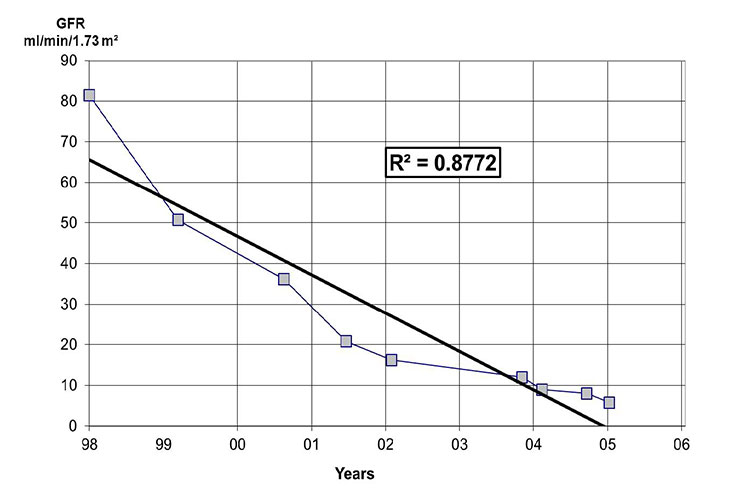
Figure 1
An example from a patient of a graph with the estimated GFR plotted against time. The intersection of the line with a GFR of 10 ml/min/ 1.73m2 roughly denotes the time needed for renal replacement therapy.
DOI: https://doi.org/10.4414/smw.2012.13713
Chronic kidney disease (CKD) represents a relevant medical and financial burden. In Norway, between 1995 and 1997, the prevalence of CKD was 10 percent, which is similar to that reported in the United States in 1988 through to 1994 [1]. A few years ago, every sixth patient over 50 years consulting a general practitioner in Ireland suffered from impaired kidney function [2]. The adjusted rates for incident and prevalent end-stage renal disease (ESRD) in the United States (US) in 2008 were 351 and 1,699 cases per million population (pmp), respectively [3]. The estimated incidence and prevalence of renal replacement therapy in 25 countries of the European Union was 137 pmp, and 786 pmp, respectively [4]. Currently in Switzerland, about 3,500 patients are treated with haemodialysis, 300 to 400 patients perform peritoneal dialysis, and more than 4,000 patients are living with a functioning kidney transplant (SVK: personal communication). The total cost of the ESRD programme in the US was approximately $39.46 billion in 2008, ranging from $26,668 for transplant patients to $77,506 for those receiving haemodialysis therapy [3]. Data from Finland and Spain reveal a similar financial burden [5, 6]. As costs of ESRD are substantial for all health systems around the world, early detection of kidney disease and followed by prevention of ESRD is essential [7].
It is very likely that many physicians are involved in the care of patients with CKD. Therefore, the present article provides a guideline for the management of CKD patients with imminent end-stage renal disease. The accurately timed evaluation of best renal replacement therapy is discussed.
CKD is defined as the presence of kidney damage, usually detected as pathologic urinary protein or albumin excretion or a pathologic urine sediment, or decreased kidney function, defined as a glomerular filtration rate (GFR) less than 60 ml/min/1.73 m2 for three or more months, irrespective of the cause [8]. Estimation of GFR should be done with a formula based on creatininaemia and the decline of the renal function should be monitored. The management of patients with CKD is directed by the stage of impaired kidney function. Table 1 shows the classification of CKD according to the GFR. Every stage demands specific diagnostic and therapeutic procedures (table 1). Independent of the stage, it is crucial to obtain a specific diagnosis of the underlying kidney disease. A kidney biopsy should be performed in all cases in which a diagnosis cannot be made by the medical history and/or non-invasive analyses. Careful management of those patients avoiding harmful therapies and dehydration can help to preserve their renal function. Close cooperation of the general practitioner with a nephrologist improves patient management and helps to lower costs [9–11]. A prognosis of the decline in renal function in CKD can be made with a graph of the reciprocal of the serum creatinine concentration or with the estimated GFR (eGFR) calculated by the MDRD formula [12]. This concept was first established by Walser and Mitch [13]. eGFR can be used instead of reciprocal serum creatinine since the GFR varies inversely with the reciprocal of the serum creatinine concentration. The graph helps to roughly predict the estimated time for need of a renal replacement therapy. Figure 1 denotes an example using the eGFR.

