Imaging in arthritis: quantifying effects of therapeutic intervention using MRI and molecular imaging
DOI: https://doi.org/10.4414/smw.2012.13326
MA
Cimmino, F
Barbieri, G
Zampogna, D
Camellino, F
Paparo, M
Parodi
Summary
Modern imaging techniques are becoming increasingly important in assessing the course of arthritis and in permitting measurement of response to treatment as part of the follow-up of patients. They include ultrasonography (US), MRI, PET/CT, and biofluorescence. In patients with rheumatoid arthritis, clinical evaluation is significantly less sensitive than either US or MRI in detecting synovitis. As a result, imaging is a useful alternative to achieving proper assessment of disease activity. The different areas in which the new imaging techniques could help practicing rheumatologists and internal physicians include the following: early and differential diagnosis of arthritis, evaluation of disease activity, prognosis, assessment of treatment efficacy, assessment of remission, and evaluation of subclinical disease. MRI is probably the best imaging method to study disease activity in RA, because it can study all the joints with similar efficacy, has been sufficiently standardised, and yields data on inflammation that can be quantified. Different methods, developed to score synovitis activity, are increasingly used in clinical trials. The main application of PET/CT in rheumatology is the diagnosis and follow-up of large vessel vasculitis. More recently, also RA disease activity has been evaluated, allowing a panoramic view of the patient. Molecular imaging studies molecular and cellular processes in intact living organisms in a non-invasive fashion. In fluorescence, dyes, that emit light upon excitation by a light source and are read by a camera, can be used to show inflamed areas where neoangiogenesis, vasodilatation, and increased vessel permeability are present. These dyes can be coupled with different compounds including antibodies and drugs.
Introduction
Modern imaging techniques are becoming increasingly important in assessing the course of arthritis and as part of patient follow-up in evaluating response to treatment [1]. The more effective schedule plan administration of traditional disease modifying drugs, as well as the highly effective new drugs, have changed the outcome of rheumatoid arthritis (RA), not only in the individual patient but also at the population level [2]. These therapeutic strategies can achieve low disease levels or even remission in a higher percentage of patients than before. Hence the need for precise monitoring of disease activity and assessment of even subtle changes in the course of arthritis. Sensitivity to change is the key feature of a quantifying method. Several studies have shown that, in patients with RA, clinical evaluation, even after careful training and standardisation, is significantly less sensitive than either US [3] or MRI [4]. As a result, imaging is a useful alternative to achieve proper assessment of disease activity. The author’s view on the relative efficiency of traditional and new imaging techniques in evaluating arthritic joints, is shown in table 1. The different areas in which the new imaging techniques could help practicing rheumatologists and internal physicians include the following:
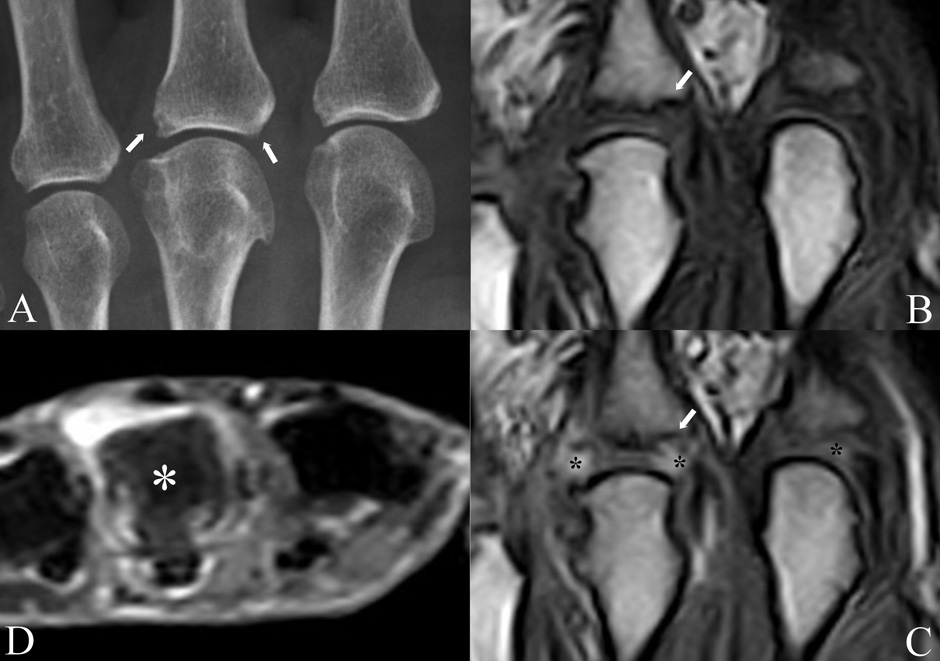
Figure 1
Conventional radiography and MRI study of the metacarpophalangeal joints of a patient with undifferentiated oligoarthritis lasting from less than 6 months. A) the radiogram shows degenerative changes (osteophytes) of the 3rd metacarpophalangeal head and small erosions (arrows) of the proximal end of the corresponding phalanx. The erosion on the right is only suspected because of an interrupted bony cortex; pre (B) and post-contrast (C) coronal Turbo 3D MRI sequences showing synovial enhancement of the painful 3rd metacarpophalangeal joint (asterisks) and confirmation of the suspected erosion (arrow). Inflammation is present also in the asymptomatic and clinically normal 2nd metacarpophalangeal joint (asterisk). Axial STIR MRI sequence (D) showing intense bone marrow oedema of the 3rd metacarpal head (asterisk), which appears gray in comparison with the normal bone of the other metacarpals, which appears black. All MRI images are obtained with an extremity-dedicated 0.2 T machine (Esaote, Genova, Italy).
