Challenges in transcatheter aortic valve implantation
DOI: https://doi.org/10.4414/smw.2012.13735
Stefan
Toggweiler, John
Webb
Summary
Although safety and efficacy of TAVI was improved with next-generation equipment, experience, and careful patient selection, some worrisome complications associated with the procedure remain. Current hot topics in transcatheter aortic valve implantation include patient selection, valve sizing, paravalvular regurgitation, cerebrovascular accidents, vascular complications and need for a permanent pacemaker. In this article we review the pathophysiology, avoidance and treatment options for these complications.
Introduction
Transcatheter aortic valve replacement (TAVI) has emerged as the current therapy of choice in patients with severe aortic valve stenosis who are not candidates for open-heart surgery [1–3], and as an alternative to high risk surgery in patients who are operable [4]. Although safety and efficacy of TAVI has improved with next-generation equipment, experience and careful patient selection, some worrisome complications associated with the procedure remain. In this paper we review some of the challenges the operator faces when performing TAVI, including patient selection, valve sizing, cerebrovascular events, vascular complications and need for a permanent pacemaker. A review article by Ferrari and von Segesser discussing different valve and access routes has been previously published in this journal [5].
Patient selection
Studies with long-term follow-up have shown that with current patient selection median survival after TAVI is ~3 years [3, 6]. Therefore, TAVI should probably not be performed in the presence of relevant comorbidities that limit life-expectancy to less than 2–3 years. Also, TAVI should result in significantly improved quality of life. Patients should be discussed and selected by a multidisciplinary heart team including cardiologists and surgeons [7]. There are subgroups of patients that may be excellent candidates for TAVI (porcelain aorta, previous coronary artery bypass grafting).
Inoperable patients
The PARTNER trial (cohort B) randomised inoperable patients to medical therapy or transfemoral TAVI [1]. The Society of Thoracic Surgeons’ (STS-Score) predicted rate of periprocedural mortality was 11%, the actual 30-day mortality was 5.0% with TAVI and 2.8% with medical treatment. At 1 year mortality was 30.7% with TAVI compared with 50.7% with medical treatment, representing an impressive absolute reduction of 20%. However, the high mortality rate of 30.7% in patients treated with transfemoral TAVI indicates that many patients died from their comorbidities. Subgroup analyses have shown that patients with a very high risk (STS score >15%) did not derive benefit from TAVI. Hence in the presence of multiple (non-cardiac) comorbidities TAVI may be less effective since some patients are likely to die from other, non-cardiac causes (“futility”).
High-risk patients
In cohort A of the PARTNER trial, patients with a mean STS score of 11.8% were randomised to TAVI and surgical AVR. Actual 30 days’ mortality was 3.4% with TAVI and 6.5% with SAVR (p = 0.07) [4]. Mortality 1 year after randomisation was 24.2% with TAVI and 26.8% with surgical AVR. TAVI was associated with a lower rate of bleeding, a shorter hospital stay and more vascular complications. There was a non-significant trend to more strokes with TAVI, but the combined risk of death or major stroke was nonsignificantly lower with TAVI.
Intermediate- and low-risk patients
Large studies comparing TAVI to surgical AVR in intermediate risk patients are currently under way (Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) and PARTNER II). As a result of improvements in TAVI technology, experience and outcome in registries, it appears reasonable to offer TAVI to patients who would otherwise be considered intermediate to low risk where there is a consensus of the heart team that they are at significantly increased risk of morbidity or mortality due to special circumstances [7]. There is evidence that improved outcomes can be expected in lower surgical risk patients undergoing TAVI [8, 9].
Valve sizing / paravalvular regurgitation
Annular sizing
Unlike in open heart surgery, where the aortic annulus can be directly inspected and sized intraoperatively, TAVI operators depend on external, indirect measurements of the annulus. Correct sizing of the annulus and choice of prosthesis is of the utmost importance if paravalvular leakage, device embolisation and annular rupture are to be avoided. Traditionally the annulus has been measured with 2-dimensional echocardiography, with TTE in the parasternal long-axis view or with TEE in the ~130° view. Recent research has shown that the annulus is an oval structure where the long axis is on average 5–6 mm (approx. 20%) longer than the short axis [10, 11]. In patients with an oval-shaped annulus, one-dimensional diameter measurements may not be accurate and computed tomography (CT) may therefore be superior since it allows reconstruction and measurement of the annulus in its true plane. Three-dimensional TEE may offer the same advantages but this technique needs further investigation [12]. Several studies have shown that CT-measured mean annular diameters are usually 1–2 mm larger than TEE-measured one-dimensional diameters, thus resulting in recommendation of a larger prosthesis size in up to 40% of patients [10, 11, 13] (fig. 1). So far there is no evidence that this practice leads to increased frequency of annular rupture, but a recent study has shown that the degree of paravalvular regurgitation was less if the prosthesis was chosen on the basis of CT instead of echocardiographic measures [14]. Currently CT is on its way to becoming the gold standard for annulus measurement and choice of valve prosthesis size. Mean annular diameter, perimeter, and annular area have all been proposed to serve as the main parameter for prosthesis selection. During the cardiac cycle the generally elliptic aortic annulus assumes a more round shape in systole, thus increasing cross-sectional area. Circumference remains relatively constant during the cardiac cycle and may therefore be the better parameter to measure [15]. After implantation of a balloon-expandable valve (to a lesser degree also after implantation of a self-expanding valve), the area increases when an oval annulus becomes more circular, but there are no major changes to the circumference. Implanting a valve based on annular area may therefore lead to undersizing in the case of a very oval annulus. However, there is some evidence that area measurements may be more reproducible than circumference measurements with a higher inter-observer and a higher intra-observer correlation [11, 16]. Overall, it is currently unclear which of the measurements will prevail and will become the new gold standard for annular sizing.
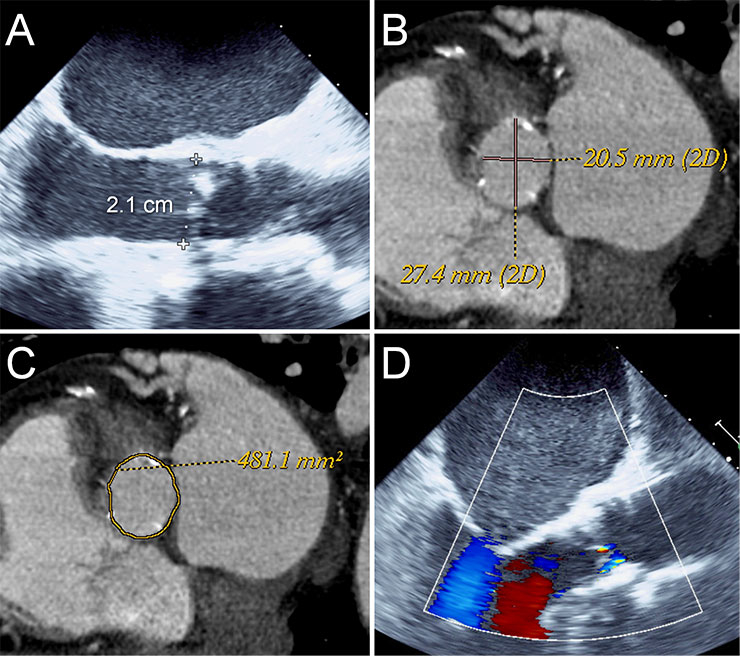
Figure 1
Annulus sizing using TEE and CT angiography.
TEE measured an annulus of 2.1 cm suggesting a 23 mm Edwards Sapien or a 26 mm Medtronic CoreValve (A). CT angiography measured an area of 481 mm2 (C) and a mean diameter of 24 mm (B) suggesting a 26 mm Edwards Sapien or a 29 mm Medtronic CoreValve valve with ~10% oversizing. A 26 mm Edwards Sapien valve was implanted with trace paravalvular leak (D).