Figure 1
An example from a patient of a graph with the estimated GFR plotted against time. The intersection of the line with a GFR of 10 ml/min/ 1.73m2 roughly denotes the time needed for renal replacement therapy.
| Table 1: Classification of CKD and screening scheme for complications of CKD [8, 44]. | ||
| GFR Stage | GFR (ml/min/1.73 m2) | Screen for and treat if necessary |
| G1 | ≥90 | Cause of kidney disease (all stages) |
| Presence of renal abnormalities e.g. proteinuria, pathologic urine sediment | ||
| G2 | 60–89 | Hypertension (all stages) |
| G3a | 45–59 | Renal anaemia |
| G3b | 30–44 | Renal bone disease |
| G4 | 15–29 | Metabolic acidosis |
| G5 | <15 | Nausea, neuropathy, pericarditis, itching, central nervous abnormalities |
Once it has been determined that renal replacement therapy will eventually be required, the patient should be counselled to consider the advantages and disadvantages of haemodialysis (in-centre or at home), peritoneal dialysis (continuous or intermittent modalities), and renal transplantation (living or deceased donor). The 2006 K/DOQI guidelines recommend that patients with a GFR less than 30 ml/min per 1.73 m2 should be educated concerning these issues [14]. The following key questions should be answered: (1.) Is the patient suitable for any kind of renal replacement therapy? (2.) Is kidney transplantation an option? (3.) If transplantation is not an option: does the patient qualify for or want a dialysis treatment?
With the rising life expectancy during the last decades, there is a growing number of elderly patients with ESRD. The universal availability of renal replacement therapy in Switzerland forces the nephrologist to consider its application in every patient in whom it might be indicated, also in the elderly. However, the elderly and terminally ill in particular may refuse dialysis. Therefore, a conservative treatment (i.e. no beginning of dialysis) must be considered in every patient. This issue can be a source of conflict among physicians, patients and their families. During the last few years several investigations were realised in the field of conservative treatment in ESRD in the elderly. The French REIN-group established and evaluated a prognostic score in elderly patient (>75 years) with ESRD to help clinical decision-making about whether to start dialysis or not [15] (table 2). The factors which were independently predictive for mortality at 6 months were cardiovascular risks, nutritional status and mobility, whereas age was not independently predictive. In patients with the highest REIN-score, the 6 month mortality rate was up to 70% whereas for patients with the lowest score it was only 8–17% [16]. Murtagh showed that there is an equal survival rate in elderly patients with high burden of co-morbidity whether they started dialysis or were conservatively managed [17], and hospital free days were found to be not significantly different [18]. In addition there is a dramatic loss of independence in very elderly patients starting dialysis with only 47% of patient with no support or assistance at home after 12 months [19].
Based upon the above data, renal replacement therapy should be started with caution in the very elderly with multiple co-morbidities as the balance of benefit and harm of dialysis in these patients is often delicate. Early referral to a nephrologist and a multidisciplinary approach to balance the individual benefice should be aimed for. Dignity, quality of life and the patients’ will should guide us in the decision-making process in these patients and not the theoretical survival benefit alone.
| Table 2: REIN score to help clinical decision-making about whether to start dialysis or not in patients older than 75 years [15]. A Score ≥9 is associated with 70% 6-months mortality on dialysis [16]. | |||
| Risk factors | Points | Score | 6-months mortality |
| BMI <18.5 kg/m2 | 2 | 8% | |
| Diabetes | 1 | 1 | 8–10% |
| Congestive heart failure stage III to IV | 2 | 2 | 14–17% |
| Peripheral vascular disease stage III to IV | 2 | 3–4 | 21–26% |
| Dysarythmia | 1 | 5–6 | 33–35% |
| Active malignant disease | 1 | 7–8 | 50–51% |
| Severe behavioural disorder | 1 | ≥9 | 62–70% |
| Total dependency for transfer | 3 | ||
| Unplanned dialysis | 2 | ||
Since the first successful acute dialysis performed by Willem J. Kolff in 1946 and the opening of the first chronic outpatient dialysis centre by Belding Scribner in Seattle in 1962, chronic haemodialysis has become a standard of care in patients with ESRD [20]. Today, nearly 300,000 patients in Europe and 1.5 million worldwide are undergoing chronic dialysis [20, 21]. Haemodialysis treatment offers the opportunity of long-term survival in case of ESRD with a good quality of life and can bridge the time to successful kidney transplantation. Technique, equipment and care of dialysis patients have significantly improved over the last 50 years. Nevertheless, patients on haemodialysis still experience a significant lower life expectancy compared to the general population (see: fig. 2). The one, three, and five year survival rate of haemodialysis patients at the University hospital Basel, Switzerland, with a mean age of 65 (15–90), is 88%, 68% and 46%, respectively [22]. Usually, three dialysis sessions a week, of 4 hours each, are the standard of care but new regimens (e.g. daily short term dialysis or nocturnal dialysis) are also propagated [23]. The place of dialysis treatment can vary depending on the patient’s self-dependence: full care in-centre dialysis, limited care dialysis, and home haemodialysis are possible options. Older, unmated patients in particular can profit from social contacts with others during the treatment session. On the other side the freedom of travelling can be limited by the lack of dialysis opportunities at the preferred destination. Dialysis treatment is generally well supported but intradialytic hypotension, nausea, gastrointestinal bleeding, malnutrition, post-dialytic fatigue and orthostatic hypotension can occur. Diet restriction may be necessary to gain better control of volume status and electrolyte disturbances. Haemodialysis requires a stable access to the bloodstream to permit dialysis to be performed. Suitable vessels are crucial to create a functioning shunt and can limit dialysis treatment in patients with severe peripheral vascular disease. In summary, haemodialysis offers a successful option of renal replacement therapy with a good quality of life in patients not suitable for kidney transplantation or awaiting a kidney transplant.
