R, Housová J, Haluzíková D, Kunesová M, et al. The influence of very-low-calorie-diet on serum leptin, soluble leptin receptor, adiponectin and resistin levels in obese women. Physiol Res. 2006;55:277–83.
26 Hainer V, Štich V, Kunešová M, Parízková J, Žák A, Wernischová V, et al. Effect of 4-wk treatment of obesity by very-low-calorie diet on anthropometric, metabolic, and hormonal indexes. Am J Clin Nutr. 1992;56:281S–2S.
27 Solá E, Vayá A, Contreras T, Falcó C, Corella D, Hernández A, et al. Effect of a hypocaloric diet on lipids and rheological profile in subjects with severe and morbid obesity. A follow-up study. Clin Hemorheol Microcirc. 2004;30:419–22.
28 Moreno O, Meoro A, Martinez A, Rodriguez C, Pardo C, Aznar S, et al. Comparison of two low-calorie diets: a prospective study of effectiveness and safety. J Endocrinol Invest. 2006;29:633–40.
29 Larsen TM, Dalskov SM, van Baak M, Jebb S, Papadaki A, Pfeiffer AF, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102–13.
30 Moloney M. Dietary treatments of obesity. Proc Nutr Soc. 2000;59:601–8.
31 Morgan WD, Ryde SSJ, Birks JL, Thomas DW, Kreitzman SN. Changes in total body nitrogen during weight reduction by very-low-calorie diets. Am J Clin Nutr. 1992;56:262S–4S.
32 Kamrath RO, Plummer LJ, Sadur CN, Weinstein RL. Body composition and weight maintenance with a very-low-calorie diet for the treatment of moderate obesity. Am J Clin Nutr. 1992;56:286S–7S.
33 Hoie LH, Bruusgaard D, Thom E. Reduction of body mass and change in body composition on a very low calorie diet. Int J Obes Relat Metab Disord. 1993;17:17–20.
34 Foster GD, Wadden TA, Peterson FJ, Letizia KA, Bartlett SJ, Conill AM. A controlled comparison of three very-low-calorie diets: effects on weight, body composition and symptoms. Am J Clin Nutr. 1992;55:811–7.
35 Ryttig KR, Rössner S. Weight maintenance after a very low calorie diet (VLCD) weight reduction period and the effects of VLCD supplementation. A prospective, randomized, comparative, controlled long-term trial. J Intern Med. 1995;238:299–306.
36 Høie LH, Bruusgaard D. Compliance, clinical effects, and factors predicting weight reduction during a very low calorie diet regime. Scand J Prim Health Care. 1995;13:13–20.
37 Wadden TA, Foster GD, Letizia KA, Stunkard AJ. A multicenter evaluation of a proprietary weight reduction program for the treatment of marked obesity. Arch Intern Med. 1992;152:961–6.
38 Handjieva-Darlenska T, Handjiev S, Larsen TM, van Baak MA, Jebb S, Papadaki A, et al. Initial weight loss on an 800-kcal diet as a predictor of weight loss success after 8 weeks: the Diogenes study. Eur J Clin Nutr. 2010;64:994–9.
39 Saris WHM. Exercise with and without dietary restriction and obesity treatment. Int J Obes. 1995;19:S113–6.