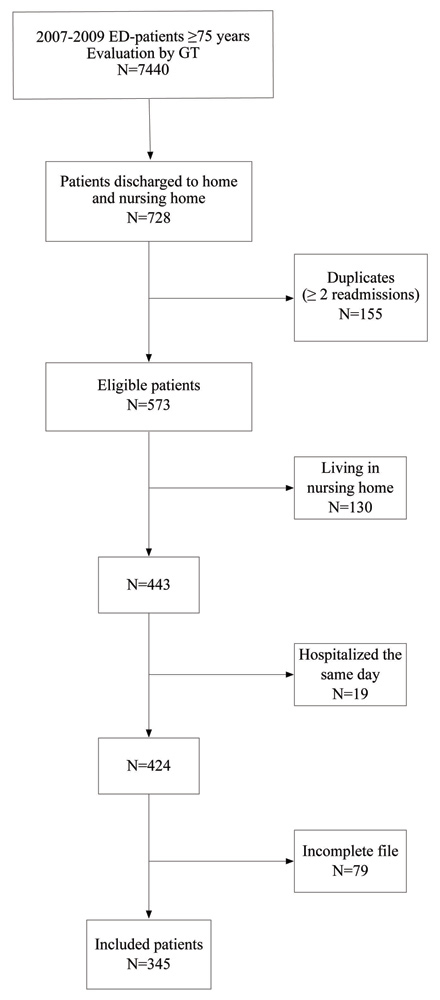
Figure 1
Flow diagram of the studied population.
ED: Emergency Department. GT: Geriatric Team.
DOI: https://doi.org/10.4414/smw.2012.13327
Comparison of two screening tools
Emergency department (ED) admissions of patients 65 years and older are increasing and account for up to 20% of all consultations [1]. After an ED-visit, this population is at high risk for adverse outcome like ED-readmission [1]. In a systematic review, Aminzadeh et al. described a readmission rate of 10% at one month, 24% at 3 months and 44% at 6 months.
The comprehensive geriatric assessment (CGA), the best well-known geriatric tool related to clinical decision making, is a reproducible procedure which includes a multidisciplinary tool with cognitive and mood evaluation, comorbidities and polypharmacy examination, assessment of the risk of falls and functional status (basic activities of daily living [BADL] and instrumental activities of daily living [IADL]), as well as nutritional status and social support [2]. Caplan et al. showed that CGA performed after an ED-visit with further ambulatory implementation or coordination of recommendations could decrease ED-readmission at 1 month (16.5 vs. 22.2%; p = 0.048) and at 18 months (44.4 vs. 54.3%; p = 0.007) [3].
However, CGA is a time-consuming process, which takes at least 30 minutes to be performed and thus cannot be applied to every older patient admitted to the ED [4]. Therefore, a two-step approach by using a targeted CGA and further ambulatory recommendations only among patients identified at high risk for ED-readmission with shorter validated tools has been developed [5]. Screening older patients in this way could thus avoid slowing down the flow in the ED. The Identification of Seniors At Risk Tool (ISAR) and the Triage Risk Stratification Tool (TRST) are the two most studied instruments in the literature and the only ones validated with a two-step approach among patients 65 years and older in the ED [6, 7]. These scales are interesting in that the ED-presenting illness is not included. Moons et al. showed that these tools could predict ED-readmission [8].
Some questions remain about the efficiency of ISAR and TRST among patients 75 years and older. This population is particularly interesting because they have less specific complaints than younger patients at the ED-visit, and they need more time to be taken in care. This challenges the management of the flow [9]. Warburton et al. reported their experience during a patient safety quality improvement programme and highlighted the lack of specificity of ISAR to predict ED-readmission among patients over 75 years [10]. In other words, almost all patients aged 75 years and older were classified as high risk, overwhelming available referral resources. Therefore, this study aims to evaluate the performances of the ISAR and TRST tools to predict unplanned readmission after an ED-visit among patients 75 years and older as well as to test their usefulness in avoiding unnecessary CGA in ED.
We carried out a historical cohort study from systematic routine data collection at Geneva University Hospitals (Switzerland). The local Ethics Committee approved the study.

Figure 1
Flow diagram of the studied population.
ED: Emergency Department. GT: Geriatric Team.