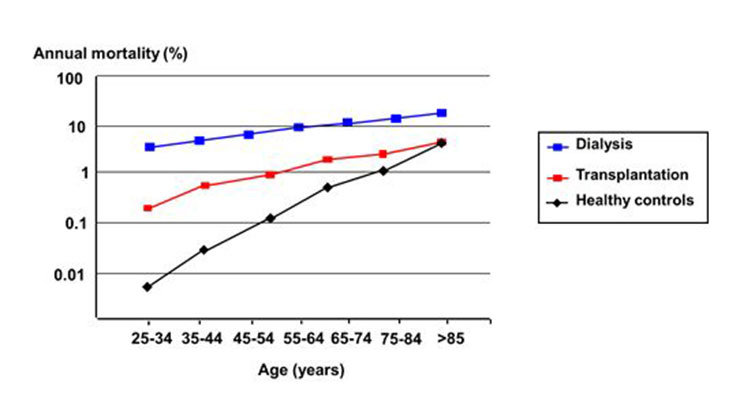
Figure 2
Age dependent mortality rate of US patients with a kidney transplant and of patients on haemodialysis, compared to healthy controls. Data from 1994 to 1996. ( Adapted from [45]: Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, et al. Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis. 1998;32(5):853–906. With kind permission from Elsevier, Oxford, UK).
Peritoneal dialysis has been a well-established renal replacement therapy since the late seventies. In general it is considered an equivalent treatment option compared to haemodialysis for ESRD [24] and is the preferred home-dialysis technique. About 11% of the global dialysis population is treated with peritoneal dialysis [25]. There are great differences of the prevalence of peritoneal dialysis around the world due to local policy of reimbursement and health care systems. Peritoneal dialysis is performed via regular exchanges of fluid through a permanent tube in the abdomen. This is made throughout the day (continuous ambulatory peritoneal dialysis) or every night while the patient sleeps (automatic peritoneal dialysis). The primary advantage of peritoneal dialysis is the self-responsibility of the patient and the ability to undertake treatment without visiting a medical facility. Patients treated with automatic peritoneal dialysis in addition have the advantage of dialysis independence during daytime with the possibility to hold down a job or time for recreational activities. Even in the elderly, peritoneal dialysis is a suitable treatment technique, which has several advantages in this patient group. It has been shown that quality of life is better in the elderly when they are on peritoneal dialysis compared to haemodialysis [26]. Other advantages are less orthostatic hypotension or intra-dialytic hypotension, no post-dialytic fatigue, no need for transport and therefore it is less disruptive for elderly patients. Peritoneal dialysis is less expensive than haemodialysis with significant cost saving per patient year [5, 6]. The primary complication of peritoneal dialysis is infection (peritonitis) due to the presence of a permanent tube in the abdomen. However when comparing the overall infection rate between peritoneal dialysis and haemodialysis patients, infection rate is equal in both but the types of infections are different [27]. There is even a higher rate of hospital admission due to septicaemia in haemodialysis than in peritoneal dialysis patients [28]. There are only a few medical contraindications for peritoneal dialysis (e.g., recurrent diverticulitis, inflammatory bowel disease). Unfortunately technique failure in peritoneal dialysis and transfer to haemodialysis is observed in up to 34% of patients after a median follow up of 1.3 years (range 0–11 years) due to different reasons (e.g., psychosocial problems, recurrent peritonitis, hernias) [29].
