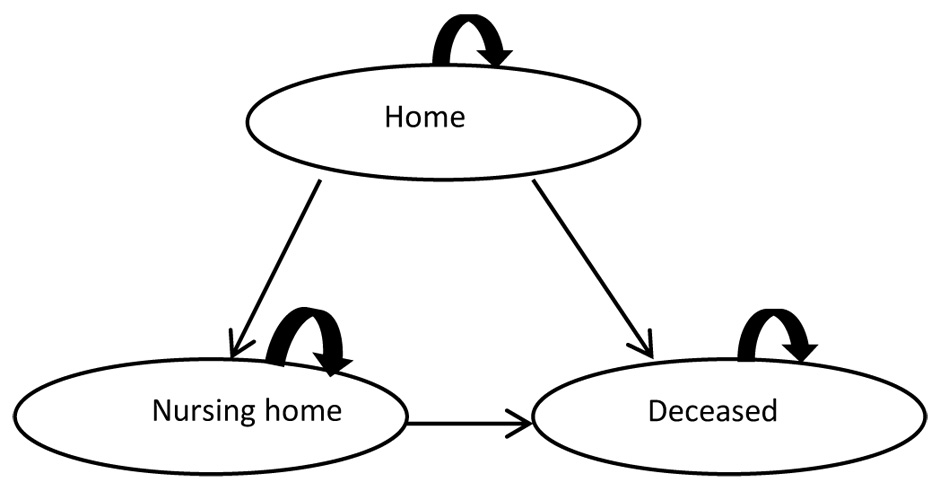
Figure 1
Structure of the Markov model from Lachaine et al. [23].
DOI: https://doi.org/10.4414/smw.2012.13676
Due to aging of the world’s population, age-related diseases are becoming a growing economic burden not only for the Swiss population but also for the world. Alzheimer’s disease (AD) – a form of dementia – refers to a progressive and irreversible neurodegenerative disease characterised by decline in cognition, impaired social behaviour in the late stage of the disease, memory loss, disorientation and other neuropsychiatric symptoms [1]. The prevalence of dementia highly increases with age from approximately 0.1% in the 30–64 year old age group to more than 30% in people aged 90 and older [2, 3]. The worldwide prevalence of dementia has been estimated to increase from 35.6 million people in 2010 to 115.4 million people in 2050 [4]. Assuming that at least 60% of dementia cases are caused by AD [5, 6], many more people will be affected by dementia due to AD in the following years.
About 70% of worldwide costs of dementia occur in Western Europe and North America, although China has the largest dementia population [7]. Worldwide annual costs per case in 2009 were USD 12,200, whereas lowest annual costs per case were found in Africa (USD 4,400) and highest annual costs per case were reported in North America (USD 26,700) [7]. Total estimated worldwide costs of dementia were USD 604 billion in 2010 [4]. As mentioned before, about 60% of dementia cases are caused by AD. Assuming that around 60% of the dementia costs are also related to AD, the total worldwide cost of Alzheimer’s dementia in 2010 would have been USD 362 billion. A Swiss analysis of the costs of AD in 1998 performed by Volz et al. reported total costs of about CHF 3.24 billion (USD 3.34 billion) [5].
The standard treatment of Alzheimer’s dementia in Switzerland is either a mono treatment with a cholinesterase inhibitor or memantine, depending on the Mini Mental State Examination (MMSE) score of the patient. Several studies have shown that the combination treatment of a cholinesterase inhibitor and memantine prolongs the time an Alzheimer’s dementia patient is able to live at home and therefore, delays the admission to a nursing home due to a statistically significant and clinically relevant better effectiveness of the combination treatment [8–11]. However, these benefits only start occurring after one year of combination therapy [10]. After two combination treatment years nursing home admission rate decreases by 20% and after five years of treatment by 40% [10]. Further, the combination treatment has no association with time to death [10]. A recent prospective randomised study performed by Howard et al. found no significant benefit of adding memantine to donepezil [12]. However, the study duration was only one year. In contrast, a recent systematic review by Farrimond et al. [13] including sources of unpublished data concluded that there is a small but significant effect of memantine combination treatment on cognitive, global and behavioural measures.