The Geneva hospital is a 1200-bed primary and tertiary care university hospital, and our ED admits 60,000 patients per year. Our ED uses a 4 level triage scale adapted from the Canadian Emergency Department Triage and Acuity Scale (CTAS) [11]. This scale is used to determine for each level the acceptable time delay before the patient should be evaluated. Triage is performed by trained nurses specialised in emergency care. A geriatric team (GT) is present from 8:00 am to 19:00 pm, weekends excepted. The GT takes care of all patients 75 years and older with a CTAS ≥3/4 (i.e. patients who should be seen within 2 hours or non-urgent patients), which accounts for 2,500 patients per year. Among them, 10% are discharged to home after the ED-visit. The GT routinely collects medical and social information, but does not perform standardised CGA. There is also no systematic referral to home care services.
We included all patients seen by the GT during a 3-year period (2007–2009) and discharged to home from the ED. Patients living in nursing homes were excluded. All data were systematically collected when the patient was seen in the ED by a physician of the GT, and included in a database. The information was obtained either from the patient or from the caregiver.
The identification of patients at high risk was performed with the ISAR and the TRST tool. The ISAR consists of six assessment items: presence of home help, increased dependency, history of hospital admissions, visual problems, memory problems, and polypharmacy (≥3 drugs). The TRST tool is a five-item instrument: cognitive impairment, difficulties with walking or transfer, admission to ED during the last month or hospitalisation within the last 3 months, polypharmacy (≥5 drugs), and professional recommendation for home care services. Responses to these questionnaires are dichotomous (i.e., yes–no). A patient is considered to be at high risk (i.e., for unplanned readmission) when the answers to two or more questions are positive (ISAR ≥2/6, TRST ≥2/5).
Functional disabilities were assessed using Basic and Instrumental Activities of Daily Living (BADL, IADL) scores. The Katz index evaluates BADL and includes six items: bathing, dressing, toilet use, transfer, feeding and continence [12]. For the IADL, the most widely used tool is the Lawton scale, which explores 9 items: using the telephone, travel, shopping, meal preparation, housework, taking medicine and management of finances, laundry and mode of transportation [13]. For both scales, each item scores from 1 (dependent) to 3 (independent). In case of total independence, the maximum score reaches 18 for BADL and 27 for IADL.
Co-morbidities were assessed by the Cumulative Illness Rating scale-Geriatrics (CIRS) [14], and took into account active and previous medical problems at the time of the ED-visit, via an extensive review of the patient's medical records and administrative data for diagnoses established at or before enrolment in this study. This scale, previously validated in the Geneva hospital, has been shown to predict length of stay, institutionalisation and death [15]. The CIRS incorporates medical conditions according to 14 categories (heart disease, hypertension, haematopoietic, respiratory, eyes and ears, upper gastro-intestinal, lower gastro-intestinal, liver, kidneys, genito-urinary, musculo-skeletal, neurological, endocrine/metabolic, psychiatric/dementia), rated each on a scale from 0 (no disease) to 4 (life-threatening disease). The total score is calculated as the sum of the 14 scores (maximum at 56 points).
Prescribing of inappropriate medication (PIM) could lead to adverse drug events and unexpected hospitalisation [16]. PIM was assessed according to the Screening Tool of Older People’s potentially inappropriate Prescriptions (STOPP). STOPP is based on physiological systems, and includes 65 criteria for avoidance of certain drug-drug interactions in older people [17]. Gallagher et al. showed that STOPP could identify twice as many PIM that Beer’s criteria.
One investigator reviewed retrospectively the file of each patient to obtain all sociodemographic data (age, sex, having a general practitioner (GP), living alone). The CIRS, STOPP criteria and ADL scales were extracted from the collected data. The ISAR and TRST tools were systematically asked of the patients during the ED-visit. Patients with incomplete files were excluded.
Unplanned readmission was considered as a binary data and defined by a new visit to the ED or a direct hospitalisation at 1, 3, 6 and 12 months after the index visit. In our city, patients over 75 years are mostly addressed to the University hospital. Elective hospitalisation for a scheduled intervention (for example cataract operation) was not considered as a readmission. If a patient had more than one readmission (i.e., considered as duplicates), only the first one was taken into account. Patients hospitalised the same day after an ED-visit were excluded. Readmission data were obtained from the hospital information system.