In summary, peritoneal dialysis is an equivalent treatment option in ESRD compared to haemodialysis and should be evaluated in every patient. It provides several advantages whereof freedom of medical facility and high quality of life are the most important ones.
Kidney transplantation is the treatment of choice for patients with end-stage renal disease. A successful kidney transplant reduces the mortality risk compared to maintenance dialysis in all age groups as shown in figure 2 and improves the quality of life (fig. 3). In contrast to dialysis treatment it offers a normal professional life in many cases and has a beneficial impact on costs [3, 5, 6]. Nowadays one-year graft survival is above 95% after living donated kidney transplantation and 90 to 95% after deceased donor transplantation (fig. 4). Short-term success rate of kidney transplantation has been markedly improved in recent times and long term results are also encouraging. Between 1985 and 2010 the deceased donor kidney transplant half-life in Europe has been 15 years, if death is censored even 24.6 years, which is much better than in previous times [30]. A more sophisticated pre-transplant assessment, better allocation rules, and more effective immunosuppression have contributed to this improvement Therefore kidney transplantation should be offered to every suitable patient independent of age as older patients can also profit from this procedure. There are only a few generally accepted contraindications to transplantation [31]: untreated infection, active malignancy life expectancy less than 2 years, chronic illness with short life expectancy, poorly controlled psychosis, and active substance abuse. Relative contraindications include: active infection, untreated or untreatable coronary heart disease, and symptomatic peripheral vascular disease especially in the pelvis axis. Advanced age, prior transplantation, and renal diagnosis are no contraindications to transplantation. Long-term immunosuppressive therapy contributes to an increased risk for malignancy in organ transplant recipients. Skin is the most common site for the development malignancy; in particular, cutaneous squamous cell carcinomas and basal cell carcinomas are frequently detected [32]. Poor drug adherence, especially in young patients is also a substantial problem [33].
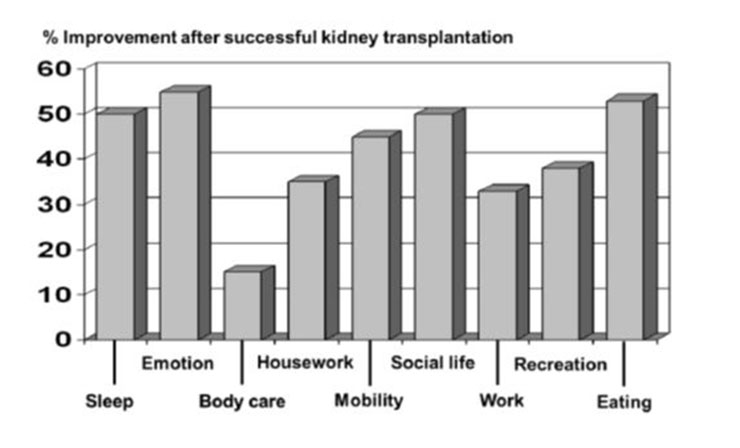
Figure 3
Changes in quality of life factors after successful kidney transplantation in patients previously undergoing haemodialysis. The Karnofsky Scale and the Sickness Impact Profile were used as indicators for quality of life (adapted from [46]: Jofre R, Lopez-Gomez JM, Moreno F, Sanz-Guajardo D, Valderrabano F. Changes in quality of life after renal transplantation. Am J Kidney Dis. 1998;32(1):93–100. With kind permission from Elsevier, Oxford, UK).
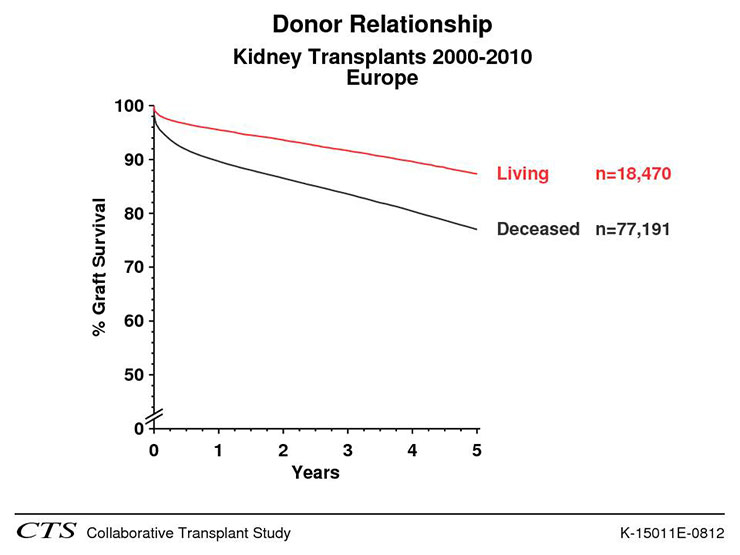
Figure 4
Comparison of graft survival between living donated kidneys and kidneys from deceased donors in Europe between 1998 and 2010 (CTS K-15011E-0212). (Source: http://www.ctstransplant.org, with kind permission.)