As the majority of costs are caused by informal or institutional care (about 90%) and medication costs are very low (about 0.4%) [5, 14], using the combination treatment instead of the standard treatment could result in overall cost savings due to major savings in informal and institutional care despite the increase in medication costs. Several studies performed in different countries [15–22] found memantine alone compared to standard care (treatment with cholinesterase inhibitors or no treatment) to be either the dominant strategy with health benefits and cost savings or a cost effective strategy with an additional health benefit. Combining the treatment of a cholinesterase inhibitor with memantine was found to be the cost saving strategy compared to the use of a cholinesterase inhibitor alone [23, 24]. Combination treatment is already reimbursed in several European countries, e.g. Germany, France, Spain, Italy, Sweden, Finland, Portugal, Belgium, Denmark (restricted reimbursement), Greece and Hungary, but not yet in Switzerland.
The aim of this study was to estimate the potential budget impact and cost-effectiveness of the combination treatment of a cholinesterase inhibitor and memantine in Alzheimer’s dementia over five years in Switzerland assuming that reimbursement of the combination treatment would be available from 2012.
The following analyses are mainly based on the results of the prevalence based cost of illness study performed by Kraft et al. [14]. The study by Kraft et al. estimated the costs of dementia from a societal perspective by including direct and indirect costs. Mainly Swiss data sources were used for calculating the costs. The amount of hours of informal care per person was retrieved from a synthesis of 27 international studies. Medical resource use and costs were assessed using aggregate data from publicly available databases (top-down) as well as survey data and expert opinions (bottom-up). Detailed methods regarding cost calculations have been described and published in earlier reports [14, 25].

Figure 1
Structure of the Markov model from Lachaine et al. [23].
Due to the lack of actual patient registry data on the prevalence of Alzheimer’s dementia in Switzerland, we estimated the number of patients with dementia by using European dementia prevalence rates based on Harvey et al. [2] for people less than 65 years of age. For people older than 65 years of age, we assigned prevalence rates reported by Hofman et al. [3]. Both prevalence rates have already been used by Kraft et al. [14] and ECOPLAN [25]. We calculated the number of dementia cases in the year 2011 by applying these prevalence data to the annual Swiss demographic figures 2011 of the Swiss Federal Statistical Office (SFSO) [26]. Dementia cases in Switzerland for the years 2012, 2013, 2014, 2015, and 2016 were calculated by using future Swiss demographic figures of the SFSO [27] and applying the same age-dependent prevalence rates as for the calculation of 2011. Sixty per cent of the calculated dementia cases were assumed to be caused by AD.
Because Swiss healthcare costs for 2011 had not been published at the time the two analyses were conducted, we based our healthcare cost calculations for the years 2011, 2012, 2013, 2014, 2015, and 2016 on the updated ECOPLAN [25] healthcare cost calculations for dementia for 2009 [28]. Direct costs included hospital costs, nursing home costs, outpatient nursing (Spitex) costs, physician costs, medication costs and costs for the memory clinics. Indirect costs consisted of informal care of family members of Alzheimer’s dementia patients. As mentioned in the introduction, about 60% of dementia cases are due to AD [5, 6]. We assumed that 60% of dementia healthcare costs are also caused by AD. Direct and indirect costs (except medication costs) were estimated to annually increase by 5% [29].
Medication costs for the mono treatment (either a cholinesterase inhibitor or memantine) only and the combination treatment (a cholinesterase inhibitor and memantine) were calculated separately. We asked the largest health insurer in Switzerland, Helsana, to provide us with their available data regarding the frequency of memantine and cholinesterase inhibitor use and the average annual medication cost per person in Switzerland for each substance separately. According to the Swiss Alzheimer’s Association and an IMS Health study, only about 25% of Swiss Alzheimer dementia patients are treated with antidementia drugs [30, 31]. Modelling prevalence estimates for the year 2012 until 2016, we could calculate total medication cost per year for the mono treatment. As the combination treatment is not yet reimbursed in Switzerland and hence no Swiss prescription data were available, we utilised empirical values from a French study [32]. In France, combination treatment has been reimbursed since 2007 for moderate to severely affected AD patients with an MMSE score of 10–20 [33]. The study by Tifratene et al. [32] included 26,809 AD patients, of which 52% had an MMSE score of 10–20, among which about 19% were treated with the combination treatment. Based on these values, we calculated additional treatment costs for Switzerland due to the implementation of the reimbursed combination treatment for the years 2012 to 2016 from a payer perspective.