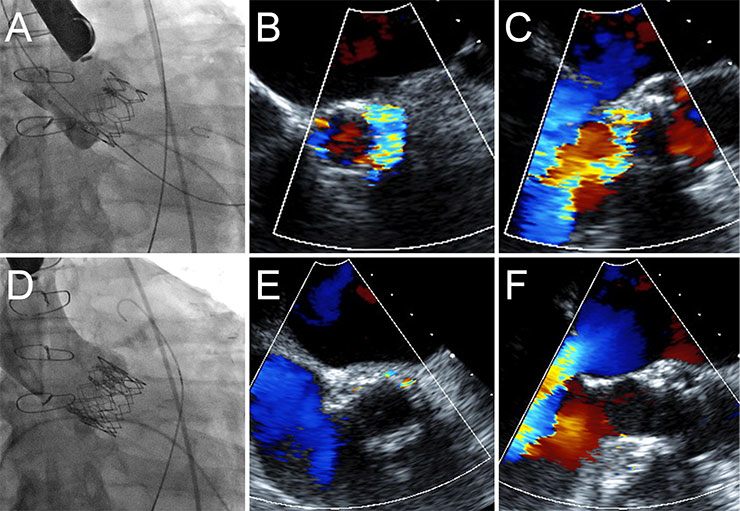
Figure 2
Treatment of paravalvular regurgitation by implantation of a second transcatheter heart valve.
In patient, the TEE measured an annulus of 22 mm. A 26 mm Edwards Sapien XT valve was implanted (A). A low implantation resulted in severe paravalvular aortic regurgitation (B, C). A second 26 mm valve was subsequently implanted in a higher position, thus extending the annular seal (D) resulting in a mild paravalvular leak (E, F).
Paravalvular regurgitation
Paravalvular regurgitation (PAR) is the result of undersizing and/or malpositioning of valves. Several studies have demonstrated an association between the degree of calcification of the native aortic valve cusps and the severity of PAR [17–19]. Grading of PAR has been done with echocardiography, angiography, and simultaneous pressure measurements of the aorta and the left ventricle [20]. In the PARTNER trial, postprocedural moderate or severe PAR was reported in 11.8 and 12.2% of patients after TAVI [1, 4]. At 1 year these rates were 7.0% after TAVI vs. 1.9% after surgical AVR. At 2 years, the respective rates were 6.9% and 0.9% [6]. In other publications the proportion of patients with postprocedural moderate or severe PAR ranged from 5.2–21% [21–26]. Several studies have consistently shown that moderate or severe paravalvular regurgitation is associated with increased mortality after both surgical AVR [27] and after TAVI with odds/hazard ratios ranging from 2.4–3.8 [20, 22, 28]. In the PARTNER trial, even mild paravalvular regurgitation was found to be significantly associated with increased mortality (hazard ratio of 2.11 for ≥ mild PAR compared to none or trace PAR) [6]. However, some questions about this finding remain. First of all, mild regurgitation of native valves is usually very well tolerated and it would be surprising if mild PAR affected clinical outcome during such a short follow-up period. One explanation for the excess mortality may be that these patients had more calcified valves, complex procedures and comorbidities, and that mild PAR was a bystander rather than the main culprit for mortality. In the PARTNER patients mortality between 2 and 3 years was higher in patients with mild PAR than in patients with moderate to severe PAR, a finding that is not intuitive [6]. Furthermore, severity of PAR is difficult to measure since multiple and eccentric jets may be present.
Treatment of paravalvular regurgitation
If relevant PAR occurs, the next step is to assess whether or not the valve is deployed in the correct position. If the valve is in the correct position postdilation may reduce the degree of PAR. If the valve appears undersized after postdilation the options are more limited, although paravalvular leak closure may be considered an option. However, most of the published data relate to paravalvular leak closure with surgically implanted valves, and experience with transcatheter valves is limited [29]. With transcatheter heart valves the shape of the leak may be circumferential and more than one leak may be present, making paravalvular leak closure more difficult or even impossible. In the event of malpositioning of the first valve, a second valve can be implanted higher (if the initial implant is too low) or lower (the initial implant is too high) than the first valve (fig. 2 ). The goal is to extend the sealing fabric in such a way that the sealing fabric of both valves overlaps and that the second valve ensures sealing with the native valve annulus [30, 31]. The same valve size is used for both valves.
Stroke
Clinical relevance of cerebrovascular accidents
Cerebrovascular accidents (CVA) have emerged as one of the most worrisome and most discussed complications in TAVI. In the PARTNER trial the rate of strokes at 30 days (4.6% vs. 2.4%), 1 year (6.0% vs. 3.2%) and 2 years (7.7% vs. 4.9%) was not significantly higher after TAVI than after SAVR [4, 6, 32]. Predictors for CVAs were prior CVA, a smaller indexed aortic valve area, higher NYHA class, prior stroke, history of PCI, and absence of COPD [32]. It has been shown that some 50% of CVAs occur within the first 2 days after TAVI and are associated with a 3.5–5 fold increased mortality [22, 32, 33].
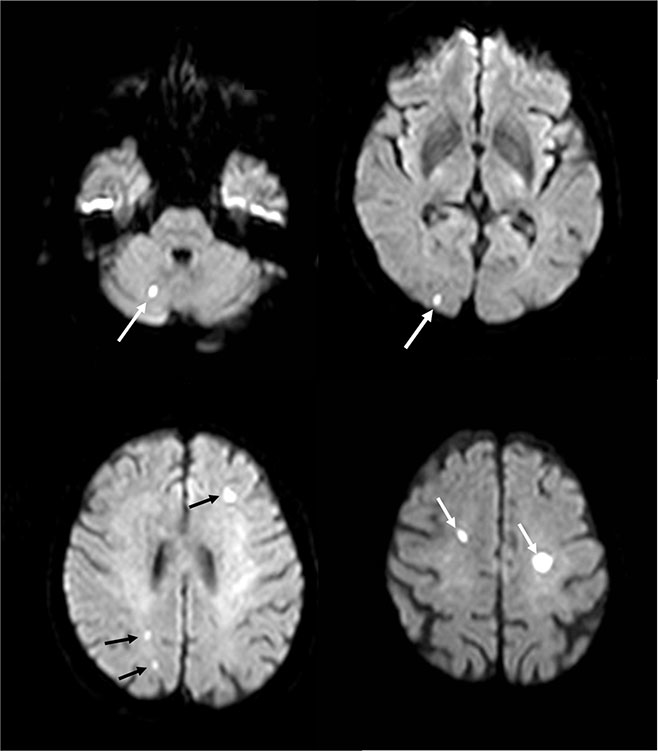
Figure 3
Silent bilateral cerebrovascular emboli after transcatheter aortic valve implantation.
Such diffuse, bihemispheric ischaemic lesions can be detected by MRT in 68–77% of patients after TAVI.
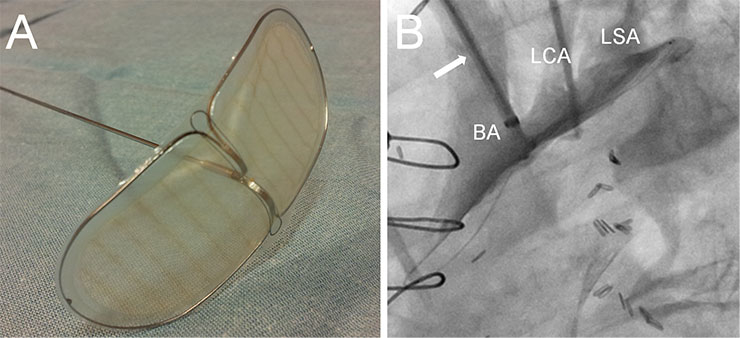
Figure 4
Example of an embolic protection device.