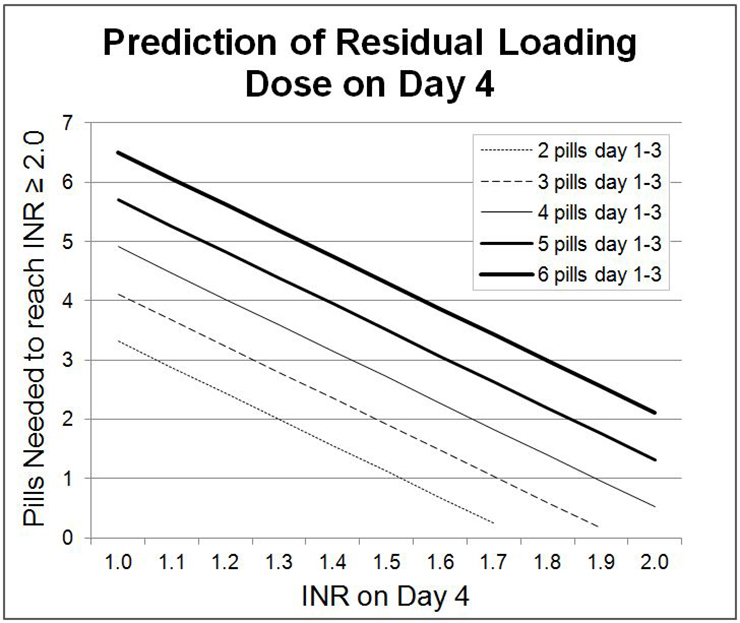
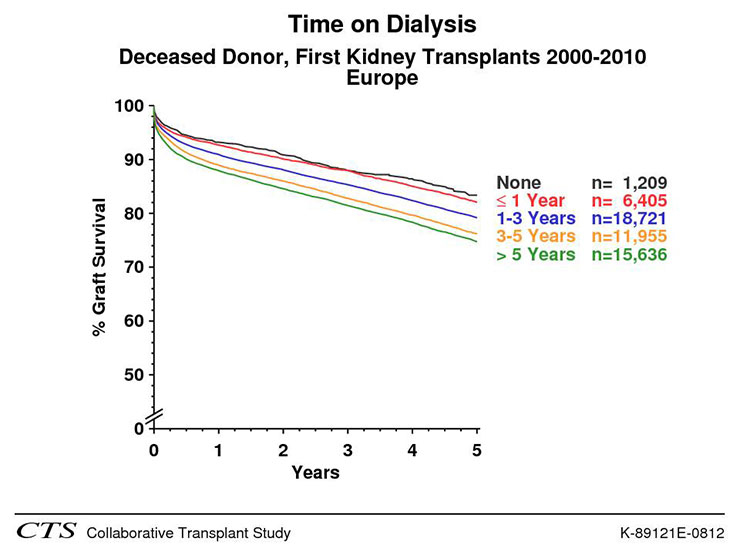
Figure 5
Impact of time on dialysis on graft survival after living donated (left panel) and deceased donor (right panel) kidney transplantation (CTS K-89201-0212 and K-89121-0212). (Source: http://www.ctstransplant.org, with kind permission.)
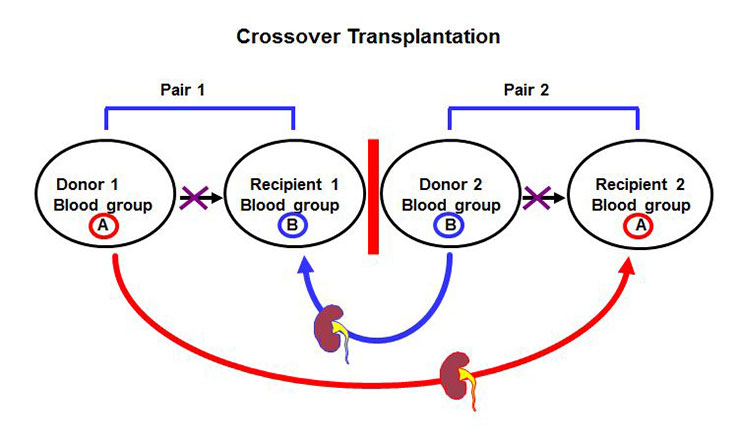
Figure 6
Principle of crossover kidney transplantation between two pairs with incompatible blood group.