For the cost-utility analysis we used the Markov state transition model (fig. 1) developed by Lachaine et al. [23] in Microsoft Office Excel. The Monte Carlo simulation based cost-utility analysis was performed from two perspectives: a health care system perspective (only direct costs) and a societal perspective (direct and indirect costs). One Markov cycle corresponded to one year. The time horizon of the model was limited to seven years because most input data were based on the findings of Lopez et al. [10]. The model assumed that initially all patients are at home and not yet institutionalised. Two different scenarios were compared: costs of the mono treatment over seven years compared to costs of the combination treatment over seven years. The primary outcome measures of the model were incremental cost-effectiveness ratios (ICERs, costs per quality adjusted life year [QALY]).
For the model calculations, most input data already used by a French adaptation [24] of the original Canadian model [23] and mainly based on the findings of Lopez et al. [10] were adopted due to lack of data specific for Switzerland (table 1). Lopez et al. conducted an observational study in 943 eligible AD patients receiving cholinesterase inhibitors and memantine, only cholinesterase inhibitors or neither to examine time to nursing home admission and death. The utility value 0.6 used in the model for an Alzheimer’s patient being at home is a combination of the utility value “before full-time care” [34] and “mild to moderate” AD [35]. A patient admitted to a nursing home had a utility value of 0.34 referring to “severe to very severe” AD [35]. The transition probabilities from “home to nursing home” and “home to death” determined by Lopez et al. [10] were maintained. In the French adaptation [24], a survival function was developed for the model with a median survival time of 4.5 years from onset of dementia based on a French prospective community based cohort study reported by Helmer et al. [36]. Compared to the treatment with a cholinesterase inhibitor alone, the combined treatment with a cholinesterase inhibitor and memantine prolonged the time in community about 8.9 months [10]. Swiss-specific indirect and direct costs were used for the model [25]. Average costs per patient were calculated by dividing the individual cost components (e.g. hospital costs, physician costs, etc.) by the number of patients either at home (58% [14, 25] of 68,053 patients in 2011) or in a nursing home (42% [14, 25] of 68,053 patients in 2011). In the base-case cost-utility analysis, costs and benefits were discounted at a rate of 3%.
A sensitivity analysis was conducted based on the variation of the original model [23]. Costs and utilities were varied between 80% and 120% of the original values (variation factor of 1.2). The transition probabilities were varied from 50 to 200% (variation factor 2) of the original values. Discount rates of the costs and benefits in the sensitivity analysis were either 0% or 5%.
| Table 1: Input data for the cost-utility analysis. | ||||
| Input data | Values used for model | Explanatory notes | ||
| Utilities | Home Nursing home | 0.60 0.34 | Mild-moderate: 0.69–0.53 Severe-end stage: 0.38–0.27 | |
| Transition probabilities to nursing home | Only ChE-I ChE-I & M | Cycle: 1 Cycle: 2 Cycle: 3 Cycle: 4 Cycle: 5 Cycle: 6, 7 Cycle: 1, 2, 3 Cycle: 4 Cycle: 5 Cycle: 6, 7 | 0.0167 0.1031 0.0947 0.0808 0.0891 0 0 0.0167 0.0418 0 | |
| Transition probabilities to death | Only ChE-I ChE-I & M | 0.1428 0.1428 | ||
| Time to nursing home | ChE-I & M | + 8.9 months | Compared to ChE-I only | |
| Annual costs per patient [CHF] (calculation based on the total costs of 2011) | Drugs Direct Indirect Death | Mono treatment Combination treatment Hospital Physician Spitex Memory clinics Nursing home Nursing home Home | 1,438 2,431 2,916 253 5,764 106 77,279 0 50,612 0 | For a patient not admitted to a nursing home, we included all direct costs, except nursing home costs. To calculate the average nursing home costs, we also added physician costs. |
About 60% of the dementia cases are caused by AD leading to approximately 68,000 patients who suffered from Alzheimer’s dementia in Switzerland in 2011 (table 2). When the European prevalence rates were applied to future Swiss demographics in the years 2012 to 2016, we calculated the following numbers of people being affected by Alzheimer’s dementia: 69,919 in 2012, 71,873 in 2013, 73,899 in 2014, 75,993 in 2015, and 78,075 in 2016 (table 3). If we assume that 25% of all Alzheimer’s dementia patients receive treatment in the following five years (2012–2016), 17,480 would be treated in 2012, 17,968 in 2013, 18,475 in 2014, 18,998 in 2015, and 19,519 in 2016.