The distribution of continuous data (age, CIRS, Katz and Lawton) was considered as normal because of the number of observations (N >50). Data for continuous variables are presented as means ± 1 standard deviation (SD), and binary data (sex, living alone, GP, PIM, ISAR and TRST) are reported as proportions (%). First, we compared all the data at 1, 3, 6 and 12 months with an unpaired t-test for continuous data and a Chi2 test for binary data. Significance was set at p <0.05. After binarisation of the 2 scores (ISAR ≥2/6, TRST ≥2/5), we measured the univariate relationship between ISAR and TRST to the outcome (i.e. readmission) using Cox regression models. Censoring data included mortality data according to the population registrar of the State of Geneva. For the multiple Cox regression model, we adjusted for all variables significantly (p <0.05) associated with readmission at each time (sex, CIRS), or the variables with a significant confounding effect (>20% of variation effect on the main predictor) (Katz, Lawton). Continuous variables without a log-linearity relationship with the outcome were binarised for the Katz (normal or not) or categorised for the Lawton (quartiles). The hazard proportional assumption was checked for each Cox model. Hazards ratios (HR) with their 95% confidence intervals (CI) were calculated. We plotted Kaplan-Meier curves for readmission at 1, 3, 6 and 12 months, and compared them with log-rank tests. We reported the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for the ISAR ≥2 and TRST ≥2 at each time, as well as their area (AUC) under the receiver operating characteristics curve (ROC). Statistical analyses were performed with Stata software version 11, College Stata, TX, US.
Among 7,440 patients aged 75 years and older evaluated in ED by the GT during the 3-yr period, 728 were discharged to home. Among them, 155 were readmitted more than once and were considered as duplicates. Of the 573 eligible patients, 228 were excluded: 130 because they were living in nursing homes, 19 because of hospitalisation during the same day, and 79 because of incomplete file. In the end 345 patients were included (fig. 1). Table 1 summarises the characteristics of the population. The mean (SD) age was 83.9 (±5.7) yrs, 62.9% were women, 58% lived alone, 97% had a general practitioner and a half had at least one inappropriate medication. Patients were relatively independent (Katz index: 17.1 (±1.5)/18, Lawton scale: 20.8 (±5.6)/27) and had quite low CIRS scores (7.8 (±3.7)/56). The cumulative readmission rates at 1, 3, 6 and 12 months were respectively 25%, 38%, 49% and 60%. Diagnoses at ED-admission were mostly due to an orthopedic problem or a trauma (30%), a cardiac pathology (25%), or a psychiatric illness (12%). At 1 month, 39% of readmission diagnoses were the same as the initial ones.
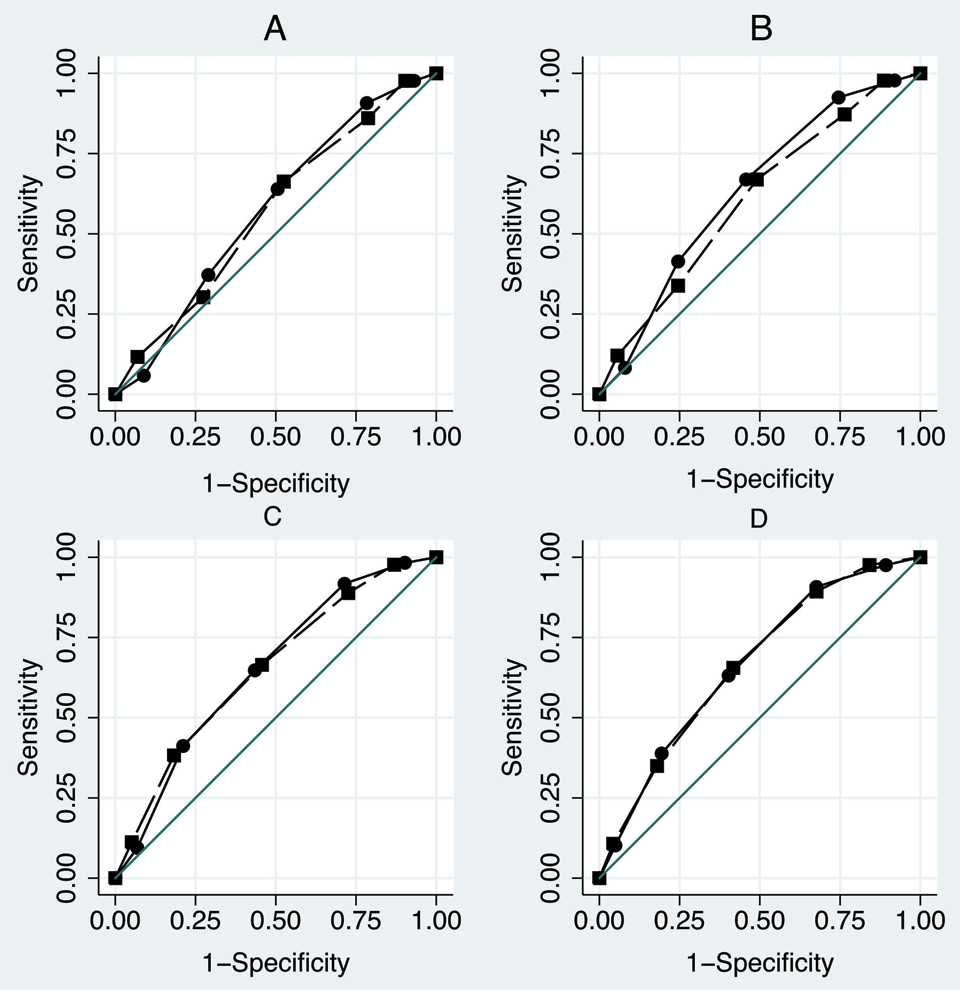
Figure 2
ROC curves of the Identification of Senior at Risk (ISAR – plain circle line) and the Triage Risk Stratification Tool (TRST- long-dash square-line) for readmission at 1, 3, 6 and 12 months.