– Early diagnosis of arthritis
– Differential diagnosis among arthritides
– Evaluation of disease activity
– Prognosis
– Assessment of treatment efficacy
– Guiding treatment
– Definition of remission
– Evaluation of subclinical disease
Early diagnosis of arthritis can be facilitated by showing synovial inflammation, defined as synovial fluid effusion, neoangiogenesis, and vasodilatation, in painful but otherwise clinically normal or only doubtful joints [5]. This can be achieved by both Doppler US [6] and contrast-enhanced MRI [7] (fig. 1B, C). In addition, MRI can show an early inflammatory bone lesion characterised by oedema (fig. 1D), which is a potent predictor of disease progression [8] and radiographic erosions. Bone marrow oedema (BME) is best seen on fat-suppressed or STIR sequences. US and MRI could represent a useful adjunct to the 1987 ACR classification criteria for RA [9] because they often identify subtle levels of inflammation even in asymptomatic joints, which facilitates reaching the minimum number of criteria required for diagnosis. Despite its advantages, imaging has been not included in the new 2010 criteria for RA [10], probably because of erratic availability of these techniques. Nonetheless, most tertiary referral rheumatological centres today routinely use advanced imaging to corroborate diagnosis in patients who present early.
Differential diagnosis of arthritis is not easily reached on the basis of imaging alone. A dichotomy has been suggested between entheseal and synovial forms of arthritis according to the results of MRI and US [11]. However, from a practical viewpoint, the positive predictive value of these findings is low and imaging alone cannot differentiate RA (the prototype synovial arthritis) from psoriatic arthritis (PsA, the prototype entheseal arthritis) in most patients [12]. What is more, the degree of synovial inflammation, measured by dynamic contrast enhanced MRI, appears the same in both diseases [13]. This observation suggests that, after correction for disease severity, PsA and RA are equally aggressive. However, recent data acquired with prolonged post contrast acquisition times, have shown that the outflow of the contrast agent – and hypothetically also of inflammatory mediators – from the joint is more rapid in PsA than in RA [14], in agreement with the view that prognosis could be better in the former disease. The pattern of joint and extra-articular locations of inflammation, rather than the study of a target joint, could be more useful to identify different diseases. In particular, scintigraphy has shown entheseal and joint uptake in patients with early PsA, and could highlight sites deserving further study with more precise imaging techniques [15]. Although a baseline scan could help in the differential diagnosis, scintigraphy cannot be used for the follow-up of patients due to its poor spatial resolution and to the administration of radioactive tracers.
Disease activity can be measured by Doppler US, contrast enhanced MRI, scintigraphy, PET-CT, and fluorescence, but only the first two techniques have been sufficiently standardised [16]. In the near future, scintigraphy with tracers conjugated with cytokines, antibodies or antibody fragments will be likely employed for evaluation of disease activity. In RA, these types of conjugates could be used to evaluate cytokine receptor expression and their anatomical location [17].
Prognosis of arthritis is predicted by the presence of BME and, to a lesser degree, by the intensity of synovial inflammation [18]. In undifferentiated arthritis, the observation of persistent Doppler signal predicts recurrences after treatment-induced remission [19]. In the same setting, MRI BME predicts the evolution toward RA with a precision of 82% [20]. MRI changes were more predictive of the evolution from undifferentiated arthritis to RA than anti-CCP antibodies in one study [21], but not in another one [7]. However, both features are additive; it can be suggested that finding a patient with early arthritis and various risk factors of erosive progression (i.e., female gender, shared epitope, positive anti-CCP antibodies and rheumatoid factor, MRI changes) allows a diagnosis of RA or at least of progressive arthritis and indicate the need of aggressive treatment.
Treatment changes could be theoretically evaluated by advanced imaging, but no formal evaluation of this aspect has been published to date. Imaging should be an appropriate tool for guiding treatment decisions because it is more sensitive than clinical evaluation in assessing disease activity. Finding a decrease or even an absence of synovitis could indicate the need to taper or interrupt treatment, whereas persistence of inflammation by imaging could indicate the need for prompt therapy increase. Experience regarding the prospective use of this approach, however, is lacking.