The Embrella embolic deflector (A, Embrella Cardiovascular Inc, Wayne, PA) is introduced through the right radial artery to protect the brachiocephalic artery (BA), the left carotid artery (LCA) and the left subclavian artery (LSA) during the procedure.
Periprocedural CVAs may result from embolisation of calcified microdebris during positioning and deployment of the valve, but also during passage of the aortic arch with the guidewire and the catheter, during balloon valvuloplasty or re-capture of a valve. Air embolism may be a concern, especially in patients undergoing transapical TAVI. Some of the next generation valves offer the possibility of re-capturing and re-positioning in the event of initial valve malposition [34]. However, additional manipulation and replacement of valves, as well as postdilation, have been associated with higher stroke rates [35, 36]. Cerebrovascular accidents not infrequently occur during the first 1–2 days after the procedure. These postprocedural strokes may be due to the non-endothelialised and thrombogenic bioprosthesis itself, new-onset atrial fibrillation, late calcific embolism, or possibly late thrombosis or haemorrhage following earlier embolism [37, 38].
In larger recent studies,the rate of acute strokes ranged from 0.8 [39] to 6.7% [1, 4, 22–25, 40–44], but the rate of silent cerebral ischaemia after TAVI was found to be much higher. Risk factors for cerebral embolisms are summarised in table 1.
Magnetic resonance and Doppler ultrasound studies
To investigate silent cerebrovascular embolism, Kahlert et al. performed diffusion-weighted magnetic resonance tomographies (MRT) in patients undergoing TAVI (n = 32) and open surgical aortic valve replacement (n = 21) [45]. After a median of 3.4 days post procedure new foci were found in 84% of patients undergoing TAVI (fig. 3 ). There was no clinical stroke in the TAVI group (but one stroke in the SAVR group), and there was no difference between the balloon-expandable Sapien valve (Edwards Lifesciences Inc, Irvine, CA) and the self-expanding CoreValve (Medtronic Inc., Minneapolis, MN). At 3 months the majority (80%) of the acute lesions had resolved. These findings were confirmed by other studies, showing that diffuse bihemispheric ischaemic lesions can be detected by MRT in 68–77% of patients after TAVI [46–49]. No differences between the transfemoral and the transapical approach were found, although some have suggested that transapical TAVI may result in less trauma to the aortic valve and aortic arch, since less manipulation of catheters is needed [46, 50]. However, there is no evidence to support this. The incidence of clinically relevant strokes correlated with the number and size of lesions on MRT in some [49] but not all studies [47]. Therefore the clinical significance of these lesions remains unclear, although evidence from the surgical literature indicates that multiple, apparently silent, cerebral infarctions increase the risk of neurological dysfunction and cognitive decline [51].
Several studies used transcranial Doppler ultrasound (TCD) with the aim of quantifying the high-intensity transient (HITS) and microembolic signals (MES) during different stages of the procedure. In transapical balloon-expandable TAVI with the Edwards Sapien valve, most HITS and MES were counted during balloon valvuloplasty and valve delivery [52], but also during manipulation of the wire in the aortic arch [53, 54]. Severe calcification of the arch was identified as a predictor for HITS [54], but the number of HITS did not differ between transfemoral and transapical procedures [53, 54]. One study showed an overall higher rate of HITS during TAVI with the self expanding CoreValve than the balloon-expandable Sapien valve, but with both valves most HITS occurred during valve deployment [53]. However, the number of HITS did not correlate with new lesions on cerebral CT. Furthermore, the high amount of HITS and new lesions on cerebral CT contrasts with the low rate of clinical cerebrovascular events.
Embolic protection devices
Several strategies to reduce acute stroke rate have been suggested. These include not performing BAV, minimising the passage of guide wires and catheters across the aortic arch, and utilisation of embolic protection devices [32, 55–57]. Strategies to reduce the rate of late CVAs include oral anticoagulation in the event of new onset atrial fibrillation.
Different embolic protection devices are currently in use and under investigation [57–59]. These devices are introduced at the beginning of the procedure either through the right radial or the femoral artery and their aim is to protect the brachiocephalic and left carotid artery from embolic material (fig. 4 ). An ideal protection device would be minimally invasive, easy and rapid to use with minimal risk of vascular injury, and should not interfere with the actual TAVI procedure. The current devices are composed of a thin membrane with pores of about 100–200 µm size designed to allow normal blood flow but deflect or capture embolic material. While these devices certainly have the potential to reduce cerebral embolism, experience with TAVI is limited [57]. As TAVI is currently performed in elderly patients who often have very calcified arteries, it is possible that deployment of the protection device itself results in embolisation of some debris. In the future these devices may become more important if TAVI is performed in younger patients with a less calcified aortic arch where the main source of embolism is the valve itself.
|
Table 1: Predictors of cerebrovascular embolism/accidents after TAVI. |
|
First author
|
Modality
|
Predictors of cerebrovascular embolism/accidents
|
| Kahlert [45] |
MRT |
Renal dysfunction, lower aortic atheroma thickness, porcelain aorta. |
| Fairbairn [49] |
MRT |
Age, severity of aortic arch atheroma. |
| Szeto [54] |
TCD |
Severe calcification of aortic arch. |
| Miller [32] |
Clinical |
Cerebrovascular disease, smaller indexed aortic valve area, higher NYHA class, prior stroke, history of PCI, COPD (lower risk). |
| Nuis [37] |
Clinical |
New onset atrial fibrillation, baseline aortic regurgitation ≥3. |
| Rodes-Cabau [46] |
Clinical |
New onset atrial fibrillation. |
Vascular complications
Clinical relevance of vascular complications
Despite the gradual decrease in sheath and catheter size, vascular complications remain one of the challenges in TAVI. Table 2 summarises the currently required sheath sizes for various valves. The Valve Academic Research Consortium (VARC) has proposed standardised definitions for clinical endpoints [60, 61]. Recently, VARC 2 has revised the VARC definitions and recommendations. According to the VARC, vascular complications include all complications that can be caused by a wire and/or are related to vascular access (e.g. major complications such as ventricular perforation, aortic dissection/rupture, iliofemoral rupture, iliofemoral dissection requiring treatment, and minor complications such as pseudoaneurysms or closure failure). Most of the recent publications reported the incidence of vascular complications according to these definitions, allowing direct comparison between studies. In such studies, major vascular complications have been reported in 5.6 to 17.3% [1, 4, 23, 41, 62–64]. In earlier studies, where various definitions were used, vascular complication rates ranged from 1.9 to 11.7% [22, 44, 65–67].
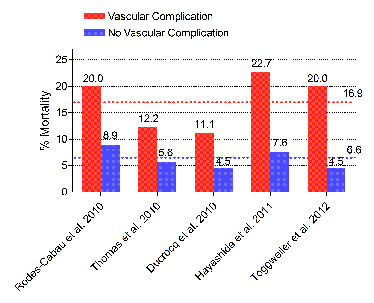
Figure 5
Impact of vascular complications on 30-day mortality.
30-day mortality in patients with a (major) vascular complication was consistently higher than in those without. In pooled analysis of all 5 studies including >1000 patients, mortality was 16.9% (red dotted line) and 6.6% (blue dotted line) in patients with and without vascular complications.
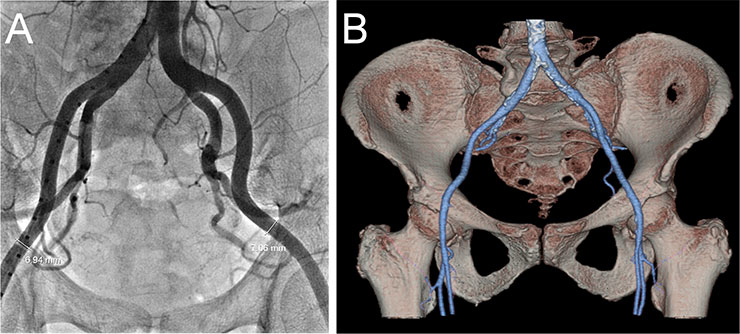
Figure 6
Screening of the iliofemoral arteries by fluoroscopy (A) and CT angiography (B).