A: 1 month ISAR ROC area = 0.612 - TRST ROC area = 0.607
B: 3 months ISAR ROC area = 0.655 - TRST ROC area = 0.621
C: 6 months ISAR ROC area = 0.660 - TRST ROC area = 0.651
D: 12 months ISAR ROC area = 0.664 - TRST ROC area = 0.664
Table 1 shows that men were consistently more readmitted than women, and that readmitted patients had always more comorbidities than those who were not readmitted. Functional dependence, as assessed by Katz or Lawton, was positively associated with readmission only at 12 months. Age was not predictive of readmission. In order to be sure that the same population was targeted with ISAR and TRST, we performed a Fisher’s exact test and found an association between the 2 classifications everytime (F exact <0.001). There was also no difference between the discordance pairs (McNemar >0.05). In the Cox regression models (table 2), the ISAR ≥2 predicted the readmission at 1, 3, 6 and 12 months in the univariate analysis. The association remained significant in the multiple analysis models. In contrast, a positive TRST score did not predict readmission at 1 month (HR 1.77, 95% IC 0.94–3.33, p = 0.078) in the univariate and multiple regression models. The ISAR prediction of readmission measured by the HR was higher than that of the TRST at each time point, but this difference did not achieve statistical significance.
The ISAR had a sensitivity >90% and a negative predictive value (NPV) between 70-90% at each analysed time point of readmission (table 3). The TRST results were quite similar. Figure 2 shows the ISAR and TRST ROC curves for readmission at 1, 3, 6 and 12 months. Their area under the curve range was 0.607–0.664 without significant difference.
Figure 3 shows the Kaplan-Meyer estimation curves for readmission according to a positive or negative ISAR or TRST scores. The curves of patients readmitted or were not statistically different (p <0.001) with both screening tools.
| Table 1: Demographic and presenting characteristics of the study patients at baseline and according to their readmission at 1, 3, 6 and 12 months. | |||||||||||||||||||
| Total | 1 month | 3 months | 6 months | 12 months | |||||||||||||||
| Readmission | No | Yes | No | Yes | No | Yes | No | Yes | |||||||||||
| N (%) | 345 | (100) | 260 | (75.4) | 85 | (24.6) | 213 | (61.7) | 132 | (38.3) | 175 | (50.7) | 170 | (49.3) | 139 | (40.3) | 206 | (59.7) | |
| Women N(%) | 217 | (62.9) | 173 | (66.5) | 44 | (51.8)* | 145 | (68.1) | 72 | (54.6)* | 121 | (69.1) | 96 | (56.5)* | 98 | (70.5) | 119 | (57.8)* | |
| Age M(SD) | 83.9 | (5.7) | 83.9 | (5.7) | 84.0 | (5.8) | 84.1 | (5.8) | 83.6 | (5.6) | 83.9 | (5.9) | 83.9 | (5.6) | 83.6 | (6.0) | 84.1 | (5.6) | |
| Living alone N(%) | 201 | (58.3) | 153 | (58.9) | 48 | (56.5) | 122 | (57.3) | 79 | (59.9) | 99 | (56.6) | 102 | (60.0) | 80 | (57.6) | 121 | (58.7) | |
| GP N(%) | 336 | (97.4) | 255 | (98.1) | 81 | (95.3) | 210 | (98.6) | 126 | (95.5) | 172 | (98.3) | 164 | (96.5) | 138 | (99.3) | 198 | (96.1) | |