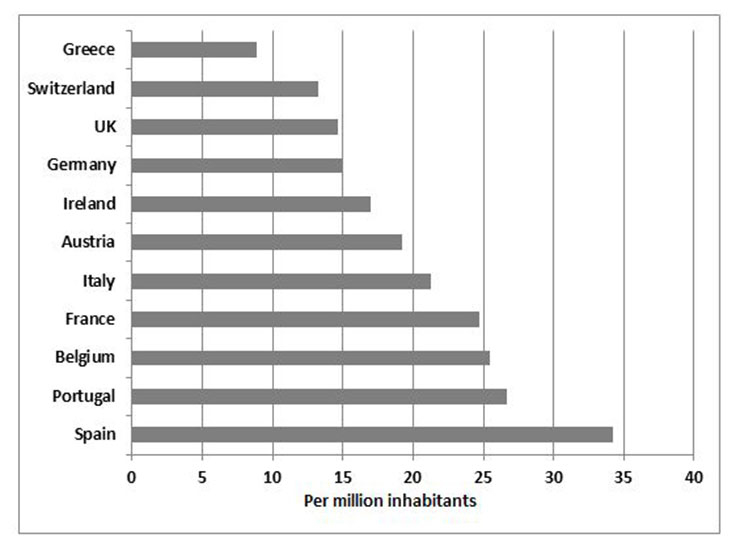
Figure 7
Number of deceased organ donors per million inhabitants in Europe 2009 (adapted from [47]: Van Gelder F, Manyalich1 M, Nanni Costa A, Paez G. 2009 International donation and transplantation activity. IRODaT preliminary data. Organs, Tissues and Cells 2010;13:5‒8. With kind permission from Editrice Compositori, Bologna, Italy.).
The first clinically successful renal replacement treatment, a pre-emptive living donor kidney transplantation, took place on the 23rd December 1954 in Boston [34]! Ronald Herrick donated a kidney to his identical twin brother Richard. Richard Herrick died eight years after his kidney transplantation with a functioning graft. His brother survived him by 48 years [35]. Therefore, living donor kidney transplantation preceded successful dialysis treatment by many years. Nowadays living donor kidney transplantation counts for about half of all kidney donors in Switzerland in the last 10 years [36]. It helps to compensate the critical shortage of deceased donors in many countries. Living donor kidney transplantation offers the most successful renal replacement therapy and the graft survival is superior as compared to grafts from deceased donors (fig. 4). Graft survival can further be improved by a pre-emptive transplantation as time on dialysis has a negative impact on graft survival from living and deceased donors (fig. 5). Since 2008 33% of all living donated kidneys and 6% of all deceased donor kidney transplantations in Switzerland could be performed pre-emptively (Swiss Transplant cohort study: personal communication). Beside the superior graft survival the pre-emptive approach offers the possibility to transplant at the best time (i.e., recipient has best medical and social conditions) and to minimise the risk of delayed graft function. Avoiding a time on dialysis is also cost saving as a dialysis treatment is more expensive than a patient with a kidney transplant [5, 6], and transplanted patients have a greater opportunity to avoid unemployment or dependence from welfare. Opponents of a pre-emptive transplantation argue that patients first have to experience the ‘hard school of dialysis’ before they get a transplant in order to improve drug adherence post-transplant. However this argument proved to be wrong [37]. Beside all the advantages for living donor kidney transplantation the possible harm of a healthy person always has to be considered. Data of the Swiss Organ Living Donor Health Registry, a prospective donor registry with current follow up of 19 years, is showing a very low rate of short and long-term complications (e.g., arterial hypertension, proteinuria) in living kidney donors confirming the acceptable risks for the donors [38, 39].
In recent years the barrier of blood group incompatibility has been successfully overcome [40, 41]. Today, blood group incompatible living donor kidney transplantation offers equal short and long term results as blood group compatible transplantation and is performed in up to 20% of living donor transplantations. Kidney paired donation (fig. 6) between two donor-recipient pairs with blood group incompatibility or presence of donor specific antibodies against the own partner is another promising new strategy to further increase the number of living donor kidney transplantation [42]. Living donor kidney transplantation offers the best solution for a patient with ESRD and has an acceptable risk for the donor. It should therefore be the treatment of choice and its possibility should be evaluated in every patient with ESRD.