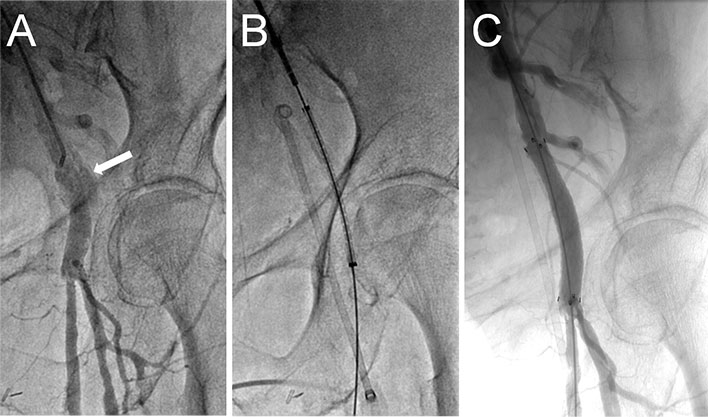
Figure 7
Treatment of femoral artery injury by a covered stent.
This patient presented with acute haemorrhage of the femoral artery (A). Bleeding was controlled by balloon tamponade, after which a Fluency Plus 9 x 60 mm covered self-expanding stentgraft (Bard Canada Inc., Oakville, Canada) was implanted with a good result (B, C).
Vascular complications have been associated with mortality, morbidity, reduced quality of life, and costs. 30 day mortality was found to be consistently higher in patients with vascular complications than those without (odds ratios ranging from 2.4 to 8.5) [42, 43, 62, 64, 68]. Pooled together, all 5 studies included >1,000 patients. 30-day mortality in patients with vascular access complications was 16.9%, in those without 6.6% (odds ratio 2.88, 95% confidence interval 1.71–4.86, fig. 5 ). Furthermore, length of hospital stay was ~ 5-7 days longer in patients with vascular complications than in those without [62, 64].
The following risk factors for vascular complications have been identified: external sheath diameter > minimal artery diameter, moderate or severe femoral calcification, presence of peripheral vascular disease, and centre experience (table 3) [62, 64, 67]. A smaller minimal iliofemoral diameter may be acceptable in the absence of circumferential or horseshoe shaped calcification, as the ability of the artery to expand is preserved. The presence of extensive tortuosity and aneurysms has not been associated with higher complication rates. The most important step to reduce vascular complication rates is therefore systematic access screening.
Access screening and current sheaths
Screening of iliofemoral access is usually accomplished using fluoroscopic angiography and/or CT angiography (fig. 6 ). Several protocols have been developed to reduce the amount of contrast dye required for CT scans in patients with reduced renal function [69, 70]. Current sheaths used for TAVI are 30–35 cm long, have an inner diameter of 14–20 French and an outer diameter of 5.9–7.8 mm. However, there are some exceptions. Following the slogan “think big”, the larger 22–24 F sheaths are still required for TAVI in the USA. There are several expandable sheaths which are introduced in a low profile configuration reducing the risk of iliofemoral injury. The Edwards expandable introducer sheath (Edwards Lifesciences, Irvine, CA) has a compliant seam that allows transient expansion as the delivery catheter is passed through it [71]. After passage of the prosthesis the sheath contracts to some degree towards its unexpanded size. The SoloPath sheath (Onset Medical Corporation, Irvine, CA) is a balloon expandable sheath that is inserted in its unexpanded state with an outer diameter of only 4.3 mm, facilitating delivery through difficult anatomy. A balloon is then inflated to expand the sheath and reach the intended internal diameter of 18–21 F.
Iliofemoral complications
Most centres have initially used a planned surgical cut-down to expose the iliofemoral artery to expose and control the artery above and below the puncture site [1, 4, 21, 44, 68, 72, 73]. More recently the preclosure technique has been used employing ProStar or ProGlide devices (Abbott Vascular Inc, Redwood City, CA) [22, 26, 40, 41, 62–67, 74–76]. This facilitates performance of the procedure under conscious sedation with local anaesthesia, and allows earlier patient ambulation [77, 78]. However, a surgical cut-down might be particularly desirable in patients where a high puncture is needed due to extensive calcification, to a high femoral bifurcation, obesity, or the presence of a femoral stent/graft.
Iliofemoral complications are the most frequent vascular complications in transfemoral TAVI. On average, dissection of the iliofemoral arteries has been reported in 6.5% (range: 1.6–21.5%), and iliofemoral rupture in 3.4% (range 0.7–9.3%) [21, 24, 44, 62, 64, 68, 74, 76, 70–81]. Mortality was 5.6% after iliofemoral dissection, but 30% after iliofemoral rupture. Other iliofemoral complications include bleeding (~7.3%), percutaneous closure failure (~6.8%), access site infection (~2.3%, chiefly after a planned surgical cut-down), pseudoaneurysms requiring intervention (~1.5%), and sheath avulsion (rare) [1, 4, 21–23, 26, 39–41, 44, 62–68, 72–76, 82].
Treatment of iliofemoral dissections depends on the extent and haemodynamic relevance. Usually, prolonged inflation of a balloon with appropriate diameter results in successful apposition of the intima and underlying media. More extensive dissection may warrant self-expanding or balloon-expandable stent deployment [83].
Similarly, treatment of iliofemoral rupture depends on the size of the rupture and the severity of bleeding. A smaller rupture may be difficult to diagnose since symptoms tend to be delayed and nonspecific (haemodynamic instability, groin/flank/abdominal/back pain). A computed tomography of the abdomen and pelvis allows rapid diagnosis. If the perforation is smaller, cross-over balloon inflation for up to 5–10 minutes may be enough to stop the bleeding. If there is ongoing bleeding, implantation of a covered stent (fig. 7 ) is often technically successful with high patency rates at mid-term follow-up [84]. Alternatively, surgical repair should be considered. In the presence of major injury, aortic balloon tamponade with an occlusion balloon may be life-saving.
Failed percutaneous closure may be treated with prolonged manual compression alone, or, in the case of ongoing or more severe bleeding, with balloon angioplasty, stent implantation or surgery. Pseudoaneurysms close spontaneously in >50% of cases and can therefore be observed if the size is <3–3.5 cm, in the absence of pain, and if oral anticoagulation is not needed [85–87]. The treatment of choice is ultrasound-guided thrombin injection, which has a success rate of almost 100% and a complication rate of <1% [88–94].
Complications of the aorta and ventricles
These severe complications include aortic dissection, rupture and left-right ventricular perforation, and are reported in <2% of transfemoral TAVI but usually associated with a >50% mortality rate [64, 74, 76, 81]. Annular rupture may occur after balloon valvuloplasty or after implantation of an oversized valve. The risk of perforation may be higher with a balloon expandable valve and in patients with a very bulky, calcified valve which can perforate the sinus during valve implantation. Left ventricular perforation is usually caused by perforation with the stiff guidewire, by the crossing catheter (usually AL-1), or by the device nosecone. Right ventricular perforation is usually due to perforation with the temporary pacemaker wire. Annular or ventricular perforation often leads to pericardial tamponade, which causes hypotension and can be rapidly detected by transoesophageal or transthoracic echocardiography. If tamponade occurs, pericardiocentesis should be performed immediately. If haemostasis is unlikely, rapid surgical exploration and suture of the injury is usually technically successful. However, even with immediate treatment tamponade is often associated with (intraprocedural) death [79, 95].