| PIM ≥1 N(%) | 169 | (49) | 120 | (46.2) | 49 | (57.7) | 100 | (47.0) | 69 | (52.3) | 81 | (46.3) | 88 | (51.8) | 65 | (46.8) | 104 | (50.5) | |
| Katz M(SD) | 17.1 | (1.5) | 17.1 | (1.5) | 17.2 | (1.4) | 17.1 | (1.5) | 17.1 | (1.4) | 17.2 | (1.5) | 17.0 | (1.5) | 17.3 | (1.4) | 17.0 | (1.5)* | |
| Lawton M(SD) | 20.8 | (5.6) | 20.7 | (5.5) | 20.8 | (6.0) | 21.1 | (5.4) | 20.4 | (5.8) | 21.3 | (5.3) | 20.4 | (5.8) | 21.9 | (4.7) | 20.1 | (6.0)* | |
| CIRS M(SD) | 7.8 | (3.7) | 7.4 | (3.5) | 8.9 | (4.0)* | 7.1 | (3.4) | 8.8 | (3.9)* | 7.0 | (3.4) | 8.6 | (3.8)* | 6.9 | (3.4) | 8.4 | (3.8)* | |
| ISAR ≥2 N(%) | 281 | (81.5) | 203 | (78.1) | 78 | (91.8)* | 158 | (74.2) | 123 | (93.2)* | 125 | (71.4) | 156 | (91.8)* | 94 | (67.3) | 187 | (90.8)* | |
| TRST ≥2 N(%) | 278 | (80.6) | 204 | (78.5) | 74 | (87.1) | 162 | (76.1) | 116 | (87.9)* | 127 | (72.6) | 151 | (88.8)* | 94 | (67.3) | 184 | (89.3)* | |
| * p <0.05 GP: General Practitioner, PIM: Prescribing of Inappropriate Medication, CIRS: Cumulative Illness Rating Scale-Geriatrics, ISAR: Identification of Senior at Risk, TRST: Triage Risk Stratification Tool. | |||||||||||||||||||
| Table 2: Cox proportional hazards models for ISAR and TRST to predict readmission at 1, 3, 6 and 12 months (N = 345). | |||||||
| Readmission | N (%) | HR | [95% CI] | p value | Ad_HR# | [95% CI] | p value |
| 1 month | 85 (24.6) | ||||||
| ISAR ≥2/6 | 78 (91.8) | 2.79 | [1.29–6.05] | 0.009 | 2.91 | [1.29–6.59] | 0.010 |
| TRST ≥2/5 | 74 (87.1) | 1.77 | [0.94–3.33] | 0.078 | 1.89 | [0.94–3.78] | 0.072 |
| 3 months | 132 (38.3) | ||||||
| ISAR ≥2/6 | 123 (93.2) | 3.73 | [1.90–7.34] | <0.001 | 3.70 | [1.81–7.56] | <0.001 |
| TRST ≥2/5 | 116 (87.9) | 1.99 | [1.18–3.35] | 0.010 | 1.90 | [1.06–3.38] | 0.030 |
| 6 months | 170 (49.3) | ||||||
| ISAR ≥2/6 | 156 (91.8) | 3.30 | [1.91–5.70] | <0.001 | 3.20 | [1.78–5.73] | <0.001 |
| TRST ≥2/5 | 151 (88.8) | 2.32 | [1.44–3.73] | 0.001 | 2.27 | [1.35–3.82] | 0.002 |
| 12 months | 206 (59.7) | ||||||
| ISAR ≥2/6 | 187 (90.8) | 3.16 | [1.97–5.03] | <0.001 | 3.00 | [1.80–5.00] | <0.001 |
| TRST ≥2/5 | 184 (89.3) | 2.67 | [1.71–4.16] | <0.001 | 2.64 | [1.63–4.27] | <0.001 |
| # HR adjusted for sex, Katz index, Lawton scale, comorbidities index (CIRS) HR: Hazard Ratio, Ad_HR: Adjusted Hazard Ratio, ISAR: Identification of Senior at Risk, TRST: Triage Risk Stratification Tool. | |||||||
| Table 3: Predictive value of ISAR and TRST for readmission at 1, 3, 6 and 12 months (N = 345). | ||||||||
| Screening tool | Readmission (months) | Cutoff | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC | [95% CI] |
| ISAR | ||||||||
| 1 | ≥2 | 91.8 | 21.9 | 27.8 | 89.1 | 0.612 | [0.55–0.68] | |
| 3 | ≥2 | 93.2 | 25.8 | 43.8 | 85.9 | 0.655 | [0.60–0.71] | |
| 6 | ≥2 | 91.8 | 28.6 | 55.5 | 78.1 | 0.660 | [0.60–0.72] | |
| 12 | ≥2 | 90.8 | 32.4 | 66.6 | 70.3 | 0.664 | [0.61–0.72] | |
| TRST | ||||||||
| 1 | ≥2 | 87.1 | 21.5 | 26.6 | 83.6 | 0.607 | [0.54–0.67] | |
| 3 | ≥2 | 87.9 | 23.9 | 41.7 | 76.1 | 0.621 | [0.56–0.68] | |
| 6 | ≥2 | 88.8 | 27.4 | 54.3 | 71.6 | 0.651 | [0.59–0.71] | |
