
Table 1
Epidemiologic data on the relation between air pollutants and neuropsychological outcomes in children.
DOI: https://doi.org/10.4414/smw.2012.13322
Mental capital, the cognitive and emotional resources, is a life-course function increasing (or developmental phase) during early childhood, that starts to flatten after age 4 up to youth when it reaches a plateau phase until the fourth-fifth decade of life. Afterwards, it starts a small decline that accelerates after the seventh decade [1]. In the growing phase, maturation of the cortex (i.e., wiring: synaptic changes, and axonal myelination) during the first years of life is very intensive, and the frontal cortex is the last to mature [2]. This period of life is considered an important window for brain development, since the brain’s plasticity decreases with age, and a long period of vulnerability in the developmental process where susceptibility to environmental insults is elevated [3]. Environmental factors may also play a role in accelerating the decline phase.
Traffic-related air pollution, basically urban outdoor pollution, is a global public health problem. Cardio-respiratory effects and mechanisms have been fully investigated [4–7]. By contrast, the influence of air pollution on the brain is unknown, with only some preliminary evidence [8].
The major suspected culprit of the systemic health effects of traffic air pollution are the ultrafine particles (UFP; i.e., atmospheric particles with aerodynamic diameter of <100 nm) [9]. Particles from vehicle emissions can be divided into primary particles formed in the vehicle and secondary particles formed in the atmosphere after emission. Primary particles are insoluble agglomerates of carbonaceous material which may contain metallic ash and adsorbed or condensed hydrocarbons; secondary particles, volatile and comprised mainly of hydrocarbons, are generally in the nanoparticle size range (below 30 nm) and mostly soluble. Small insoluble particle size allows better penetration and diffusion and major particle deposition in the respiratory tract, translating to a systemic reaction as well as direct translocation in the brain [10]. There is little information on the trend in UFP in European urban atmospheres, but the increased load of diesel vehicles and recent data [11–12] suggest an upward trend. In cities such as Barcelona, traffic is the origin of 90% of the UFP [13].
In rats, intratracheal instillation of particles less than 100 nm labeled with radioactivity was subsequently detected in several organs, including the brain [14]. Ultrafine carbon particles [15] and Manganese (Mn) nanoparticles [16] have been found in the olfactory bulb and the cerebrum and cerebellum after inhalation. Another pathway of deposition of particles into the brain suggested particulate matter (PM) >200 nm (TiO2) may be phagocytised by macrophages and dendritic cells which may carry the particles to lymph nodes in the lung or to those closely associated with the lungs [17]. Changes in cytokine expression in brain mice have been directly linked to intranasal exposure to ultrafine carbon [18].
Animals exposed to high levels of air pollution, such as fine and ultrafine PM, lipopolysaccharides associated with PM, ozone, and diesel engine exhaust, showed an increase of proinflammatory cytokines in brain tissue [19–23]. Of special interest is the sequence of studies by Calderon-Gacidueñas et al. with dogs exposed to Mexico City air. Healthy dogs younger than 1 year exhibited neuroinflammation along with disruption of the blood–brain-barrier and accumulation of beta amyloid 42 [24] which was also observed in adult dogs [25]. Furthermore, dogs exhibited frontal white matter upregulation of two important inflammatory genes: Cyclooxygenase-2 (COX2) and interleukin-1beta (IL-1β), as well as diffuse vascular changes [19]. Noteworthy also a study on rats showing that levels of the pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-1alpha (IL-1α) were dependent on the region analysed and increased in the striatum after exposure to diesel exhaust [21]. Recently, Levesque et al. found that rats exposed to diesel exhaust by inhalation had increased levels of IL-6 protein, nitrated proteins, and ionised calcium binding adaprot molecule-1 (IBA-1) protein (microglial marker) in the whole brain, indicating generalised neuroinflammation [22]. Moreover, diesel exhaust increased TNFα, IL-1β, IL-6, macrophage inflammatory protein-1alpha (MIP-1α), receptor for advanced glycation (RAGE), fractalkine, and the IBA-1 microglial marker in most regions tested, showing a greater response in the midbrain.
Autopsies from children and young adult residents in Mexico City showed a significant upregulation of COX2, IL1β and cluster of differentiation 14 (CD14) in olfactory bulb, frontal cortex, substantia nigrae and vagus nerves, disruption of the blood-brain-barrier, endothelial activation, oxidative stress, and inflammatory cell trafficking [26]. In a second study on autopsies from children and young adults residents in Mexico, UPF were found to accumulate in the respiratory nasal epithelium as well as in olfactory bulb neurons and in the endothelium and basement membranes of olfactory bulb arterioles together with immunoreactivity to beta-amyloid 42 and alpha-synuclein [27].