|
Table 2: Commonly used large sheaths. |
|
Valve
|
Commonly used sheaths (examples)
|
Sheath internal diameter
|
Sheath external diameter
|
| Edwards Sapien 23 mm |
RetroFlex 3 introducer sheath |
22 F |
8.4 mm |
| Edwards Sapien 26 mm |
RetroFlex 3 introducer sheath |
24 F |
9.2 mm |
| Edwards Sapien XT 23 mm |
NovaFlex introducer sheath |
18 F |
7.2 mm* |
| |
Edwards expandable sheath |
16 F |
6.6 mm* |
| Edwards Sapien XT 26 mm |
NovaFlex introducer sheath |
19 F |
7.5 mm |
| |
Edwards expandable sheath |
18 F |
7.2 mm* |
| Edwards S3 26 mm |
Edwards expandable sheath |
14F |
5.9 mm* |
| Edwards Sapien XT 29 mm |
Edwards expandable sheath |
20 F |
7.8 mm* |
| Medtronic CoreValve 26‒29 mm and St. Jude Portico 26 mm |
St. Jude Medical Ultimum |
18 F |
6.8 mm |
| |
Cook Check-Flo Introducer |
18 F |
7.2 mm |
| |
Onset Medical SoloPath Balloon Expandable Transfemoral Introducer |
19 F |
7.3 mm† |
| |
Gore Medical DrySheath |
18 F |
6.8 mm |
| * The pre-expanded diameter is indicated. The fully expanded diameter depends on the size of the catheter used for the procedure. † The final outer diameter is indicated for the SoloPath Balloon Expandable Transfemoral Introducer. The unexpanded outer diameter is 4.3 mm. |
|
Table 3: Predictors of vascular complications. |
|
First author
|
N
|
Predictors
|
| Lange [67] |
412 |
Centre experience, planned surgical cut-down reduced vascular complication rate. |
| Hayashida [64] |
130 |
Sheath to femoral artery ratio (SFAR), femoral calcification, centre experience. |
| Toggweiler [62] |
137 |
Sheath diameter > minimal artery diameter, moderate/severe femoral calcification, learning curve. |
Need for a permanent pacemaker
Post-procedural conduction disorders requiring implantation of a permanent pacemaker (PPM) are more frequent after TAVI than after surgical AVR [96–98]. Mechanical trauma to the His bundle in the region of the subvalvular membranous septum, localised oedema and inflammation may contribute to atrioventicular conduction disorders following TAVI. Trauma may occur either directly by the implanted transcatheter heart valve or by compression through the calcified native aortic valve cusps. The most frequent disorder is a new left bundle branch block. Conduction disorders such as complete atrioventricular block or symptomatic bradycardia may occur acutely or delayed (more often with the Medtronic CoreValve), chiefly within the first week [96, 99].
Frequency of pacemaker implant
Need for a permanent pacemaker has been reported in ~6% (range: 3.4–22%) of patients undergoing TAVI with the Edwards Sapien valve and ~21% (Range: 16.6–42.5%) with the Medtronic CoreValve [1, 4, 21, 23, 40, 43, 44, 63, 73, 79, 97]. In centres using both the Edwards Sapien and the CoreValve, a pre-existing RBBB may be a reason for using the Edwards Sapien valve to decrease the need for a PPM, but a recent study has shown that lower PPM rates can be achieved with higher implantation of the CoreValve [100]. In this study the targeted implantation depth of 4–6 mm was achieved in 69%. A transient or sustained complete atrioventricular block occurred in 12.7%, a pacemaker was implanted in 10.6% after a mean of 4.4 ± 5.9 days post TAVI. Another study reported a PPM rate of 11.7% after CoreValve implantation without balloon predilatation [101]. Using smaller balloons for valvuloplasty may also reduce the need for PPM.
Predictors of pacemaker requirement
In a meta-analysis including a total of 5,258 patients a pre-existing right bundle branch block (RBBB) and implantation of a CoreValve were the only predictors of pacemaker requirement [102]. Other studies have identified low implantation depth (of both Edwards Sapien and Medtronic CoreValve), valve oversizing, bradycardia, a pre-existing atrioventricular block, and higher age as risk factors for a new PPM [97, 98, 103–105]. In patients at high risk of PPM, prophylactic implantation of a PPM may be performed.
Relevance of pacemaker implantation
Compared to other complications such as stroke, major vascular injury, death and acute severe paravalvular regurgitation, PPM implantation is considered a benign event. However, it does require an additional procedure, adds costs and may result in prolonged hospitalisation. Chronic right ventricular pacing may result in left ventricular dyssynchrony and reduced left ventricular contractility [106]. Survival up to 1 year follow-up was not worse in patients requiring a PPM after TAVI, but the long-term effects of right ventricular pacing are unknown [107].
Conclusion
The safety and efficacy of TAVI have improved with next-generation low-profile equipment, experience and careful patient selection. Computed tomography allows more accurate annular and iliofemoral measurements, improving both valve sizing and selection of an optimal access route. Some of the next generation valves will allow repositioning and are designed to reduce paravalvular leaks. Hopefully stroke rates will decrease with experience, optimal antithrombotic treatment and patient selection.
Correspondence: Stefan Toggweiler, MD, Luzerner Kantonsspital, Spitalstrasse, CH-6000 Luzern 16, Switzerland, stefan.toggweiler[at]luks.ch
References
1 Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607.
2 Thomas M. The global experience with percutaneous aortic valve replacement. JACC Cardiovasc Interv. 2010;3:1103–9.
3 Gurvitch R, Wood DA, Tay EL, Leipsic J, Ye J, Lichtenstein SV, et al. Transcatheter aortic valve implantation: Durability of clinical and hemodynamic outcomes beyond 3 years in a large patient cohort. Circulation. 2010;122:1319–27.
4 Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98.
5 Ferrari E, von Segesser LK. Transcatheter aortic valve implantation (tavi): State of the art techniques and future perspectives. Swiss Med Wkly. 2010;140:w13127.
6 Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686–95.
7 Webb J, Rodes-Cabau J, Fremes S, Pibarot P, Ruel M, Ibrahim R, et al. Transcatheter aortic valve implantation: A Canadian cardiovascular society position statement. Can J Cardiol. 2012;28:520–8.
8 Piazza N, Lange R, Martucci G, Serruys PW. Patient selection for transcatheter aortic valve implantation: Patient risk profile and anatomical selection criteria. Arch Cardiovasc Dis. 2012;105:165–73.
9 Lange R, Bleiziffer S, Mazzitelli D, Elhmidi Y, Opitz A, Krane M, et al. Improvements in transcatheter aortic valve implantation outcomes in lower surgical risk patients: A glimpse into the future. J Am Coll Cardiol. 2012;59:280–7.
10 Messika-Zeitoun D, Serfaty JM, Brochet E, Ducrocq G, Lepage L, Detaint D, et al. Multimodal assessment of the aortic annulus diameter: Implications for transcatheter aortic valve implantation. J Am Coll Cardiol. 2010;55:186–94.
11 Willson AB, Webb JG, Labounty TM, Achenbach S, Moss R, Wheeler M, et al. 3-dimensional aortic annular assessment by multidetector computed tomography predicts moderate or severe paravalvular regurgitation after transcatheter aortic valve replacement: A multicenter retrospective analysis. J Am Coll Cardiol. 2012;59:1287–94.
12 Ng AC, Delgado V, van der Kley F, Shanks M, van de Veire NR, Bertini M, et al. Comparison of aortic root dimensions and geometries before and after transcatheter aortic valve implantation by 2- and 3-dimensional transesophageal echocardiography and multislice computed tomography. Circ Cardiovasc Imaging. 2010;3:94–102.
13 Willson AB, Webb JG, Labounty TM, Achenbach S, Moss R, Wheeler M, et al. 3-dimensional aortic annular assessment by multidetector computed tomography predicts moderate or severe paravalvular regurgitation after transcatheter aortic valve replacement a multicenter retrospective analysis. J Am Coll Cardiol. 2012
14 Jilaihawi H, Kashif M, Fontana G, Furugen A, Shiota T, Friede G, et al. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol. 2012;59:1275–86.
