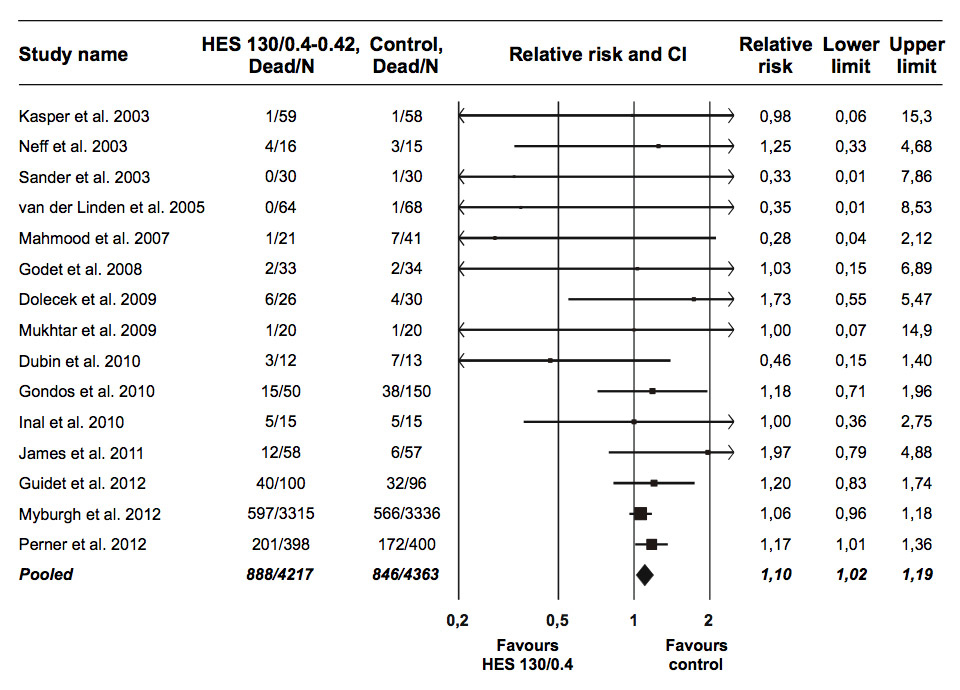
Figure 1
Relative mortality risk after HES 130/0.4–0.42 administration in randomised trials. Error bars depict CI. Scaling of data points according to meta-analytic weight.
CI = 95% confidence interval; HES = hydroxyethyl starch.
DOI: https://doi.org/10.4414/smw.2013.13747
The clinical utility of hydroxyethyl starch (HES) solutions shows substantial limitations due to characteristic complications such as kidney failure, impairment of coagulation, pruritus, and has the potential to increase mortality [1, 2]. Nevertheless, it continues to be argued that “modern” HES solutions with a comparatively low average molecular weight of 130 kDa and low average substitution of 0.4–0.42 may mitigate the risks. However, a recent meta-analysis of 13 randomised controlled trials with a total of 1131 patients revealed a trend toward increased mortality in recipients of HES 130/0.4, as judged by a pooled relative risk (RR) of 1.14 with a 95% confidence interval (CI) of 0.89 to 1.46 [3]. This trend became even stronger after adjustment for significant publication bias (RR: 1.25; CI: 0.98 to 1.58) but still did not reach statistical significance (p = 0.069). It was noted in the meta-analysis that major new evidence on the safety of modern HES would be shortly forthcoming from the Scandinavian Starch for Severe Sepsis/Septic Shock (6S) trial and the Crystalloid versus Hydroxyethyl Starch Trial (CHEST). Those trials have now been reported [4, 5], and their results have been used to update the meta-analysis.

Figure 1
Relative mortality risk after HES 130/0.4–0.42 administration in randomised trials. Error bars depict CI. Scaling of data points according to meta-analytic weight.
CI = 95% confidence interval; HES = hydroxyethyl starch.
The 6S trial compared 6% HES 130/0.42 with Ringer’s acetate in 798 patients with severe sepsis, while CHEST compared 6% HES 130/0.4 with 0.9% NaCl in 7000 intensive care unit (ICU) patients. Renal replacement therapy and blood product transfusion were significantly increased by modern HES in both trials. Additionally, in the 6S trial, mortality at 90 days was significantly increased by HES 130/0.42 (RR: 1.17; CI: 1.01‒1.36). Mortality at 90 days was also higher among patients allocated to HES 130/0.4 in CHEST (RR: 1.06; CI: 0.96‒1.18), although the difference was not statistically significant (p = 0.26). The inability to demonstrate a significant difference in CHEST may have been at least partly due to the low mortality risk in this general ICU population, as indicated by the control group mortality rate of only 17% at 90 days, and to the low average daily HES 130/0.4 dose of 526 ml per patient.
Addition of the 6S and CHEST data increases the total patients in the meta-analysis up to 8580 (fig. 1). There was no evidence of heterogeneity (I2: 0%; CI: 0%‒27%; p = 0.84). Mortality was significantly increased by HES 130/0.4-0.42 (RR: 1.10; CI: 1.02‒1.19; p = 0.018). Despite the addition of the two new trials, significant publication bias persisted in the meta-analysis (p = 0.048), although its impact was greatly reduced compared with that in the original meta-analysis, and after adjustment by trim and fill the pooled effect size was hardly changed (RR: 1.11; CI: 1.02‒1.20; p = 0.014).
This is the first meta-analysis to demonstrate a statistically significant increase in mortality attributable to modern HES. Evidence has previously been reported of increased mortality among patients receiving HES 450/0.7 [6] and HES 200/0.5 [1]. Taken together with those results, the present meta-analysis suggests that increased mortality is a class effect of HES solutions.
1 Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–39.
2 Navickis RJ, Haynes GR, Wilkes MM. Effect of hydroxyethyl starch on bleeding after cardiopulmonary bypass: a meta-analysis of randomized trials. J Thorac Cardiovasc Surg. 2012;144(1):223–30.
3 Wiedermann CJ, Joannidis M. Mortality after hydroxyethyl starch 130/0.4 infusion: an updated meta-analysis of randomized trials. Swiss Med Wkly. 2012;142(30 July):w13656.
4 Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Åneman A, et al. Hydroxyethyl starch 130/0.4 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367(2):124–34.
5 Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):190–11.
6 Sedrakyan A, Gondek K, Paltiel D, Elefteriades JA. Volume expansion with albumin decreases mortality after coronary artery bypass graft surgery. Chest. 2003;123(6):1853–7.
Funding / potential competing interests: CJW has received fees for speaking from CSL Behring, Kedrion and Baxter. MJ has received fees for speaking from Fresenius, Baxter and CSL Behring.