Currently, there are some epidemiological studies in children that translate for the first time evidence of the neuropsychological developmental hazards of air pollution from animal studies into humans (table 1) [26, 28–38]. All these studies have used well validated and widely used neuropsychological tests in order to assess several cognitive areas including global intelligence quotient (IQ), language development, or executive function, and motor development. These neuropsychological tests were administered in general by trained interviewer or psychologist and recently by computerised testing. Furthermore, behavioural outcomes such as attention-deficit hyperactivity disorder (ADHD) symptoms or autism disorder were assessed by questionnaires reported by the mother/teacher.

Table 1
Epidemiologic data on the relation between air pollutants and neuropsychological outcomes in children.
The first study assessed the relation between polycyclic aromatic hydrocarbons (PAH) in particulate mode – collected with individual pumps during two consecutive days in a small sample of nonsmoking pregnant women from New York City (USA) – and mental and psychomotor development and behaviour problem measured in the offspring at different ages from 1 to 5 years old [31–32]. They found that a reduced cognitive development emerged at 3 years old, while no association was shown at younger ages. Moreover, no psychomotor development delay or increased behaviour problem was found at any age. A small cohort was set-up in Krakow (Poland) following the same design and measurements for the air pollution exposure during pregnancy [28]. A reduced IQ score was shown at 5 years old. A similar inverse association between PAH exposure and child IQ at 5 years old has been found in these both studies despite the different levels of PAH observed in each study. However, though these studies were adjusted for potential confounders, such as socio-economic conditions, maternal IQ, or internal doses of lead, they were based on a short measurement of the exposure – only two days – and an air pollution biomarker with low specificity, the PAH. The principal source of PAH is tobacco smoke. Though only nonsmoking pregnant women were included and results were adjusted for second-hand smoking exposure or cotinine levels, these design limitations resulted in preliminary research being not very conclusive. Regarding PAH exposure, another study was carried out in Tongliang, Chongquing (China) where a seasonal coal-fired power plant was operating [30, 35]. Two identical small prospective cohorts enrolled nonsmoking pregnant women and their newborn until 2 years old, one before and the other after the shutdown of the coal-burning plant. Prenatal PAH exposure was measured by PAH-DNA adducts in umbilical cord blood. Before the power plant shutdown, decreases in motor and global IQ were associated with increased cord blood levels of PAH-DNA adducts [35]. However, the closure of the power plant was followed by a significant reduction of the mean PAH-DNA adduct levels, a significant improvement of the social developmental quotient, and a non significant association between PAH-DNA adducts and any infant developmental quotients [30]. These studies were the only using biomarkers of exposure, although PAH-DNA is not specific of urban air pollution.
Some other studies have been focused on environmental assessment of more specific markers of traffic-related air pollution using geographic systems such as land use regression modeling based on nitrogen dioxide (NO2), benzene, black carbon environmental measures and, distance to main road. In the largest study conducted so far, adverse effects of residential NO2 and benzene exposure during the whole pregnancy – based on land use regression modeling – on infant mental development around 14 months were observed among subjects with low exposure to maternal consumption of fruits and vegetables during pregnancy in 4 Spanish regions [38]. These results were based on a large sample size and were very stable in several sensitivity analyses such as adjusting and stratifying for socio-economic factors, noise, cord blood lead, indoor air pollution, or smoking exposure. This was the only study that took into account noise exposure as a potential confounder although it was self-reported noise annoyance instead of a direct measure of noise levels. However, children were only 1.5 years old. Another study in a small cohort of children 4 years old carried out in Granada (Spain) assessed the role of residential NO2 exposure – also based on land use regression modeling – on cognitive and motor development [29]. A nonsignificant reduction of several cognitive subareas was found. A case-control study aimed to examine the association between autism and proximity of residence to freeways and major roadways during pregnancy and near the time of delivery, as a surrogate for air pollution exposure [37]. An increase risk of autism among the 10% of children living within 309 m of a freeway was observed after adjusting for socioeconomic factors and maternal smoking during pregnancy compared to the 50% of children living at a distance higher than 1,419 m. Living near other major roads at birth was not associated with autism. In a birth cohort study carried out in Boston (USA), exposure to black carbon was estimated on the basis of the children’s residence – derived by spatial modeling – during the study period where the neurocognitive assessment was done [34]. A significant decrease of global IQ, non-verbal IQ, and visual memory was observed in children around 9 years old, even after adjustment for socioeconomic status, birth weight, tobacco smoke exposure, and blood lead levels.