Remission is the goal of RA treatment, but is achieved only rarely. This is true for clinical remission [22] and even more for imaging remission, which is reached only in a minority of patients with clinical remission. At present, it is still not known which should be the definition of imaging remission, the absence of any sign of disease, i.e., no power Doppler signal or absence of contrast enhancement, or a decrease of inflammation below a given cut-off. In healthy individuals, imaging of the normal synovial membrane is only virtual. Therefore the first hypothesis, no imaging at all of inflammation, should be theoretically accepted. However, several papers highlight the difficulty of finding normal imaging in patients in clinical remission [23], supporting the view that a low level inflammation could be acceptable to define the “cured” synovium, but again, this level has not been established yet.
Sensitive imaging techniques can appreciate subclinical joint involvement. This point has been demonstrated in psoriasis by both US [24] and MRI [25]. Patients with mere cutaneous disease and no history of joint involvement can show typical imaging signs of arthritis or enthesitis. In addition, patients with overt arthritis have more joints involved by imaging than by clinical examination.
In the following paragraphs, an appraisal of three modern imaging techniques that are routinely used (MRI) or could be used (PET-CT, fluorescence) for evaluating patients with RA will be discussed more in detail. We will not further review the role of US, which is presently the most widespread and easily performed among the advanced imaging techniques because it is a particularly familiar technique, which has been recently and often reviewed [26].
|
Table 1: Imaging techniques and their relative efficacy in the evaluation of inflammatory joint diseases. |
|
Method
|
Disease activity
|
Damage
|
Practicability
|
Follow-up
|
Panoramic view
|
| radiography |
– |
++ |
+++ |
+++ |
+ |
| CT |
– |
+++ |
+ |
+ |
+ |
| scintigraphy |
+ |
– |
+ |
|
+++ |
| US |
++ |
+ |
++ |
++ |
– |
| MRI |
+++ |
++ |
+ |
++ |
++ |
| PET-CT |
++ |
+ |
– |
– |
+++ |
| fluorescence |
++ |
– |
– |
– |
+ |
MRI
MRI is in our opinion the best imaging method to study disease activity in RA. In contrast with US, it can study all the joints with similar efficacy, has been sufficiently standardised, and yields data on inflammation that can be quantified. Relative disadvantages are its high costs, administration of an intravenous contrast agent, and the long duration of the diagnostic procedure. In addition, MRI examinations on high-field, total body machines are not always well accepted by rheumatic patients especially if they need to be repeated over time. Conversely, extremity-dedicated machines are more patient-friendly and cheap, and can be easily used for follow-up of treatment. The study of a RA patient by MRI is limited to a single or few joints, whereas US can be performed on multiple joints in a single session. Recently, whole body MRI has been studied especially in ankylosing spondylitis patients showing an excellent capacity to show simultaneously inflammatory changes of the axial and peripheral skeleton [27]. In RA, the reference areas are the hand and wrist, with the majority of studies having been based on the evaluation of these joints [28]. The hand and wrist are of particular interest because they are affected early during the course of the disease, are involved in almost all patients with RA, and because they are a good indicator of the inflammatory state of RA as a whole [29]. OMERACT has developed a score to evaluate RA [30]: this Rheumatoid Arthritis MRI Score (RAMRIS) includes assessment of synovitis, BME, and erosions [31]. Synovitis is defined as an area of the synovial compartment that shows enhancement thicker than the width of the normal synovium after contrast agent infusion. It is assessed in three wrist regions (distal radioulnar joint, radiocarpal joint, and intercarpal and carpometacarpal joints) and in each metacarpophalangeal joint. BME is a lesion within the trabecular bone, with ill-defined margins and a signal typical of increased water content. The scale is from 0 to 3, based on the proportion of involved bone. Bone erosions are defined as bone defects with sharp margins visible on T1-weighted images in 2 planes, with a cortical break seen in at least one plane. A score is given which ranges from 0 to 10 according to the percentage of the bone volume, which is occupied by the erosion (i.e., 10%, 20% etc.). More recently, a sub-score for evaluation of tenosynovitis has been added [32]. The resulting total score offers a comprehensive evaluation of the global burden of RA, including disease activity and extent of damage. The main drawback of RAMRIS is that it is a time-consuming procedure, the reproducibility of which is low if the investigator is not well trained. To overcome these problems, quantification of synovial membrane enhancement has been obtained through the study of curves generated after contrast agent injection and acquisition of fast, consecutive sequences [29, 33]. This method is known as dynamic, contrast-enhanced MRI (DCE-MRI). Automated analysis of the resulting curves has been developed to further increase the rapidity and objectivity of the examination [34, 35]. This method needs only minor interaction with the investigator and has been easily standardised [36]. For all these reasons, DCE-MRI is an excellent candidate for the follow-up of treatment in RA patients, a field in which it has shown more sensitivity than RAMRIS [37]. MRI shows an increased sensitivity also in the detection of erosions when compared with traditional radiology [38]. Radiographic articular erosions have been always considered a hallmark of rheumatoid arthritis. Synovitis and BME, revealed by the new imaging techniques, although less specific than erosions, appear much earlier in the course of the disease and allow timely diagnosis. However, the appearance of synovitis and BME is the same in a wide spectrum of joint diseases, which fact emphasises the obvious need to correlate imaging findings with the patient’s clinical features. In particular, BME can be considered an unspecific response of bone to different insults and is seen also in trauma, osteoarthritis [39], infection, gouty tophi, Südeck’s syndrome, beyond arthritis. Erosions tend also to be identified earlier by MRI, thanks to its multiplanar imaging capacity [38]. The increased sensitivity of MRI for the identification of erosive bone damage also allows it to be used to follow their progress, including healing, a feature that was only rarely appreciated on radiographs in the past [40]. On the other hand, caution should be implemented not to over-diagnose erosions. Sometimes bone cysts on MRI can be mistaken for erosions [41]. In addition, with this sensitive method, a few erosions can be seen also in normal controls [42]. In clinical trials, evaluation of MRI results could decrease the number of patients involved and the duration of the study because of the increased sensitivity of the method in comparison with both clinical examination and conventional radiography [43]. As a result, novel drugs for rheumatoid and psoriatic arthritis have been evaluated with MRI after only three [44] or six [45] months from treatment start.