| 12 | ≥2 | 89.3 | 32.4 | 66.2 | 67.2 | 0.664 | [0.61–0.72] | |
| AUC: Area Under the Curve, CI: Confident Internal, ISAR: Identification of Senior at Risk, NPV: Negative Predictive Value, PPV: Positive Predictive value, TRST: Triage Risk Stratification Tool. | ||||||||
Among ED-patients ≥75 years, both the ISAR and the TRST tools predicted unplanned readmission (new ED-visit or direct hospitalisation) with moderate accuracy, due to their low specificity. Nevertheless, there was overall a non significant trend for the ISAR to consistently better predict unplanned readmission than the TRST. Furthermore, in contrast with the later, the ISAR discriminates significantly high-risk patients already at 1 month. More interestingly, in case of negative ISAR or TRST, their high NPV permitted, in our population, avoiding 64 useless CGA with safety (ISAR <2 readmitted: 7/64 at 1 month, 9/64 at 3 months). To the best of our knowledge, these two screening tools have been evaluated in a Swiss population for the first time.
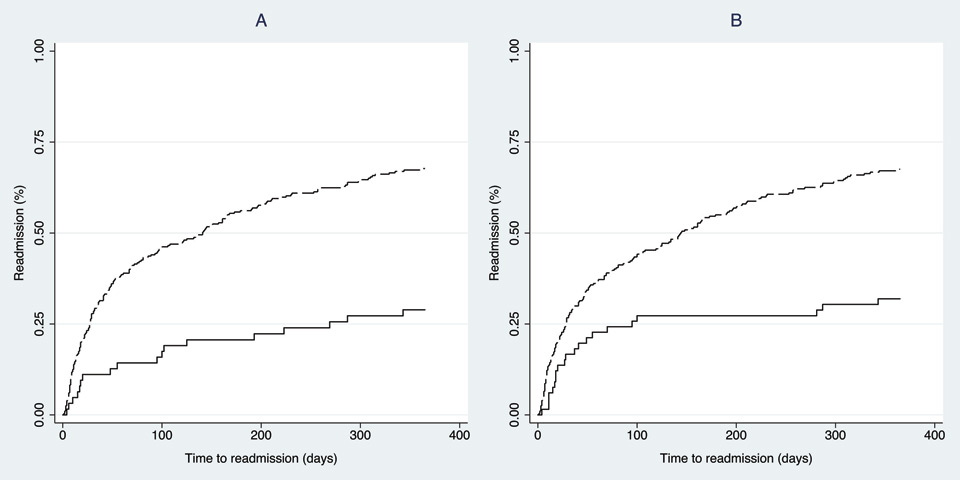
Figure 3
Kaplan-Meyer estimations curves for readmission stratified by the Identification of Senior at Risk (ISAR) and by the Triage Risk Stratification Tool (TRST).
A: ISAR <2 (plain line) - ISAR ≥2 (long-dash line), logrank test: p <0.001
B: TRST <2 (plain line) - TRST ≥2 (long-dash line), logrank test: p <0.001
Readmission rates in this study are basically in line with those in previous reports [7, 18]. However, the rates at 12 months were higher in our study than in that of Lee et al. (60% vs 44%) [19]. The fact that our population is older (84 vs 77 years) and that EDs in our country are highly accessible could explain this difference.
Regarding the ISAR tool, its performance is consistent with previous studies, and comparable with that described among younger people. In the original paper, McCusker et al. included 1,122 patients over 65 years from 4 urban Canadian hospitals and described an AUC of 0.630 for readmission at 1 month [20]. Similarly, Salvi et al. performed a recent prospective cohort study of 200 patients ≥65 years from 2 urban Italian ED, and reported that the ISAR was efficient to predict ED-readmission at 3 months (odds ratio: 2.02, CI 95%: 1.06–3.87, p = 0.035) [18]. Moons et al. conducted a prospective cohort study in one Belgian University hospital including 83 patients over 65 years discharged from ED [8]. The authors compared four screening tools, including the ISAR and the TRST. The predictive value of the ISAR was the same as in our study (AUC: 0.610 and 0.630 at 1 and 3 months respectively, NPV of 89% and 82%). However, in our cohort, sensitivities are constantly better and specificities lower that theirs (sensitivity: 92% vs 79% and 93% vs 79% at 1 and 3 months, specificity: 22% vs 37% and 26% vs 41%). This could be explained by the older age of our population (median age: 84 vs 74 years), and enhances the hypothesis that the specificity of the ISAR could decrease with age.