Some studies have been carried out comparing cognitive and behavioural assessments of children from two areas, one significantly more polluted than the other. A study in Quanzhou, China, compared neurobehavioural performance tests of 431 children from a school in a dense traffic area with 430 children from a school in a clean air area [36]. NO2, PM less than 10 µm aerodynamic diameter (PM10), and lead were measured by passive samplers in both schools. Only NO2 concentrations were statistically different between schools. Regarding neurobehavioural outcomes, they found a significant reduction in psychomotor, attention, and sensory scales, although no changes in cognitive function, among children from the school of a dense traffic area compared to children from the school in the clean air area. This study adjusted for a large number of potential confounders including socioeconomic factors, indoor air pollution, and smoking exposure. Calderón-Garcidueñas et al. recruited 55 children from Mexico City with chronically very high concentrations of pollutants and 18 children from Polotitlán, a control city with low levels of pollutants [26]. Results suggested that Mexico City children, but no Polotitlán children, performed significantly behind their normative level of cognitive development, including global IQ, verbal IQ, and several sub-tests of the Wechsler Intelligence Scale for Children-Revised (WISC-R) such as memory or executive function. However, this study did not perform measurement of the exposure and did not adjust for contextual cofactors that differed between the two areas. Similarly another study carried out in India aimed to compare 969 children from Delhi and 850 children from two rural areas of the region of Delhi [33]. Ambient air pollution level was much less in the rural areas due to lesser number of automobiles and air-pollution industries. Prevalence of ADHD symptoms was significantly higher in children of Delhi than those from the two rural areas. Indoor levels of PM10 were measured at households and schools of the 60 participants from Delhi and 60 participants from the two rural areas. PM10was found to be positively and strongly associated with ADHD symptoms.
Two ecological studies were carried out in order to assess the association between levels of air pollution around schools and academic performance comparing areas with different air pollution levels, but without any measurement of the exposure. The unit of analyses in these studies were the school [39–40]. Pastor et al. calculated a total respiratory hazard index associated with outdoor air toxics exposures for each public school of California and examined its relationship with the school academic performance index, a summary score of overall school performance [39]. Results indicated that schools located in areas with higher respiratory hazards associated with air toxics also tend to have lower academic performance, even after controlling for a set of school-level variables including student socio-economic status, teacher quality, parent education, and other factors. Another study set up in Michigan found that schools located in areas with the highest air pollution levels – measured as the distance to major industrial facilities and major highways – had the highest proportions of students who failed to meet state educational testing standards [40]. The analyses were adjusted for school attendance rates, number of students in each school, school expenditures, number of students eligible for the free lunch program, and the racial and ethnic makeup of the school.
Two other studies explored the association between hazardous air pollutants and autism spectrum disorders at 8–9 years, one in the San Francisco Bay area and another in North Carolina and West Virginia following a case-control design [41–42]. Hazardous air pollutants include hundreds of metals, particulate, and volatile organic compounds known to harm human health. The National Air Toxics Assessment (NATA) programme from the US Environmental Protection Agency uses emissions data to model annual-average of hazardous air pollutants levels for each census tract. Individual exposure to hazardous air pollutants was assigned to each subject (cases and controls) using the modelled levels corresponding to the census tract of the birth address. Windham et al. found a significant association of autism spectrum disorders with higher ambient air concentrations of metals such as cadmium, mercury, and nickel, although results were not adjusted for multiple testing [42]. However, no relationship was shown with aromatic solvents such as benzene, ethyl benzene, styrene, toluene, or xylene neither with PAH or diesel PM. Kalkbrenner et al. estimated null associations between several pollutants including PAH, arsenic, lead, manganese, mercury, and toluene and autism spectrum disorders [41]. The main limitation of this study was the selection of controls that had speech and language impairments. They assume that these problems would not be appreciably affected by air pollution. Nevertheless, as we showed in this review, some studies have pointed out a potential relationship between air pollutants and language development [26, 32].
In related research, early-life exposure to household gas appliances and indoor NO2 levels was found to be negatively associated with general cognitive functioning and with a higher risk for development of ADHD symptoms at age 4 on 398 preschool children from a birth cohort [43]. Gas appliances produce complex mixtures including NO2 and UFP. These findings were replicated on four birth cohorts recruited 7 years later though at younger age [44].
Another related research refers to second hand smoking, measured by cotinine levels in children [45–46] and in adults [47] though direct effects of nicotine and cotinine on cognitive impairment and behaviour problems could be the explanation rather than air pollutans.
Overall, these studies open a new horizon for research on the hazards of air pollution for neuropsychological development during childhood, an issue of major worldwide impact.