PET/CT
A positron emission tomography (PET) scan is an imaging test that uses a radioactive tracer to evaluate physiological and pathological processes [46]. Tracers are obtained by labelling compounds with short-lived positron-emitting radionuclides to measure cell metabolism. The most frequently used tracer is [18F] fluorodeoxyglucose (18F-FDG or FDG), a glucose analogue, which reflects glucose uptake by cells and thus their metabolism. FDG uptake is greater in cells with increased metabolism and its localisation allows the diagnosis of inflammation and neoplastic proliferation. PET is a functional imaging technique, not a morphological technique. However, in the last thirteen years integrated PET/CT machines have been introduced, allowing simultaneous acquisition of CT and PET without moving the patients from the examination table. PET and CT images are spatially co-registered, giving the opportunity to fuse functional and morphological information.
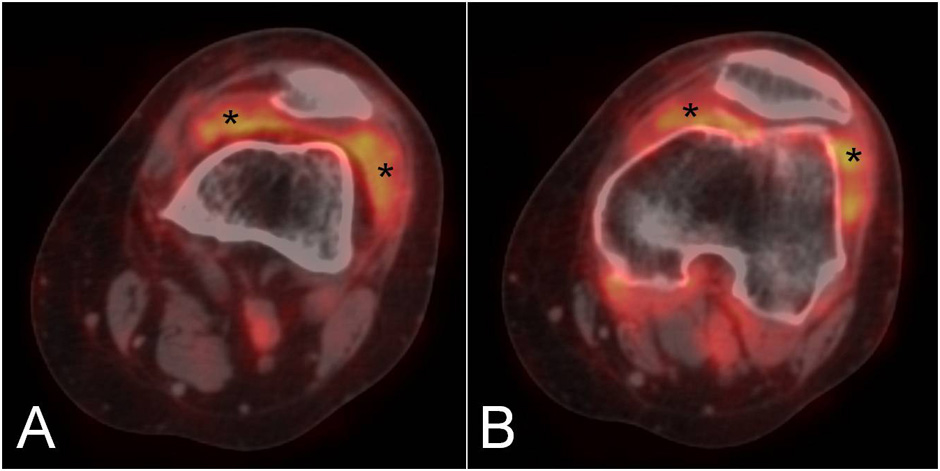
Figure 2
Two sequential PET/CT fused images of the knee in a patient with rheumatoid arthritis showing diffuse F18-FDG uptake in the medial and lateral paracondylar recesses (asterisks).
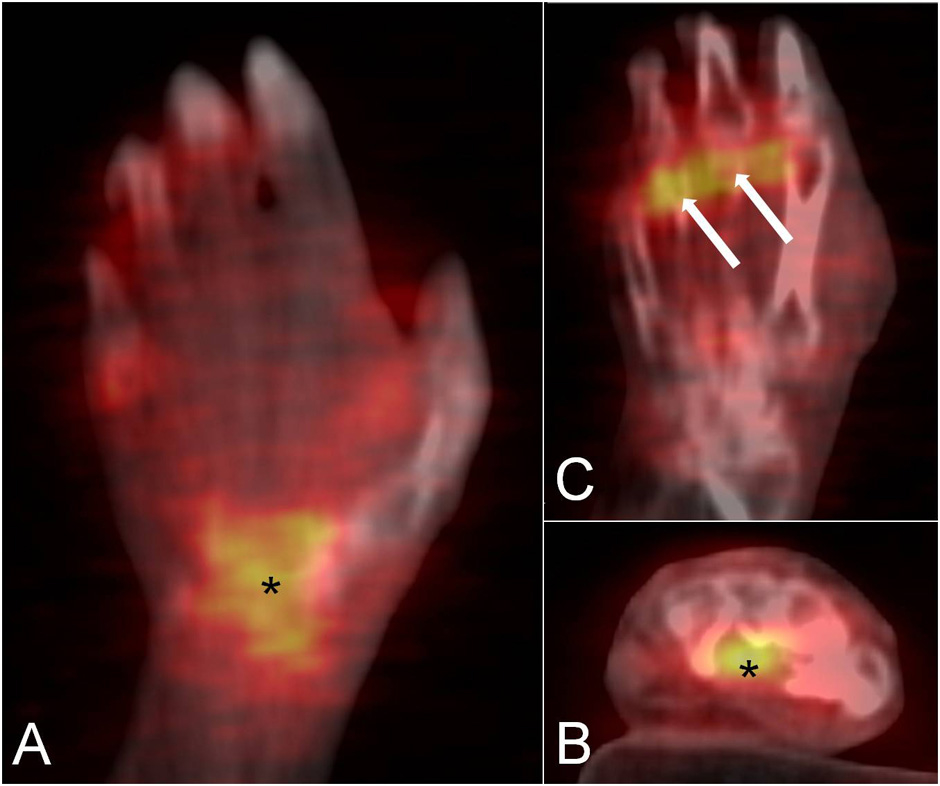
Figure 3
The coronal (A) and axial (B) PET/CT fused images of the wrist in a patient with rheumatoid arthritis demonstrate a focal area of F18-FDG uptake in the carpal tunnel, suggestive of flexor tendons tenosynovitis (asterisk). Tracer uptake is also observed in the metacarpophalangeal joints (C, arrows).