15 Hamdan A, Guetta V, Konen E, Goitein O, Segev A, Raanani E, et al. Deformation dynamics and mechanical properties of the aortic annulus by 4-dimensional computed tomography insights into the functional anatomy of the aortic valve complex and implications for transcatheter aortic valve therapy. J Am Coll Cardiol. 2012;59:119–27.
16 Gurvitch R, Webb JG, Yuan R, Johnson M, Hague C, Willson AB, et al. Aortic annulus diameter determination by multidetector computed tomography: Reproducibility, applicability, and implications for transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2011;4:1235–45.
17 Ewe SH, Ng AC, Schuijf JD, van der Kley F, Colli A, Palmen M, et al. Location and severity of aortic valve calcium and implications for aortic regurgitation after transcatheter aortic valve implantation. Am J Cardiol. 2011;108:1470–7.
18 Gripari P, Ewe SH, Fusini L, Muratori M, Ng AC, Cefalu C, et al. Intraoperative 2d and 3d transoesophageal echocardiographic predictors of aortic regurgitation after transcatheter aortic valve implantation. Heart. 2012;98:1229–36.
19 Colli A, D’Amico R, Kempfert J, Borger MA, Mohr FW, Walther T. Transesophageal echocardiographic scoring for transcatheter aortic valve implantation: Impact of aortic cusp calcification on postoperative aortic regurgitation. J Thorac Cardiovasc Surg. 2011;142:1229–35.
20 Sinning JM, Hammerstingl C, Vasa-Nicotera M, Adenauer V, Lema Cachiguango SJ, Scheer AC, et al. Aortic regurgitation index defines severity of peri-prosthetic regurgitation and predicts outcome in patients after transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;59:1134–41.
21 Webb JG, Pasupati S, Humphries K, Thompson C, Altwegg L, Moss R, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation. 2007;116:755–63.
22 Tamburino C, Capodanno D, Ramondo A, Petronio AS, Ettori F, Santoro G, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308.
23 Gurvitch R, Toggweiler S, Willson AB, Wijesinghe N, Cheung A, Wood DA, et al. Outcomes and complications of transcatheter aortic valve replacement using a balloon expandable valve according to the valve academic research consortium (varc) guidelines. EuroIntervention. 2011;7:41–8.
24 Eltchaninoff H, Prat A, Gilard M, Leguerrier A, Blanchard D, Fournial G, et al. Transcatheter aortic valve implantation: Early results of the france (french aortic national corevalve and edwards) registry. Eur Heart J. 2011;32:191–7.
25 Ye J, Cheung A, Lichtenstein SV, Nietlispach F, Albugami S, Masson JB, et al. Transapical transcatheter aortic valve implantation: Follow-up to 3 years. J Thorac Cardiovasc Surg. 2010;139:1107–13, 1113 e1101.
26 Tchetche D, Dumonteil N, Sauguet A, Descoutures F, Luz A, Garcia O, et al. Thirty-day outcome and vascular complications after transarterial aortic valve implantation using both edwards sapien and medtronic corevalve bioprostheses in a mixed population. EuroIntervention. 2010;5:659–65.
27 Sponga S, Perron J, Dagenais F, Mohammadi S, Baillot R, Doyle D, et al. Impact of residual regurgitation after aortic valve replacement. Eur J Cardiothorac Surg. 2012.
28 Abdel-Wahab M, Zahn R, Horack M, Gerckens U, Schuler G, Sievert H, et al. Aortic regurgitation after transcatheter aortic valve implantation: Incidence and early outcome. Results from the german transcatheter aortic valve interventions registry. Heart. 2011;97:899–906.
29 Noble S, Basmadjian A, Ibrahim R. Transcatheter prosthetic paravalvular leak closure. Cardiovascular Medicine. 2012;15:245–52.
30 Toggweiler, Wood D, Rodes-Cabau J, Kapadia S, Willson AB, Ye J, et al. Transcatheter valve-in-valve implantation for failed balloon expandable transcatheter aortic valves. JACC Cardiovasc Interv. 2012; In press.
31 Ussia GP, Barbanti M, Ramondo A, Petronio AS, Ettori F, Santoro G, et al. The valve-in-valve technique for treatment of aortic bioprosthesis malposition: an analysis of incidence and 1-year clinical outcomes from the italian corevalve registry. J Am Coll Cardiol. 2011;57:1062–8.
32 Miller DC, Blackstone EH, Mack MJ, Svensson LG, Kodali SK, Kapadia S, et al. Transcatheter (tavr) versus surgical (avr) aortic valve replacement: Occurrence, hazard, risk factors, and consequences of neurologic events in the partner trial. J Thorac Cardiovasc Surg. 2012;143:832–843 e813.
33 Eggebrecht H, Schmermund A, Voigtlander T, Kahlert P, Erbel R, Mehta RH. Risk of stroke after transcatheter aortic valve implantation (tavi): A meta-analysis of 10,037 published patients. EuroIntervention. 2012;8:129–38.
34 Willson AB, Rodes-Cabau J, A. WD, Leipsic J, Cheung A, Toggweiler S, et al. Transcatheter aortic valve replacement with the St. Jude medical portico valve: First-in-human experience. J Am Coll Cardiol. 2012; In press.
35 Geisbusch S, Bleiziffer S, Mazzitelli D, Ruge H, Bauernschmitt R, Lange R. Incidence and management of corevalve dislocation during transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2010;3:531–6.
36 Nombela-Franco L, Rodes-Cabau J, DeLarochelliere R, Larose E, Doyle D, Villeneuve J, et al. Predictive factors, efficacy, and safety of balloon post-dilation after transcatheter aortic valve implantation with a balloon-expandable valve. JACC Cardiovasc Interv. 2012;5:499–512.
37 Nuis RJ, Van Mieghem NM, Schultz CJ, Moelker A, van der Boon RM, van Geuns RJ, et al. Frequency and causes of stroke during or after transcatheter aortic valve implantation. Am J Cardiol. 2012;109:1637–43.
38 Amat-Santos IJ, Rodes-Cabau J, Urena M, Delarochelliere R, Doyle D, Bagur R, et al. Incidence, predictive factors, and prognostic value of new-onset atrial fibrillation following transcatheter aortic valve implantation. J Am Coll Cardiol. 2011;59:178–88.
39 Osten MD, Feindel C, Greutmann M, Chamberlain K, Meineri M, Rubin B, et al. Transcatheter aortic valve implantation for high risk patients with severe aortic stenosis using the edwards sapien balloon-expandable bioprosthesis: A single centre study with immediate and medium-term outcomes. Catheter Cardiovasc Interv. 2010;75:475–85.
40 Zahn R, Gerckens U, Grube E, Linke A, Sievert H, Eggebrecht H, et al. Transcatheter aortic valve implantation: First results from a multi-centre real-world registry. Eur Heart J. 2011;32:198–204.
41 Stahli BE, Bunzli R, Grunenfelder J, Buhler I, Felix C, Bettex D, et al. Transcatheter aortic valve implantation (tavi) outcome according to standardized endpoint definitions by the valve academic research consortium (varc). J Invasive Cardiol. 2011;23:307–12.
42 Thomas M, Schymik G, Walther T, Himbert D, Lefevre T, Treede H, et al. Thirty-day results of the sapien aortic bioprosthesis european outcome (source) registry: A european registry of transcatheter aortic valve implantation using the edwards sapien valve. Circulation. 2010;122:62–9.
43 Rodes-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: Acute and late outcomes of the multicenter canadian experience. J Am Coll Cardiol. 2010;55:1080–90.
44 Webb JG, Altwegg L, Boone RH, Cheung A, Ye J, Lichtenstein S, et al. Transcatheter aortic valve implantation: Impact on clinical and valve-related outcomes. Circulation. 2009;119:3009–16.