Few epidemiological studies have assessed the neuropsychological effects of ambient air pollutants in adults (table 2) [48–51]. Chen et al. conducted an analysis using data of the Third National Health and Nutrition Examination Survey (NHANES III) [51]. Individuals were assigned exposure values based on distance between their residence and the monitor. In models adjusted for a large set of variables, increasing levels of estimated annual exposure to ambient ozone prior the examination was associated with a reduced performance in memory and attention tests in adults from 20 to 59 years. However, the association between PM10and cognitive and behavioural outcomes disappeared after adjustment for sociodemographic factors. A study of 399 women aged 68-79 years who lived for more than 20 years in the same residence showed a significant reduction of cognitive function in those of age less than or equal to 74 years that lived within a distance range of 50 m to the next busy road with a traffic density of more than 10,000 cars per day [48]. Nevertheless, no effect in cognitive function was found in relation to PM10 levels. Recently, Power et al. reported a significant reduction of cognitive function related to black carbon exposure at residential addresses in a cohort of 680 men aged from 51 to 97 years [49]. Exposure was assessed using land use regression based on black carbon measurements. Results remained similar after adjusting for estimates of lead exposure.
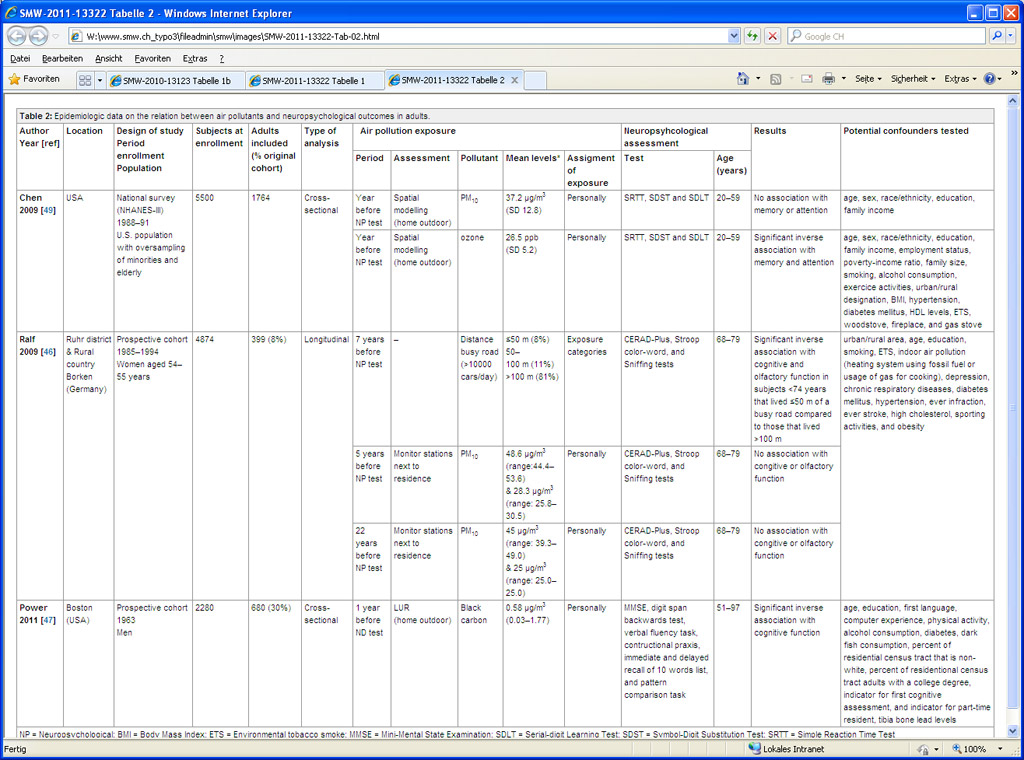
Table 2
Epidemiologic data on the relation between air pollutants and neuropsychological outcomes in adults.
A double blind randomised crossover study was carried out with 10 human volunteers aged from 18 to 39 years [50]. They were exposed to dilute diesel exhaust (300 µg/m3) as a model for ambient PM exposure and to filtered air (sham condition) during one hour, separated by a period of two to four days. Crüts et al. showed an increased activity of the left frontal cortex during and after diesel exhaust exposure that indicated a delayed response to diesel exhaust in the frontal cortex.
Genome wide studies for ADHD or cognitive function have shown that genetic variants identified explain a small proportion of the phenotypic variability [52–53], indicating the need for new approaches such as incorporating the environmental exposures in the genetic studies.
In a birth cohort study, Morales et al. found a stronger adverse effect of household gas appliances exposure and indoor NO2 concentrations in children with the GSTP1Val-105 allele [43]. In another study, Vrijheid et al. replicated these results showing a reduction of mental development associated with the presence of a gas cooker at home during pregnancy in children with the GSTP1Val-105 allele [44]. GSTP1protect against oxidative stress and it represents the most strongly expressed glutathione S-transferase isoenzyme in the human brain during early life [54]. Given that GSTP1Ile105Val results in a less active enzyme, brain cells from children with the less active GSTP1 Val-105 variant are more susceptible to biochemical changes induced by early-life air pollution exposure and that modulation in expression levels of antioxidant genes as a result of gene polymorphisms could alter the magnitude of effects caused by UFP.