Patients with no opportunity for a living donated kidney should be listed on the national kidney waiting lists. The number of deceased donors strongly depends on the local particularities and structures. Amongst others it is affected by the general acceptance of organ donation in the population, the jurisdiction (e.g., presumed or informed consent for donation), the extent of the net of transplant coordinators, and the available resources for the management of potential donors in the ICUs. In Europe, there are big differences in the number of deceased donors per million inhabitants as shown in figure 7. The acceptance criteria for listing of patients can vary between different countries. Simultaneous listing in different countries is not permitted. In Switzerland since 2008 the Swiss transplantation law regulates the allocation criteria for all organs from deceased donors [43]. The allocation is performed by Swisstransplant for all organs according to these criteria. CKD patients with a GFR below 15 ml/min/1.73 m2 can be listed. Early listing, even before start of dialysis treatment, offers the possibility of a pre-emptive transplantation (see above), reduces waiting time on dialysis, and contributes to transplant success. Transplants from deceased donors usually have an inferior outcome compared to living donor transplants as these grafts are usually from older, less healthy donors, and have a longer cold ischemia time (i.e., time between explantation and engrafting).
Despite all efforts there is a dramatic worldwide lack of deceased donor organs leading to long waiting times with substantial morbidity and mortality.
1 Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006;17(8):2275–84.
2 Glynn LG, Anderson J, Reddan D, Murphy AW. Chronic kidney disease in general practice: prevalence, diagnosis, and standards of care. Ir Med J. 2009;102(9):285–8.
3 United States Renal Data System, USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, 2010. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD; 2010 Jan 1.
4 Lameire N, Jager K, Van BW, de BD, Vanholder R. Chronic kidney disease: a European perspective. Kidney Int Suppl. 2005;(99):S30–S38.
5 Salonen T, Reina T, Oksa H, Sintonen H, Pasternack A. Cost analysis of renal replacement therapies in Finland. Am J Kidney Dis. 2003;42(6):1228–38.
6 Villa G, Rodriguez-Carmona A, Fernandez-Ortiz L, Cuervo J, Rebollo P, Otero A, et al. Cost analysis of the Spanish renal replacement therapy programme. Nephrol Dial Transplant. 2011;26(11):3709–14.
7 Levey AS, Schoolwerth AC, Burrows NR, Williams DE, Stith KR, McClellan W. Comprehensive public health strategies for preventing the development, progression, and complications of CKD: report of an expert panel convened by the Centers for Disease Control and Prevention. Am J Kidney Dis. 2009;53(3):522–35.
8 Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165–80.
9 Pereira BJ. Optimization of pre-ESRD care: the key to improved dialysis outcomes. Kidney Int. 2000;57(1):351–65.
10 Chan MR, Dall AT, Fletcher KE, Lu N, Trivedi H. Outcomes in patients with chronic kidney disease referred late to nephrologists: a meta-analysis. Am J Med. 2007;120(12):1063–70.
11 Khan SS, Xue JL, Kazmi WH, Gilbertson DT, Obrador GT, Pereira BJ, et al. Does predialysis nephrology care influence patient survival after initiation of dialysis? Kidney Int. 2005;67(3):1038–46.
12 Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70.
13 Mitch WE, Walser M, Buffington GA, Lemann J, Jr. A simple method of estimating progression of chronic renal failure. Lancet. 1976;2(7999):1326–8.
14 Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48(Suppl 1):S2–90.
15 Couchoud C, Labeeuw M, Moranne O, Allot V, Esnault V, Frimat L, et al. A clinical score to predict 6-month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol Dial Transplant. 2009;24(5):1553–61.
16 Berger JR, Hedayati SS. Renal Replacement Therapy in the Elderly Population. Clin J Am Soc Nephrol. 2012 Apr 19.
17 Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant. 2007;22(7):1955–62.
18 Carson RC, Juszczak M, Davenport A, Burns A. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol. 2009;4(10):1611–9.
19 Jassal SV, Chiu E, Hladunewich M. Loss of independence in patients starting dialysis at 80 years of age or older. N Engl J Med. 2009;361(16):1612–3.
20 2002 Albert Lasker Award for Clinical Medical Research. J Am Soc Nephrol. 2002;13(12):3027–30.
21 European Renal Association EDaTA. Annual Report. 2007 Jan 1.
22 Breidthardt T, Moser-Bucher CN, Praehauser C, Garzoni D, Bachler K, Steiger J, et al. Morbidity and mortality on chronic haemodialysis: a 10-year Swiss single centre analysis. Swiss Med Wkly. 2011;141:w13150.