Although the main applications of PET and PET/CT are in the fields of oncology, neurology, psychiatry, and cardiology [47], novel data suggest a possible role of these techniques in rheumatology [4]. The main application is the diagnosis and follow-up of large vessel vasculitis [49, 50]. More recently RA patients have also been studied with this technique and their disease activity has been evaluated by calculating the standard uptake value (SUV) in the affected joints [51]. This method has allowed the measurement of a total score that includes all the joints involved by RA in a panoramic view of the patient. It has shown a good correlation with clinical evaluation and has also revealed involvement of the atlanto-axial joint in a few asymptomatic patients [52]. In another study, the results of PET imaging of the rheumatoid knee were in agreement with those obtained by more traditional imaging techniques, such as DCE-MRI and US [53]. In particular, PET uptake was significantly correlated with all MRI enhancement parameters as well as with synovial membrane thickness measured by US. PET uptake correlated with CRP and serum concentrations of metalloproteinase-3. After treatment with a single infusion of infliximab, an anti-TNFα biological agent, total SUV, MRI and laboratory findings significantly decreased [54]. Other anecdotal observations have confirmed the potential role of PET in the evaluation of treatment efficacy in RA patients by studying the wrist after etanercept administration [55]. One of the limitations of this method is that spatial resolution is low and imaging often suboptimal for small joints. Nevertheless, studies on hand and foot joints have been performed in RA with satisfying results [56]. Figures 2 and 3 show the results of PET/CT examination of the knee and hand, respectively, in patients with RA. In addition, PET/CT could be used for differential diagnosis between RA and seronegative spondyloarthritides (SpA) [57] especially by demonstrating a different anatomical pattern of uptake between these diseases. Evaluation of images of PET/CT scans of the shoulder, hip and knee joints have shown that FDG accumulated at the entheses in SpA and in the synovium in RA patients. In general, the main advantage of PET/CT examination in arthritis is the possibility of calculating a global score of the amount of inflammation present in the joints. A clear drawback is that, due to the high amount of ionising radiation, it cannot be used in repeated follow-up examinations. To reduce radiation exposuree, PET/CT could be used to obtain functional and morphological information at baseline, and PET alone could be repeated over time.
Fluorescence
Molecular imaging is a rapidly growing field in biomedicine, aiming at studying molecular and cellular processes in intact living organisms in a non-invasive fashion [48]. Fluorescence or bio-fluorescence is a newly applied technique in rheumatology that can quantify the amount of inflammation. In fluorescence imaging, light emitted by a fluorochrome upon excitation by a light source is detected by sensitive cameras, whereas in bioluminescence imaging no excitation light is necessary because it is based on the expression of luciferase by transfected cells, which catalyses the oxidation of luciferin and results in the release of photons. In an experimental model, rats with collagen-induced arthritis were injected with different anti-TNFα agents conjugated with alexa680, a dye [58]. The accumulation of this complex in inflamed areas, where TNFα is abundant, was monitored by a biofluorescence reader. The advantage of the technique is mainly that harmless dyes, usually employed in cancer diagnosis, can be easily conjugated with other compounds. In addition, the technique is cheap. Its main disadvantage is that only superficial joints can be assessed because of the low depth penetration due to light scattering and tissue absorption. This approach has not been used to date in humans. Conversely, another method based on the intravenous injection of indocyanine green as fluorophor and its evaluation by a fluorescence reader has been used to study the hands and wrists of patients with rheumatoid arthritis. Preliminary studies comparing this novel technique with the more standardised US have confirmed its possible role in the evaluation of inflammatory activity in the joints [59]. According to one study, fluorescence might be more sensitive than both clinical examination and power Doppler US [59].