Another study identified a number of significant interactions between maternal genetic markers and PAH, as well as interactions between newborn genetic markers and PAH, on mental development from 1 to 3 years old before adjusting for multiple comparisons [55]. However, no single marker-PAH interaction remain significant after Bonferroni correction. Significant interaction effects between haplotypes and PAH were observed in mothers and their newborns after Bonferroni correction, particularly haplotypes of CYP1A1 and CYP1B1. The cytochrome P450 genes CYP1A1 and CYP1B1 have been shown to play important roles in the metabolic activation of PAH [54].
High intakes of antioxidant nutrients have been proposed as an important potential modifier of air pollution impairment [58]. Villareal-Calderon et al. found a sustained dorso vagal complex inflammation in mice exposed to Mexico City air, which were mitigated by dark chocolate administration, rich in polyphenols which are potent antioxidants [59]. Regarding other outcomes effects, Jedrychowski et al. reported a non-significant greater reduction of birth weight among those newborns whose mothers reported low fish intake during pregnancy [60]. Within data from the same study, Jedrychowski et al. showed a reduction of the adjusted risk of coughing over the first 2 years of life related with the prenatal exposure to PM less than 2.5 µm aerodynamic diameter (PM2.5) in infants whose mothers consumed more fish in pregnancy [61]. Moreover, a protective effect of antioxidant micronutriens such as alpha-tocopherol and carotenoids on the DNA damage associated with prenatal PAH exposure was reported [62].
This research on individual susceptibility will open new fields of knowledge about mechanisms underlying UFP-related neurological effects, as well as identifying susceptible subgroups.
Based on the above toxicological and epidemiological data the hypothesis that we draw is the following: UFP activates pro-inflammatory genes inducing pro-inflammatory cytokines in human bronchial epithelial cells [63], lung epithelial cells [64], and macrophages [65]. The interaction of macrophages with epithelial cells amplifies cytokine production in those cells, and these cytokines are also present in the blood of subjects during episodes of acute atmospheric air pollution. Furthermore, there is evidence that oxidative stress and induced inflammation translates systemically [66]. Even though most of the available research about inflammatory effects of air pollution refers to the lungs, there is evidence that the oxidative stress and inflammation induced by PM translate systemically beyond the lungs as a result of increased expression of inflammatory genes [9]. For example, elevated particle number counts increased markers of systemic inflammation (IL-6 and fibrinogen peripheral levels) [67], particularly in subgroups with a given genotype [68–69].
UFP are enriched in organic carbon content as well as prooxidative PAH that promote oxidative stress and inflammation. It is not only the particle number concentration, but also particle compositions since different composition may produce different neurological effects. Carbon particles themselves generally adsorb transimetals (including antimony, barium, copper, iron, zinc) emitted from traffic exhaust and also from tire and brake wear. These metals are mainly generated by traffic in current urban atmospheres [70]. Changes in cognitive function in children have been shown to be associated with relatively low internal doses of lead [71] and mercury [72]. In addition to being linked to cognitive deficits in children, lead has been related to a diversity of behavioural problems [73]. Metals have been shown to induce oxidative stress in animal brain [14].
Overall, either deposition of UFP containing metals in olfactory bulb or frontal cortical and subcortical areas, or alternatively the neuroinflammation following the inflammatory systemic responses secondary to oxidative stress triggered by air pollution, could result in white matter lesions and vascular pathology in these areas that could be the basis for the cognitive deficits and behavioural impairment observed in children and elderly. The epidemiological research in the two edges of life (during mental development and during mental decline) is recent and limited and there is need for multicentre and large studies assessing both the growth and the decline of the global mental function, and its specific areas such as memory or executive function, as well as on the clinical impact in social impairment, ADHD, autism or Alzheimer. The potential role of lead and other metals, as well as of noise, as the underlying cause must be ruled out. Furthermore, the epigenetics and the role of the susceptibility genes are a key area of interest.
1 Beddington J, Cooper CL, Field J, Goswami U, Huppert FA, Jenkins R, et al. The mental wealth of nations. Nature. 2008;455(7216):1057–60.
2 Rice D, Barone S Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108:Suppl3:511–33.
3 Landrigan PJ, Sonawane B, Butler RN, Trasande L, Callan R, Droller D. Early environmental origins of neurodegenerative disease in later life. Environ Health Perspect. 2005;113(9):1230–3.
4 Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–78.