45 Kahlert P, Knipp SC, Schlamann M, Thielmann M, Al-Rashid F, Weber M, et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: A diffusion-weighted magnetic resonance imaging study. Circulation. 2010;121:870–8.
46 Rodes-Cabau J, Dumont E, Boone RH, Larose E, Bagur R, Gurvitch R, et al. Cerebral embolism following transcatheter aortic valve implantation: Comparison of transfemoral and transapical approaches. J Am Coll Cardiol. 2011;57:18–28.
47 Arnold M, Schulz-Heise S, Achenbach S, Ott S, Dorfler A, Ropers D, et al. Embolic cerebral insults after transapical aortic valve implantation detected by magnetic resonance imaging. JACC Cardiovasc Interv. 2010;3:1126–32.
48 Ghanem A, Muller A, Nahle CP, Kocurek J, Werner N, Hammerstingl C, et al. Risk and fate of cerebral embolism after transfemoral aortic valve implantation: A prospective pilot study with diffusion-weighted magnetic resonance imaging. J Am Coll Cardiol. 2010;55:1427–32.
49 Fairbairn TA, Mather AN, Bijsterveld P, Worthy G, Currie S, Goddard AJ, et al. Diffusion-weighted mri determined cerebral embolic infarction following transcatheter aortic valve implantation: Assessment of predictive risk factors and the relationship to subsequent health status. Heart. 2012;98:18–23.
50 Hynes BG, Rodes-Cabau J. Transcatheter aortic valve implantation and cerebrovascular events: The current state of the art. Ann. N.Y. Acad. Sci. 2012; In press.
51 Goto T, Baba T, Honma K, Shibata Y, Arai Y, Uozumi H, Okuda T. Magnetic resonance imaging findings and postoperative neurologic dysfunction in elderly patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2001;72:137–42.
52 Drews T, Pasic M, Buz S, Unbehaun A, Dreysse S, Kukucka M, et al. Transcranial doppler sound detection of cerebral microembolism during transapical aortic valve implantation. Thorac Cardiovasc Surg. 2011;59:237–42.
53 Erdoes G, Basciani R, Huber C, Stortecky S, Wenaweser P, Windecker S, et al. Transcranial doppler-detected cerebral embolic load during transcatheter aortic valve implantation. Eur J Cardiothorac Surg. 2012;41:778–84.
54 Szeto WY, Augoustides JG, Desai ND, Moeller P, McGarvey ML, Walsh E, et al. Cerebral embolic exposure during transfemoral and transapical transcatheter aortic valve replacement. J Card Surg. 2011;26:348–54.
55 Bagur R, Rodes-Cabau J, Doyle D, De Larochelliere R, Villeneuve J, Bertrand OF, et al. Transcatheter aortic valve implantation with “no touch” of the aortic arch for the treatment of severe aortic stenosis associated with complex aortic atherosclerosis. J Card Surg. 2010;25:501–3.
56 Carpenter JP, Carpenter JT, Tellez A, Webb JG, Yi GH, Granada JF. A percutaneous aortic device for cerebral embolic protection during cardiovascular intervention. J Vasc Surg. 2011;54:174–81 e171
57 Nietlispach F, Wijesinghe N, Gurvitch R, Tay E, Carpenter JP, Burns C, et al. An embolic deflection device for aortic valve interventions. JACC Cardiovasc Interv. 2010;3:1133–8.
58 Etienne PY, Papadatos S, Pieters D, El Khoury E, Alexis F, Price J, et al. Embol-x intraaortic filter and transaortic approach for improved cerebral protection in transcatheter aortic valve implantation. Ann Thorac Surg. 2011;92:e95–96.
59 Naber CK, Ghanem A, Abizaid AA, Wolf A, Sinning JM, Werner N, et al. First-in-man use of a novel embolic protection device for patients undergoing transcatheter aortic valve implantation. EuroIntervention. 2012.
60 Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the valve academic research consortium. J Am Coll Cardiol. 2011;57:253–69.
61 Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: A consensus report from the valve academic research consortium. Eur Heart J. 2011;32:205–17.
62 Toggweiler S, Gurvitch R, Leipsic J, Wood D, Willson AB, Binder R, et al. Percutaneous aortic valve replacement. Vascular outcomes with a fully percutaneous procedure. J Am Coll Cardiol. 2012;59:113–8.
63 Nuis RJ, van Mieghem NM, van der Boon RM, van Geuns RJ, Schultz CJ, Oei FB, et al. Effect of experience on results of transcatheter aortic valve implantation using a medtronic corevalve system. Am J Cardiol. 2011;107:1824–9.
64 Hayashida K, Lefevre T, Chevalier B, Hovasse T, Romano M, Garot P, et al. Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC Cardiovasc Interv. 2011;4:851–8.
65 Bleiziffer S, Ruge H, Mazzitelli D, Schreiber C, Hutter A, Laborde JC, et al. Results of percutaneous and transapical transcatheter aortic valve implantation performed by a surgical team. Eur J Cardiothorac Surg. 2009;35:615–20.
66 Piazza N, Grube E, Gerckens U, den Heijer P, Linke A, Luha O, et al. Procedural and 30-day outcomes following transcatheter aortic valve implantation using the third generation (18 fr) corevalve revalving system: Results from the multicentre, expanded evaluation registry 1-year following ce mark approval. EuroIntervention. 2008;4:242–9.
67 Lange R, Bleiziffer S, Piazza N, Mazzitelli D, Hutter A, Tassani-Prell P, et al. Incidence and treatment of procedural cardiovascular complications associated with trans-arterial and trans-apical interventional aortic valve implantation in 412 consecutive patients. Eur J Cardiothorac Surg. 2011;40:1105–13.
68 Ducrocq G, Francis F, Serfaty JM, Himbert D, Maury JM, Pasi N, et al. Vascular complications of transfemoral aortic valve implantation with the edwards sapien prosthesis: Incidence and impact on outcome. EuroIntervention. 2010;5:666–72.
69 Nietlispach F, Leipsic J, Al-Bugami S, Masson JB, Carere RG, Webb JG. Ct of the ilio-femoral arteries using direct aortic contrast injection: Proof of feasibility in patients screened towards percutaneous aortic valve replacement. Swiss Med Wkly. 2009;139:458–62.
70 Joshi SB, Mendoza DD, Steinberg DH, Goldstein MA, Lopez CF, Raizon A, et al. Ultra-low-dose intra-arterial contrast injection for iliofemoral computed tomographic angiography. JACC Cardiovasc Imaging. 2009;2:1404–11.
71 Freeman M, Rodes-Cabau J, Urena M, Delarochelliere R, Dumont E, Masson JB, et al. First-in-man transfemoral transcatheter aortic valve replacement with the 29 mm edwards sapien xt valve. Catheter Cardiovasc Interv. 2012
72 Webb JG, Chandavimol M, Thompson CR, Ricci DR, Carere RG, Munt BI, et al. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation. 2006;113:842–50.
73 Grube E, Buellesfeld L, Mueller R, Sauren B, Zickmann B, Nair D, et al. Progress and current status of percutaneous aortic valve replacement: Results of three device generations of the corevalve revalving system. Circ Cardiovasc Interv. 2008;1:167–75.
74 Sharp AS, Michev I, Maisano F, Taramasso M, Godino C, Latib A, et al. A new technique for vascular access management in transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2010;75:784–93.
75 Van Mieghem NM, Nuis RJ, Piazza N, Apostolos T, Ligthart J, Schultz C, et al. Vascular complications with transcatheter aortic valve implantation using the 18 fr medtronic corevalve system: The rotterdam experience. EuroIntervention. 2010;5:673–9.
76 Genereux P, Kodali S, Leon MB, Smith CR, Ben-Gal Y, Kirtane AJ, et al. Clinical outcomes using a new crossover balloon occlusion technique for percutaneous closure after transfemoral aortic valve implantation. JACC Cardiovasc Interv. 2011;4:861–7.