Regarding the TRST, our results are surprisingly better than those described in the literature. Lee et al. followed a cohort of 788 patients over 65 years discharged from 3 Canadian ED, and found poor performances for this tool (sensibility and specificity of 62 and 57% at 1 month, 56 and 61% at 1 year) [18]. On the other hand, AUC at 1 month was similar to that reported in our study (0.610). The difference in performances could be explained either by the higher proportion of patients readmitted at 1 year in our study, or the younger population of the study of Lee et al. Because of the lack of comorbidities and functional assessment in Lee’s study, it is also not clear if the two populations are comparable. Moons et al. reported weak AUC at 1and 3 months (0.570 and 0.520 respectively) with TRST. However, the authors used a 6-items TRST tool. According to the predictive value, Meldon et al. originally decided to withdraw the “Lives alone or no available caregiver” item, in order to improve the final model [7]. Keeping this useless item in the tool could have decreased its performance.
A recent Dutch prospective study including 381 patients over 65 years reported very bad results regarding ISAR and TRST performance (sensitivity of 56% – specificity of 54% for the ISAR and sensitivity of 79% – specificity of 33% for the TRST) to predict ED readmission [21]. This could be due to the low rate of outcome’s prevalence (15% at 120 days), to the different setting of the hospital (tertiary hospital), and to the different population (younger and in better health). However, despite these results, NPV of the two tools remained acceptable (90%).
From our results, the ISAR and the TRST tools have almost the same performances. However, the ISAR seems to be easier routine use in ED. The “professional recommendation” item of the TRST tool is subjective and particularly difficult to estimate in clinical use. The fact that the ISAR remains predictive of readmission in multivariate Cox regression models shows that this tool can explore some patients’ characteristics explaining ED-readmission beyond the medical problem. The ISAR has a high sensitivity and NPV, which is particularly important for a useful screening tool in clinical practice. In our study, a negative ISAR screening prevented further geriatric investigations. Thus, a negative ISAR screening allows the discharge of old patients without risk, after having managed the acute medical problem. In contrast, some authors raised the question of the lack of specificity of these tools [8, 10]. We observed the same phenomenon in our selected population. The poor specificity could unnecessarily increase the use of the home care services, induced by CGA. Further research is needed to find a way to improve the specificity of these tools. Warburton et al. suggested to specify some questions of the ISAR in order to adapt it to an older population, but that remained to be validated [10].
As the first limitation, our work was centred in one study site and we included patients triaged as patients who should be seen within 2 hours or non-urgent patients (emergency 3 or 4), which included about 60% of patients ≥75 years admitted to the ED of our institution. This may constrain the generalisability of the research findings. Nevertheless, Rutschmann et al. described that this population often does not have specific complaints at admission, making their care more difficult [9]. These patients spend more time, and are particularly of interest in order to improve the flow in the ED. Second, our study relies upon retrospective data, rather than data collected from a cohort followed prospectively. However, because the information was collected at the ED-admission, we do not think that a prospective approach would yield substantially different results. Third, as only patients who returned to the main ED-center or were directly hospitalised were captured, patients who were readmitted to the ED of another institution may have been missed. However, in our country, most of the older people have a basic health insurance which only covers care in the public hospital. Thus, older patients are preferentially addressed by their GP to the Geneva University hospital. Finally, included patients were evaluated by the GT, which could induce a bias whether a systematic intervention would be applied to reduce the outcomes of interest. As mentioned before, the GT performed no systematic intervention nor did systematic home care referral at the time of this study.
In summary, ISAR and TRST used in ED-patients 75 years and older modestly predicted unplanned readmissions, but their lack of specificity and high sensitivity lead them to be more useful to select patients who can return home from the ED without any further geriatric evaluation (i.e., who can be managed as younger patients). These scores allow the avoidance of useless CGA with safety and, in this way, will not slow down the flow in ED. Positively screened patients need further specific geriatric evaluation, which can be performed ambulatory after discharge. Nevertheless, it remained to be analysed in further studies whether specifying some questions of these tools could improve their specificity and therefore increase their positive predictive value in regard to the high prevalence of ED-readmission among elderly patients.