5 Götschi T, Heinrich J, Sunyer J, Kunzli N. Long-term effects of ambient air pollution on lung function: a review. Epidemiology. 2008;19(5):690–701.
6 Perez L, Rapp R, Künzli N. The Year of the Lung: outdoor air pollution and lung health. Swiss Med Wkly. 2010;140:w13129
7 Künzli N, Perez L. Evidence based public health – the example of air pollution. Swiss Med Wkly. 2009;139(17-18):242–50.
8 Block ML, Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32(9):506–16.
9 Araujo JA, Nel AE. Particulate matter and atherosclerosis: role of particle size, composition and oxidative stress. Part Fibre Toxicol. 2009;6:24.
10 Morawska L, Ristovski Z, Jayaratne ER, Keogh DU, Ling X. Ambient nano and ultrafine particles from motor vehicle emissions: characteristics, ambient processing and implications on human exposure. Atmos Environ. 2008;42(35):8113–38.
11 Wichmann HE, Spix C, Tuch T, Wölke G, Peters A, Heinrich J, et al. Daily mortality and fine and ultrafine particles in Erfurt, Germany. Part I: Role of particle number and particle mass. Health Effects Institute – Research report. 2000;98.
12 Mejìa JF, Wraith D, Mengersen K, Morawska L. Trends in size classified particle number concentration in subtropical Brisbane, Australia, based on a 5 year study. Atmos Environ. 2007;41:1064–79.
13 Pey J, Querol X, Alastuey A, Rodríguez S, Putaud JP, Van Dingenen R. Source apportionment of urban fine and ultra-fine particle number concentration in a Western Mediterranean city. Atmos Environ. 2009;43(29):4407–15.
14 Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am J Respir Crit Care Med. 2001;164(9):1665–8.
15 Donaldson K, Tran L, Jimenez LA, Duffin R, Newby DE, Mills N, et al. Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part Fibre Toxicol. 2005;2:10.
16 Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114(8):1172–8.
17 Peters A, Veronesi B, Calderón-Garcidueñas L, Gehr P, Chen LC, Geiser M, et al. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part Fibre Toxicol. 2006;3:13.
18 Tin-Tin-Win-Shwe, Yamamoto S, Ahmed S, Kakeyama M, Kobayashi T, Fujimaki H. Brain cytokine and chemokine mRNA expression in mice induced by intranasal instillation with ultrafine carbon black. Toxicol Lett. 2006;163(2):153–60.
19 Calderón-Garcidueñas L, Mora-Tiscareno A, Gomez-Garza G, Carrasco-Portugal MC, Perez-Guille B, Flores-Murrieta FJ, et al. Effects of a cyclooxygenase-2 preferential inhibitor in young healthy dogs exposed to air pollution: a pilot study. Toxicol Pathol. 2009;37(5):644–60.
20 Campbell A, Araujo JA, Li H, Sioutas C, Kleinman M. Particulate matter induced enhancement of inflammatory markers in the brains of apolipoprotein E knockout mice. J Nanosci Nanotechnol. 2009;9(8):5099–104.
21 Gerlofs-Nijland ME, van Berlo D, Cassee FR, Schins RP, Wang K, Campbell A. Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain. Part Fibre Toxicol. 2010;7:12.
22 Levesque S, Taetzsch T, Lull ME, Kodavanti U, Stadler K, Wagner A, et al. Diesel Exhaust Activates & Primes Microglia: Air Pollution, Neuroinflammation, & Regulation of Dopaminergic Neurotoxicity. 2011;119(8):1149–55.
23 van Berlo D, Albrecht C, Knaapen AM, Cassee FR, Gerlofs-Nijland ME, Kooter IM, et al. Comparative evaluation of the effects of short-term inhalation exposure to diesel engine exhaust on rat lung and brain. Arch Toxicol. 2010;84(7):553–62.
24 Calderón-Garcidueñas L, Azzarelli B, Acuna H, Garcia R, Gambling TM, Osnaya N, et al. Air pollution and brain damage. Toxicol Pathol. 2002;30(3):373–89.
25 Calderón-Garcidueñas L, Reed W, Maronpot RR, Henriquez-Roldan C, Delgado-Chavez R, Calderón-Garcidueñas A, et al. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol. 2004;32(6):650–8.
26 Calderón-Garcidueñas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. 2008;36(2):289–310.
27 Calderón-Garcidueñas L, Franco-Lira M, Henriquez-Roldan C, Osnaya N, Gonzalez-Maciel A, Reynoso-Robles R, et al. Urban air pollution: influences on olfactory function and pathology in exposed children and young adults. Exp Toxicol Pathol. 2010;62(1):91–102.
28 Edwards SC, Jedrychowski W, Butscher M, Camann D, Kieltyka A, Mroz E, et al. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children’s intelligence at 5 years of age in a prospective cohort study in Poland. Environ Health Perspect. 2010;118(9):1326–31.