In conclusion, modern imaging can show joint damage and inflammation simultaneously in arthritis. In the near future, it will possibly allow better identification, quantifying and localisation of key processes of arthritis. MRI has reached a good level of standardisation and is used in most on-going pharmacological trials, because of its high sensitivity. PET-CT is a promising technique for research and clinical applications, and could gain more widespread use after the introduction of new conjugates with drugs and antibodies. Early diagnosis and monitoring of treatment efficacy are its main areas of employment.
References
1 Cimmino MA, Grassi W, Cutolo M. Modern imaging techniques: a revolution for rheumatology practice. Best Pract Res Clin Rheumatol. 2008;22:951–9.
2 Cimmino MA, Masocco M, Torre M. Hospital admissions for rheumatoid arthritis dwindled in Italy between 2001 and 2008. Rheumatology (Oxford) 2011 Sep 8. [Epub ahead of print].
3 Szkudlarek M, Klarlund M, Narvestad E, Court-Payen M, Strandberg C, Jensen KE, et al. Ultrasonography of the metacarpophalangeal and proximal interphalangeal joints in rheumatoid arthritis: a comparison with magnetic resonance imaging, conventional radiography and clinical examination. Arthritis Res Ther. 2006;8:R52.
4 Tamai M, Kawakami A, Iwamoto N, Kawashiri SY, Fujikawa K, Aramaki T, et al. Comparative study of the detection of joint injury in early-stage rheumatoid arthritis by magnetic resonance imaging of the wrists and finger joints and physical examination. Arthritis Care Res. 2011;63:436–9.
5 McGonagle D, Tan AL. What magnetic resonance imaging has told us about the pathogenesis of rheumatoid arthritis – the first 50 years. Arthritis Res Ther. 2008;10:222.
6 Haslam KE, McCann LJ, Wyatt S, Wakefield RJ. The detection of subclinical synovitis by ultrasound in oligoarticular juvenile idiopathic arthritis: a pilot study. Rheumatology. (Oxford) 2010;49:123–7.
7 Tamai M, Kawakami A, Uetani M, Takao S, Arima K, Iwamoto N, et al. A prediction rule for disease outcome in patients with undifferentiated arthritis using magnetic resonance imaging of the wrists and finger joints and serologic autoantibodies. Arthritis Care Res. 2009;61:772–8.
8 Boyesen P, Haavardsholm EA, Østergaard M, van der Heijde D, Sesseng S, Kvien TK. MRI in early rheumatoid arthritis: synovitis and bone marrow oedema are independent predictors of subsequent radiographic progression. Ann Rheum Dis. 2011;70:428–33.
9 Sugimoto H, Takeda A, Hyodoh K. Early-stage rheumatoid arthritis: prospective study of the effectiveness of MR imaging for diagnosis. Radiology. 2000;216:569–75.
10 Neogi T, Aletaha D, Silman AJ, Naden RL, Felson DT, Aggarwal R, et al. The 2010 American College of Rheumatology/European League against Rheumatism classification criteria for rheumatoid arthritis: Phase 2 methodological report. Ann Rheum Dis. 2010;62:2582–91.
11 McGonagle D, Gibbon W, Emery P. Classification of inflammatory arthritis by enthesitis. Lancet. 1998;352:1137–40.
12 Cimmino MA, Parodi M, Zampogna G, Paparo F, Silvestri E, Garlaschi G, et al. Magnetic resonance imaging of the hand in psoriatic arthritis. J Rheumatol. (Suppl) 2009;83:39–41.
13 Cimmino MA, Parodi M, Innocenti S, Succio G, Banderali S, Silvestri E, et al. Dynamic magnetic resonance of the wrist in psoriatic arthritis reveals imaging patterns similar to those of rheumatoid arthritis. Arthritis Res Ther. 2005;7:R725–31.
14 Schwenzer NF, Kötter I, Henes JC, Schraml C, Fritz J, Claussen CD. The role of dynamic contrast-enhanced MRI in the differential diagnosis of psoriatic and rheumatoid arthritis. Am J Radiol. 2010;194:715–20.
15 Scarpa R, Cuocolo A, Peluso R, Atteno M, Gisonni P, Iervolino S, et al. Early psoriatic arthritis: the clinical spectrum. J Rheumatol. 2007;35:137–41.
16 Cimmino MA, Grassi W. What is new in ultrasound and magnetic resonance imaging for musculoskeletal disorders? Best Pract Res Clin Rheumatol. 2008;22:1141–8.
17 Barrera P, Oyen WJ, Boerman OC, van Riel PL. Scintigraphic detection of tumour necrosis factor in patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:825–8.
18 Hetland ML, Stengaard-Pedersen K, Junker P, Østergaard M, Ejbjerg BJ, Jacobsen S, et al. Radiographic progression and remission rates in early rheumatoid arthritis – MRI bone oedema and anti-CCP predicted radiographic progression in the 5-year extension of the double-blind randomised CIMESTRA trial. Ann Rheum Dis. 2010;69:1789–95.
19 Scirè CA, Montecucco C, Codullo V, Epis O, Todoerti M, Caporali R. Ultrasonographic evaluation of joint involvement in early rheumatoid arthritis in clinical remission: power Doppler signal predicts short-term relapse. Rheumatology. (Oxford) 2009;48:1092–7.