Acknowledgement: We would like to thank Mme Chantal Genet for her help in collecting data.
1 Aminzadeh F, Dalziel WB. Older adults in the emergency department: a systematic review of patterns of use, adverse outcomes, and effectiveness of interventions. Ann Emerg Med. 2002;39(3):238–47.
2 Rubenstein LZ, Stuck AC, Siu AL, Wieland D. Impacts of geriatric evaluation and management programs on defined outcomes: overview of the evidence. J Am Geriatr Soc. 1991;39(9 Pt 2):8S–16S; Discussion 7S–8S.
3 Caplan GA, Williams AJ, Daly B, Abraham K. A randomized, controlled trial of comprehensive geriatric assessment and multidisciplinary intervention after discharge of elderly from the emergency department – the DEED II study. J Am Geriatr Soc. 2004;52(9):1417–23.
4 Guttman A, Afilalo M, Guttman R, Colacone A, Robitaille C, Lang E, et al. An emergency department-based nurse discharge coordinator for elder patients: does it make a difference? Acad Emerg Med. 2004;11(12):1318–27.
5 Graf CE, Zekry D, Giannelli S, Michel JP, Chevalley T. Efficiency and applicability of the comprehensive geriatric assessment in the emergency department: a systematic review. Aging Clin Exp Res. Oct 5.
6 Mccusker J, Bellavance F, Cardin S, Trepanier S, Verdon J, Ardman O. Detection of older people at increased risk of adverse health outcomes after an emergency visit: the ISAR screening tool. J Am Geriatr Soc. 1999;47(10):1229–37.
7 Meldon SW, Mion LC, Palmer RM, Drew BL, Connor JT, Lewicki LJ, et al. A brief risk-stratification tool to predict repeat emergency department visits and hospitalizations in older patients discharged from the emergency department. Acad Emerg Med. 2003;10(3):224–32.
8 Moons P, De Ridder K, Geyskens K, Sabbe M, Braes T, Flamaing J, et al. Screening for risk of readmission of patients aged 65 years and above after discharge from the emergency department: predictive value of four instruments. Eur J Emerg Med. 2007;14(6):315–23.
9 Rutschmann OT, Chevalley T, Zumwald C, Luthy C, Vermeulen B, Sarasin FP. Pitfalls in the emergency department triage of frail elderly patients without specific complaints. Swiss Med Wkly. 2005;135(9-10):145–50.
10 Warburton RN, Parke B, Church W, Mccusker J. Identification of Seniors At Risk: process evaluation of a screening and referral program for patients aged > or =75 in a community hospital emergency department. Int J Health Care Qual Assur Inc Leadersh Health Serv. 2004;17(6):339–48.
11 Beveridge R. Caep issues. The canadian triage and acuity scale: a new and critical element in health care reform. Canadian association of emergency physicians. J Emerg Med. 1998;16(3):507–11.
12 Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–9.
13 Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86.
14 Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc. 1995;43(2):130–7.
15 Zekry D, Valle BH, Michel JP, Esposito F, Gold G, Krause KH, et al. Prospective comparison of six co-morbidity indices as predictors of 5 years post hospital discharge survival in the elderly. Rejuvenation Res. Dec;13(6):675-82.
16 Winterstein AG, Sauer BC, Hepler CD, Poole C. Preventable drug-related hospital admissions. Ann Pharmacother. 2002;36(7–8):1238–48.
17 Gallagher P, Ryan C, Byrne S, Kennedy J, O’mahony D. STOPP (Screening Tool Of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83.
18 Salvi F, Morichi V, Grilli A, Spazzafumo L, Giorgi R, Polonara S, et al. Predictive validity of the Identification of Seniors At Risk (ISAR) screening tool in elderly patients presenting to two italian emergency departments. Aging Clin Exp Res. 2009;21(1):69–75.
19 Lee JS, Schwindt G, Langevin M, Moghabghab R, Alibhai SM, Kiss A, et al. Validation of the Triage Risk Stratification Tool to identify older persons at risk for hospital admission and returning to the emergency department. J Am Geriatr Soc. 2008;56(11):2112–7.
20 Mccusker J, Cardin S, Bellavance F, Belzile E. Return to the emergency department among elders: patterns and predictors. Acad Emerg Med. 2000;7(3):249–59.
21 Buurman BM, Van Den Berg W, Korevaar JC, Milisen K, De Haan RJ, De Rooij SE. Risk for poor outcomes in older patients discharged from an emergency department: feasibility of four screening instruments. Eur J Emerg Med. 2011;18(4):215–20.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article was reported.