29 Freire C, Ramos R, Puertas R, Lopez-Espinosa MJ, Julvez J, Aguilera I et al. Association of traffic-related air pollution with cognitive development in children. J Epidemiol Community Health. 2010;64(3):223–8.
30 Perera F, Li TY, Zhou ZJ, Yuan T, Chen YH, Qu L, et al. Benefits of reducing prenatal exposure to coal-burning pollutants to children’s neurodevelopment in China. Environ Health Perspect. 2008;116(10):1396–400.
31 Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect. 2006;114(8):1287–92.
32 Perera FP, Li Z, Whyatt R, Hoepner L, Wang S, Camann D, et al. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics. 2009;124(2):e195–e202.
33 Siddique S, Banerjee M, Ray MR, Lahiri T. Attention-deficit hyperactivity disorder in children chronically exposed to high level of vehicular pollution. Eur J Pediatr. 2011;170(7):923–9.
34 Suglia SF, Gryparis A, Wright RO, Schwartz J, Wright RJ. Association of black carbon with cognition among children in a prospective birth cohort study. Am J Epidemiol. 2007;167(3):280–6.
35 Tang D, Li TY, Liu JJ, Zhou ZJ, Yuan T, Chen YH, et al. Effects of prenatal exposure to coal-burning pollutants on children’s development in China. Environ Health Perspect. 2008;116(5):674–9.
36 Wang S, Zhang J, Zeng X, Zeng Y, Wang S, Chen S. Association of traffic-related air pollution with children’s neurobehavioral functions in Quanzhou, China. Environ Health Perspect. 2009;117(10):1612–8.
37 Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential Proximity to Freeways and Autism in the CHARGE Study. Environ Health Perspect. 2010;119(6):873–7.
38 Guxens M, Aguilera I, Ballester F, Estarlich M, Fernández-Somoano A, Lertxundi A, et al. Prenatal exposure to residential air pollution and infant mental development: modulation by antioxidants and detoxification factors. Environ Health Prespect. 2012;120(1):1–7.
39 Pastor M Jr, Morello-Frosch R, Sadd JL. Breathless: schools, air toxics, and environmental justice in California. Policy Studies Journal. 2006;34(3):337–62.
40 Mohai P, Kweon BS, Lee S, Ard K. Air pollution around schools is linked to poorer student health and academic performance. Health Aff (Millwood). 2011;30(5):852–62.
41 Kalkbrenner AE, Daniels JL, Chen JC, Poole C, Emch M, Morrissey J. Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8. Epidemiology. 2010;21(5):631–41.
42 Windham GC, Zhang L, Gunier R, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the san francisco bay area. Environ Health Perspect. 2006;114(9):1438–44.
43 Morales E, Julvez J, Torrent M, de Cid R, Guxens M, Bustamante M, et al. Association of early-life exposure to household gas appliances and indoor nitrogen dioxide with cognition and attention behavior in preschoolers. Am J Epidemiol. 2009;169(11):1327–36.
44 Vrijheid M, Martinez D, Aguilera I, Bustamante M, Ballester F, Estarlich M et al. Indoor air pollution from gas cooking and neurodevelopment in Infants. Epidemiology. 2012;23(1):23–32.
45 Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R. Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environ Health Perspect. 2004;113(1):98–103.
46 Bandiera FC, Richardson AK, Lee DJ, He JP, Merikangas KR. Secondhand smoke exposure and mental health among children and adolescents. Arch Pediatr Adolesc Med. 2011;165(4):332–8.
47 Llewellyn DJ, Lang IA, Langa KM, Naughton F, Matthews FE. Exposure to secondhand smoke and cognitive impairment in non-smokers: national cross sectional study with cotinine measurement. BMJ. 2009;338:b462.
48 Ranft U, Schikowski T, Sugiri D, Krutmann J, Kramer U. Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environ Res. 2009;109(8):1004–11.
49 Power MC, Weisskopf MG, Alexeeff SE, Coull BA, Spiro A, III, Schwartz J. Traffic-related air pollution and cognitive function in a cohort of older men. Environ Health Perspect. 2010;119(5):682–7.
50 Crüts B, van Etten L, Tornqvist H, Blomberg A, Sandstrom T, Mills NL et al. Exposure to diesel exhaust induces changes in EEG in human volunteers. Part Fibre Toxicol. 2008;5:4.
51 Chen JC, Schwartz J. Neurobehavioral effects of ambient air pollution on cognitive performance in US adults. Neurotoxicology. 2009;30(2):231–9.
52 Neale BM, Lasky-Su J, Anney R, Franke B, Zhou K, Maller JB, et al. Genome-wide association scan of attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(8):1337–44.