20 Duer-Jensen A, Horslev-Petersen K, Hetland ML, Bak L, Ejbjerg BJ, Hansen MS, et al. Bone edema on magnetic resonance imaging is an independent predictor of rheumatoid arthritis development in patients with early undifferentiated arthritis. Arthritis Rheum. 2011;63:2192–202.
21 Narváez J, Sirvent E, Narváez JA, Bas J, Gómez-Vaquero C, Reina D, et al. Usefulness of magnetic resonance imaging of the hand versus anticyclic citrullinated peptide antibody testing to confirm the diagnosis of clinically suspected early rheumatoid arthritis in the absence of rheumatoid factor and radiographic erosions. Semin Arthritis Rheum. 2008;38:101–9.
22 Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum. 2011;63:573–86.
23 Brown AK, Conaghan PG, Karim Z, Quinn MA, Ikeda K, Peterfy CG, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58:2958–67.
24 Gisondi P, Tinazzi I, El-Dalati G, Gallo M, Biasi D, Barbara LM, et al. Lower limb enthesopathy in patients with psoriasis without clinical signs of arthropathy: a hospital-based case-control study. Ann Rheum Dis. 2008;67:26–30.
25 Offidani A, Cellini A, Valeri G, Giovagnoni A. Subclinical joint involvement in psoriasis: magnetic resonance imaging and X-ray findings. Acta dermatol Venereol. 1998;78:463–5.
26 Machado PM, Koevoets R, Bombardier C, van der Heijde DM. The value of magnetic resonance imaging and ultrasound in undifferentiated arthritis: a systematic review. J Rheumatol. (Suppl) 2011;87:31–7.
27 Weber U, Hodler J, Kubik RA, Rufibach K, Lambert RG, Kissling RO, et al. Sensitivity and specificity of spinal inflammatory lesions assessed by whole-body magnetic resonance imaging in patients with ankylosing spondylitis or recent-onset inflammatory back pain. Arthritis Rheum. 2009;61:900–8.
28 Cimmino MA, Bountis C, Silvestri E, Garlaschi G, Accardo S. An appraisal of magnetic resonance imaging of the wrist in rheumatoid arthritis. Semin Arthritis Rheum. 2000;30:180–95.
29 Cimmino MA, Innocenti S, Livrone F, Magnaguagno F, Silvestri E, Garlaschi G. Dynamic gadolinium-enhanced magnetic resonance imaging of the wrist in patients with rheumatoid arthritis can discriminate active from inactive disease. Arthritis Rheum. 2003;48:1207–13.
30 Ostergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;30:1385–6.
31 Bird P, Conaghan P, Ejbjerg B, McQueen F, Lassere M, Peterfy C, et al. The development of the EULAR-OMERACT rheumatoid arthritis MRI reference image atlas. Ann Rheum Dis. 2005;64(Suppl 1):i8-10.
32 Haavardsholm EA, Østergaard M, Ejbjerg BJ, Kvan NP, Kvien TK. Introduction of a novel magnetic resonance imaging tenosynovitis score for rheumatoid arthritis: reliability in a multireader longitudinal study. Ann Rheum Dis. 2007;66:1216–20.
33 Boesen M, Østergaard M, Cimmino MA, Kubassova O, Jensen KE, Bliddal H. MRI quantification of rheumatoid arthritis: current knowledge and future perspectives. Eur J Radiol. 2009;76:438–9.
34 Kubassova O, Boesen M, Cimmino MA, Bliddal H. A computer-aided detection system for rheumatoid arthritis MRI data interpretation and quantification of synovial activity. Eur J Radiol. 2010;74:e67–72.
35 Kubassova O, Boesen M, Peloschek P, Langs G, Cimmino MA, Bliddal H, et al. Quantifying disease activity and damage by imaging in rheumatoid arthritis and osteoarthritis. Ann N Y Acad Sci. 2009;1154:207–38.
36 Boesen M, Kubassova O, Parodi M, Bliddal H, Innocenti S, Garlaschi G, et al. Comparison of the manual and computer-aided techniques for evaluation of wrist synovitis using dynamic contrast-enhanced MRI on a dedicated scanner. Eur J Radiol. 2011;77:202–6.
37 Parodi M, Zampogna G, Barbieri F, Paparo F, Garlaschi G, Cutolo M, et al. Short-term follow-up of RA patients treated with rituximab by extremity-dedicated MRI: dynamic MRI is more sensitive than RAMRIS in the evaluation of synovitis. Ann Rheum Dis. 2010;69(Suppl 3):380–1.
38 Østergaard M, Hansen M, Stoltenberg M, Jensen KE, Szkudlarek M, Pedersen-Zbinden B, et al. New radiographic bone erosions in the wrists of patients with rheumatoid arthritis are detectable with magnetic resonance imaging a median of two years earlier. Arthritis Rheum. 2003;48:2128–31.
39 Oliviero F, Ramonda R, Punzi L. New horizons in osteoarthritis. Swiss Med Wkly 2010;140:w13098. doi: 10.4414/smw.2010.13098.