77 Torsello GB, Kasprzak B, Klenk E, Tessarek J, Osada N, Torsello GF. Endovascular suture versus cutdown for endovascular aneurysm repair: A prospective randomized pilot study. J Vasc Surg. 2003;38:78–82.
78 Baim DS, Knopf WD, Hinohara T, Schwarten DE, Schatz RA, Pinkerton CA, et al. Suture-mediated closure of the femoral access site after cardiac catheterization: Results of the suture to ambulate and discharge (stand i and stand ii) trials. Am J Cardiol. 2000;85:864–9.
79 Himbert D, Descoutures F, Al-Attar N, Iung B, Ducrocq G, Detaint D, et al. Results of transfemoral or transapical aortic valve implantation following a uniform assessment in high-risk patients with aortic stenosis. J Am Coll Cardiol. 2009;54:303–11.
80 Cockburn J, de Belder A, Brooks M, Hutchinson N, Hill A, Trivedi U, et al. Large calibre arterial access device closure for percutaneous aortic valve interventions: Use of the prostar system in 118 cases. Catheter Cardiovasc Interv. 2012;79:143–9.
81 Lefevre T, Kappetein AP, Wolner E, Nataf P, Thomas M, Schachinger V, et al. One year follow-up of the multi-centre european partner transcatheter heart valve study. Eur Heart J. 2011;32:148–57.
82 Grube E, Schuler G, Buellesfeld L, Gerckens U, Linke A, Wenaweser P, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding corevalve prosthesis: Device success and 30-day clinical outcome. J Am Coll Cardiol. 2007;50:69–76.
83 Tsetis D. Endovascular treatment of complications of femoral arterial access. Cardiovasc Intervent Radiol. 2010;33:457–68.
84 Stortecky S, Wenaweser P, Diehm N, Pilgrim T, Huber C, Rosskopf AB, et al. Percutaneous management of vascular complications in patients undergoing transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012;5:515–24.
85 Kent KC, McArdle CR, Kennedy B, Baim DS, Anninos E, Skillman JJ. A prospective study of the clinical outcome of femoral pseudoaneurysms and arteriovenous fistulas induced by arterial puncture. J Vasc Surg. 1993;17:125–31; discussion 131–123.
86 Kresowik TF, Khoury MD, Miller BV, Winniford MD, Shamma AR, Sharp WJ, et al. A prospective study of the incidence and natural history of femoral vascular complications after percutaneous transluminal coronary angioplasty. J Vasc Surg. 1991;13:328–33; discussion 333-325.
87 Toursarkissian B, Allen BT, Petrinec D, Thompson RW, Rubin BG, Reilly JM, et al. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J Vasc Surg. 1997;25:803–8; discussion 808–9.
88 Khoury M, Rebecca A, Greene K, Rama K, Colaiuta E, Flynn L, et al. Duplex scanning-guided thrombin injection for the treatment of iatrogenic pseudoaneurysms. J Vasc Surg. 2002;35:517–21.
89 Lonn L, Olmarker A, Geterud K, Risberg B. Prospective randomized study comparing ultrasound-guided thrombin injection to compression in the treatment of femoral pseudoaneurysms. J Endovasc Ther. 2004;11:570–6.
90 Stone P, Lohan JA, Copeland SE, Hamrick RE Jr., Tiley EH, 3rd, Flaherty SK. Iatrogenic pseudoaneurysms: Comparison of treatment modalities, including duplex-guided thrombin injection. W V Med J. 2003;99:230–2.
91 Gorge G, Kunz T. Thrombin injection for treatment of false aneurysms after failed compression therapy in patients on full-dose antiplatelet and heparin therapy. Catheter Cardiovasc Interv. 2003;58:505–9.
92 Paulson EK, Sheafor DH, Kliewer MA, Nelson RC, Eisenberg LB, Sebastian MW, et al. Treatment of iatrogenic femoral arterial pseudoaneurysms: Comparison of us-guided thrombin injection with compression repair. Radiology. 2000;215:403–8.
93 Taylor BS, Rhee RY, Muluk S, Trachtenberg J, Walters D, Steed DL, et al. Thrombin injection versus compression of femoral artery pseudoaneurysms. J Vasc Surg. 1999;30:1052–9.
94 Weinmann EE, Chayen D, Kobzantzev ZV, Zaretsky M, Bass A. Treatment of postcatheterisation false aneurysms: Ultrasound-guided compression vs ultrasound-guided thrombin injection. Eur J Vasc Endovasc Surg. 2002;23:68–72.
95 Fejka M, Dixon SR, Safian RD, O’Neill WW, Grines CL, Finta B, et al. Diagnosis, management, and clinical outcome of cardiac tamponade complicating percutaneous coronary intervention. Am J Cardiol. 2002;90:1183–6.
96 Bagur R, Rodes-Cabau J, Gurvitch R, Dumont E, Velianou JL, Manazzoni J, et al. Need for permanent pacemaker as a complication of transcatheter aortic valve implantation and surgical aortic valve replacement in elderly patients with severe aortic stenosis and similar baseline electrocardiographic findings. JACC Cardiovasc Interv. 2012;5:540–51.
97 Bates MG, Matthews IG, Fazal IA, Turley AJ. Postoperative permanent pacemaker implantation in patients undergoing trans-catheter aortic valve implantation: What is the incidence and are there any predicting factors? Interact Cardiovasc Thorac Surg. 2011;12:243–53.
98 Schroeter T, Linke A, Haensig M, Merk DR, Borger MA, Mohr FW, et al. Predictors of permanent pacemaker implantation after medtronic corevalve bioprosthesis implantation. Europace. 2012.
99 Bleiziffer S, Ruge H, Horer J, Hutter A, Geisbusch S, Brockmann G, et al. Predictors for new-onset complete heart block after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2010;3:524–30.
100 Tchetche D, Modine T, Farah B, Vahdat O, Sudre A, Koussa M, et al. Update on the need for a permanent pacemaker after transcatheter aortic valve implantation using the corevalve(r) accutrak system. EuroIntervention. 2012;8:556–62.
101 Grube E, Naber C, Abizaid A, Sousa E, Mendiz O, Lemos P, et al. Feasibility of transcatheter aortic valve implantation without balloon pre-dilation: A pilot study. JACC Cardiovasc Interv. 2011;4:751–7.
102 Erkapic D, De Rosa S, Kelava A, Lehmann R, Fichtlscherer S, Hohnloser SH. Risk for permanent pacemaker after transcatheter aortic valve implantation: A comprehensive analysis of the literature. J Cardiovasc Electrophysiol. 2012;23:391–7.
103 Nuis RJ, Van Mieghem NM, Schultz CJ, Tzikas A, Van der Boon RM, Maugenest AM, et al. Timing and potential mechanisms of new conduction abnormalities during the implantation of the medtronic corevalve system in patients with aortic stenosis. Eur Heart J. 2011;32:2067–74.
104 Munoz-Garcia AJ, Hernandez-Garcia JM, Jimenez-Navarro MF, Alonso-Briales JH, Dominguez-Franco AJ, Fernandez-Pastor J, et al. Factors predicting and having an impact on the need for a permanent pacemaker after corevalve prosthesis implantation using the new accutrak delivery catheter system. JACC Cardiovasc Interv. 2012;5:533–9.
105 Laynez A, Ben-Dor I, Barbash IM, Hauville C, Sardi G, Maluenda G, et al. Frequency of conduction disturbances after edwards sapien percutaneous valve implantation. Am J Cardiol. 2012;110:1164–8.
106 Tse HF, Lau CP. Long-term effect of right ventricular pacing on myocardial perfusion and function. J Am Coll Cardiol. 1997;29:744–9.
107 Buellesfeld L, Stortecky S, Heg D, Hausen S, Mueller R, Wenaweser P, et al. Impact of permanent pacemaker implantation on clinical outcome among patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol. 2012.