53 Need AC, Attix DK, McEvoy JM, Cirulli ET, Linney KL, Hunt P, et al. A genome-wide study of common SNPs and CNVs in cognitive performance in the CANTAB. Hum Mol Genet. 2009;18(23):4650–61.
54 Raijmakers MT, Steegers EA, Peters WH. Glutathione S-transferases and thiol concentrations in embryonic and early fetal tissues. Hum Reprod. 2001;16(11):2445–50.
55 Wang S, Chanock S, Tang D, Li Z, Edwards S, Jedrychowski W, et al. Effect of gene-environment Interactions on mental development in African American, Dominican, and Caucasian mothers and newborns. Ann Hum Genet. 2010;74(1):46–56.
56 Abnet CC, Fagundes RB, Strickland PT, Kamangar F, Roth MJ, Taylor PR, et al. The influence of genetic polymorphisms in Ahr, CYP1A1, CYP1A2, CYP1B1, GST M1, GST T1 and UGT1A1 on urine 1-hydroxypyrene glucuronide concentrations in healthy subjects from Rio Grande do Sul, Brazil. Carcinogenesis. 2007;28(1):112–7.
57 Gilliland FD, Li YF, Saxon A, Diaz-Sanchez D. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: randomised, placebo-controlled crossover study. Lancet. 2004;363(9403):119–25.
58 Romieu I, Castro-Giner F, Kunzli N, Sunyer J. Air pollution, oxidative stress and dietary supplementation: a review. Eur Respir J. 2008;31(1):179–97.
59 Villarreal-Calderon R, Torres-Jardon R, Palacios-Moreno J, Osnaya N, Perez-Guille B, Maronpot RR, et al. Urban air pollution targets the dorsal vagal complex and dark chocolate offers neuroprotection. Int J Toxicol. 2010;29(6):604–15.
60 Jedrychowski W, Perera F, Mrozek-Budzyn D, Flak E, Mroz E, Sochacka-Tatara E et al. Higher fish consumption in pregnancy may confer protection against the harmful effect of prenatal exposure to fine particulate matter. Ann Nutr Metab. 2010;56(2):119–26.
61 Jedrychowski W, Flak E, Mroz E, Pac A, Jacek R, Sochacka-Tatara E, et al. Modulating effects of maternal fish consumption on the occurrence of respiratory symptoms in early infancy attributed to prenatal exposure to fine particles. Ann Nutr Metab. 2008;52(1):8–16.
62 Kelvin EA, Edwards S, Jedrychowski W, Schleicher RL, Camann D, Tang D, et al. Modulation of the effect of prenatal PAH exposure on PAH-DNA adducts in cord blood by plasma antioxidants. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2262–8.
63 Fujii T, Hayashi S, Hogg JC, Vincent R, Van Eeden SF. Particulate matter induces cytokine expression in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2001;25(3):265–71.
64 Jiménez LA, Drost EM, Gilmour PS, Rahman I, Antonicelli F, Ritchie H, et al. PM(10)-exposed macrophages stimulate a proinflammatory response in lung epithelial cells via TNF-alpha. Am J Physiol Lung Cell Mol Physiol. 2002;282(2):L237–L248.
65 van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, et al. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). Am J Respir Crit Care Med. 2001;164(5):826–30.
66 Hirano S, Furuyama A, Koike E, Kobayashi T. Oxidative-stress potency of organic extracts of diesel exhaust and urban fine particles in rat heart microvessel endothelial cells. Toxicology. 2003;187(2-3):161–70.
67 Rückerl R, Greven S, Ljungman P, Aalto P, Antoniades C, Bellander T, et al. Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ Health Perspect. 2007;115(7):1072–80.
68 Ljungman P, Bellander T, Schneider A, Breitner S, Forastiere F, Hampel R, et al. Modification of the interleukin-6 response to air pollution by interleukin-6 and fibrinogen polymorphisms. Environ Health Perspect. 2009;117(9):1373–9.
69 Peters A, Greven S, Heid IM, Baldari F, Breitner S, Bellander T, et al. Fibrinogen genes modify the fibrinogen response to ambient particulate matter. Am J Respir Crit Care Med. 2009;179(6):484–91.
70 Steiner M, Boller M, Schulz T, Pronk W. Modelling heavy metal fluxes from traffic into the environment. J Environ Monit. 2007t;9(8):847–54.
71 Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113(7):894–9.
72 Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368(9553):2167–78.
73 Froehlich TE, Lanphear BP, Auinger P, Hornung R, Epstein JN, Braun J, et al. Association of tobacco and lead exposures with attention-deficit/hyperactivity disorder. Pediatrics. 2009;124(6):e1054–e1063.
Funding/potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.