40 Barbieri F, Parodi M, Zampogna G, Paparo F, Cimmino MA. Bone ankylosis of the wrist as a possible indicator of treatment efficacy in rheumatoid arthritis. Rheumatology. (Oxford) 2010;49:1414–6.
41 Barbieri F, Parodi M, Zampogna G, Paparo F, Cimmino MA. Caveat on the interpretation of metacarpal head erosions seen by magnetic resonance imaging. J Rheumatol. 2010;37:1965–6.
42 Parodi M, Silvestri E, Garlaschi G, Cimmino MA. How normal are the hands of normal controls? A study with dedicated magnetic resonance imaging. Clin Exp Rheumatol. 2006;24:134–41.
43 Peterfy CG. Magnetic resonance imaging of rheumatoid arthritis: the evolution of clinical applications through clinical trials. Semin Arthritis Rheum. 2001;30:375–96.
44 Genovese MC, Kavanaugh A, Weinblatt ME, Peterfy C, DiCarlo J, White ML, et al. An oral Syk kinase inhibitor in the treatment of rheumatoid arthritis. Arthritis Rheum. 2011;63:337–45.
45 Mease P, Genovese MC, Gladstein G, Kivits AJ, Ritchlin C, Tak PP, et al. Abatacept in the treatment of patients with psoriatic arthritis. Arthritis Rheum. 2011;63:939–48.
46 Von Schulthess GK, Steinert HC, Hany TF. Integrated PET/CT: current applications and future directions. Radiology. 2006;238:405–22.
47 Morbelli S, Piccardo A, Villavecchia G, Dessi B, Brugnolo A, Piccini A, et al. Mapping brain morphological and functional conversion patterns in amnestic MCI: a voxel-based MRI and FDG-PET study. Eur J Nucl Med Mol Imaging. 2010;37:36–45.
48 Wunder A, Straub RH, Gay S, Funk J, Müller-Ladner U. Molecular imaging: novel tools in visualizing rheumatoid arthritis. Rheumatology. (Oxford) 2005;44:1341–9.
49 Camellino D, Cimmino MA. Imaging of polymyalgia rheumatica: indications on its pathogenesis, diagnosis and prognosis. Rheumatology (Oxford) 2011 May 12 [Epub ahead of print].
50 Camellino D, Morbelli S, Sambuceti G, Cimmino MA. Methotrexate treatment of polymyalgia rheumatica/giant cell arteritis-associated large vessel vasculitis. Clin Exp Rheumatol. 2010;28:288–9.
51 Kubota K, Ito K, Morooka M, Mitsumoto T, Kurihara K, Yamashita H, et al. Whole-body FDG-PET/CT on rheumatoid arthritis of large joints. Ann Nucl Med. 2009;23:783–91.
52 Elzinga EH, van der Laken CJ, Comans EFI, Lammertsma AA, Dijkmans BAC, Voskuyl AE. 2-Deoxy-2-[F18]fluoro-D-glucose joint uptake on positron emission tomography images: rheumatoid arthritis versus osteoarthritis. Mol Imaging Biol. 2007;9:357–60.
53 Beckers C, Jeukens X, Ribbens C, André B, Marcelis S, Leclercq P, et al. 18F-FDG PET imaging of rheumatoid knee synovitis correlates with dynamic magnetic resonance and sonographic assessments as well as with the serum level of metalloproteinase-3. Eur J Nucl Med Mol Imaging. 2006;33:275–80.
54 Beckers C, Ribbens C, André B, Marcelis S, Kaye O, Mathy L, et al. Assessment of disease activity in rheumatoid arthritis with 18F-FDG PET. J Nucl Med. 2004;45:956–64.
55 Chaudari AJ, Bowen SL, Burkett GW, Packard NJ, Godinez F, Joshi AA, et al. High resolution 18F-FDG PET with MRI for monitoring response to treatment in rheumatoid arthritis. Eur J Nucl Med Mol Imaging. 2010;37:1047.
56 Vogel WV, van Riel PLCM, Oyen WJG. FDG-PET/CT can visualize the extent of inflammation in rheumatoid arthritis of the tarsus. Eur J Nucl Med Mol Imaging. 2007;34:439.
57 Taniguchi Y, Arii K, Kumon Y, Fukumoto M, Ohnishi T, Horino T, et al. Positron emission tomography/computed tomography: a clinical tool for evaluation of enthesitis in patients with spondyloarthritides. Rheumatology. (Oxford) 2010;49:348–54.
58 Palframan R, Airey M, Moore A, Vugler A, Nesbitt A. Use of biofluorescence imaging to compare the distribution of certulizumab pegol, adalimumab, and infliximab in the inflamed paws of rats with collagen-induced arthritis. J Immunol Met. 2009;348:36–41.
59 Werner S, Ohrndorf S, Langer HE, Bahner M, Schwenke C, Burmester GR, et al. Comparison of xiralite with clinical examination and ultrasonography. Ann Rheum Dis. 2011;70(Suppl 3):361.