23 Kooistra M. Frequent prolonged home haemodialysis: three old concepts, one modern solution. Nephrol Dial Transplant. 2012;18:16–9.
24 Vonesh EF, Snyder JJ, Foley RN, Collins AJ. Mortality studies comparing peritoneal dialysis and hemodialysis: what do they tell us? Kidney Int Suppl. 2006;(103):S3–11.
25 Jain AK, Blake P, Cordy P, Garg AX. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol. 2012;23(3):533–44.
26 Brown EA, Johansson L, Farrington K, Gallagher H, Sensky T, Gordon F, et al. Broadening Options for Long-term Dialysis in the Elderly (BOLDE): differences in quality of life on peritoneal dialysis compared to haemodialysis for older patients. Nephrol Dial Transplant. 2010;25(11):3755–63.
27 Aslam N, Bernardini J, Fried L, Burr R, Piraino B. Comparison of infectious complications between incident hemodialysis and peritoneal dialysis patients. Clin J Am Soc Nephrol. 2006;1(6):1226–33.
28 Hoen B, Paul-Dauphin A, Hestin D, Kessler M. EPIBACDIAL: a multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol. 1998;9(5):869–76.
29 Descoeudres B, Koller MT, Garzoni D, Wolff T, Steiger J, Schaub S, Mayr M. Contribution of early failure to outcome on peritoneal dialysis. Perit Dial Int. 2008;28(3):259–67.
30 CTS. CTS K15108E-0212.[electronic mail system]. 212 Apr 22.
31 Evaluation of potential renal transplantation. In: Danovitch G, editor. Handbook of Kidney Transplantation. 4th edition ed. Philadelphia: Lippincott Williams and Wilkins; 2005.
32 Vajdic CM, van Leeuwen MT. Cancer incidence and risk factors after solid organ transplantation. Int J Cancer. 2009;125(8):1747–54.
33 Nevins TE, Thomas W. Quantitative patterns of azathioprine adherence after renal transplantation. Transplantation. 2009;87(5):711–8.
34 Guild WR, Harrison JH, Merrill JP, Murray J. Successful homotransplantation of the kidney in an identical twin. Trans Am Clin Climatol Assoc. 1955;67:167–73.
35 Murray JE. Ronald Lee Herrick Memorial: June 15, 1931–December 27, 2010. Am J Transplant. 2011;11(3):419.
36 Swisstransplant Jahresbericht 2011. 01.01.2012: 2012.
37 Papalois VE, Moss A, Gillingham KJ, Sutherland DE, Matas AJ, Humar A. Pre-emptive transplants for patients with renal failure: an argument against waiting until dialysis. Transplantation. 2000;70(4):625–31.
38 Thiel GT, Nolte C, Tsinalis D. Prospective Swiss cohort study of living-kidney donors: study protocol. BMJ Open. 2011;1(2):e000202.
39 Thiel GT, Nolte C, Tsinalis D. The Swiss Organ Living Donor Health Registry (SOL-DHR). Ther Umsch. 2005;62(7):449–57.
40 Oettl T, Halter J, Bachmann A, Guerke L, Infanti L, Oertli D, et al. ABO blood group-incompatible living donor kidney transplantation: a prospective, single-centre analysis including serial protocol biopsies. Nephrol Dial Transplant. 2009;24(1):298–303.
41 Oettl T, Zuliani E, Gaspert A, Hopfer H, Dickenmann M, Fehr T. Late steroid withdrawal after ABO blood group-incompatible living donor kidney transplantation: high rate of mild cellular rejection. Transplantation. 2010;89(6):702–6.
42 Gentry SE, Montgomery RA, Segev DL. Kidney paired donation: fundamentals, limitations, and expansions. Am J Kidney Dis. 2011;57(1):144–51.
43 Immer FF. What does the practicing doctor need to know about the transplantation law and its effects? Ther Umsch. 2011;68(12):673–7.
44 National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classifi cation, and stratifi cation. Am J Kidney Dis. 2002;39(2 Suppl):S1–S266.
45 Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, et al. Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis. 1998;32(5):853–906.
46 Jofre R, Lopez-Gomez JM, Moreno F, Sanz-Guajardo D, Valderrabano F. Changes in quality of life after renal transplantation. Am J Kidney Dis. 1998;32(1):93–100.
47 Van Gelder F, Manyalich1 M, Nanni Costa A, Paez G. 2009 International donation and transplantation activity. IRODaT preliminary data. Organs, Tissues and Cells. 2010;13:5–8.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.