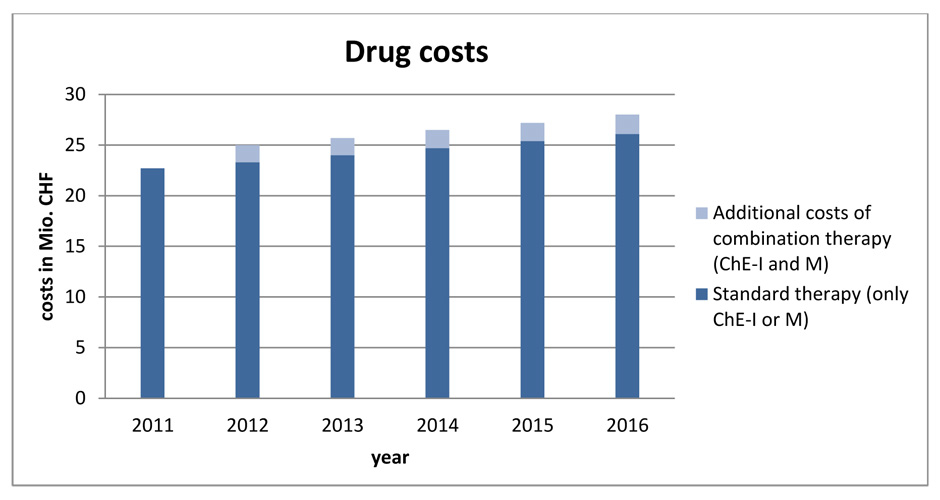
Figure 2
Calculated medication costs for the years 2011–2016.
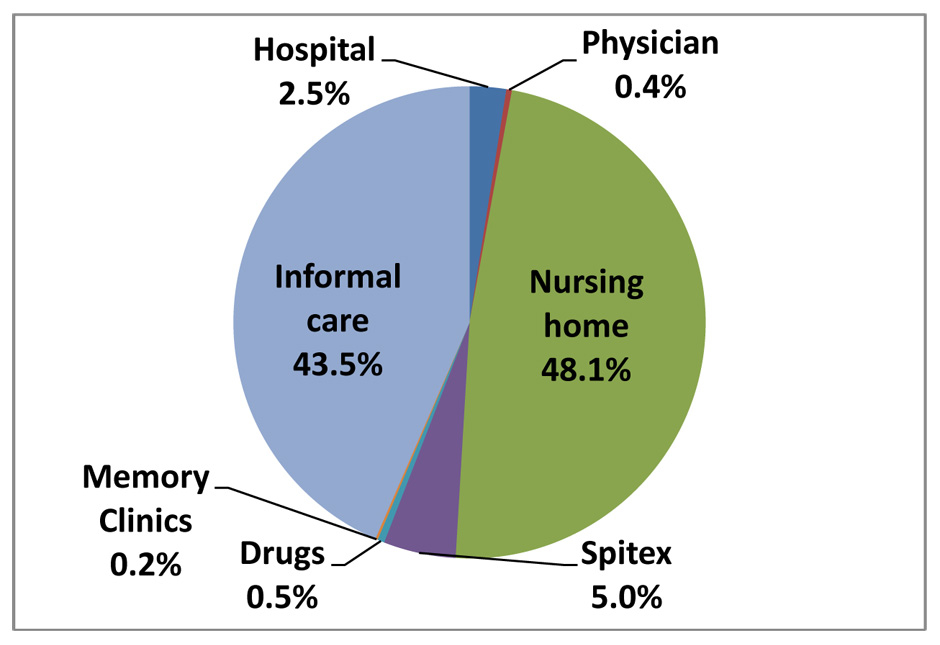
Figure 3
Estimated cost distribution in the year 2016.
According to IMS Health, antidementia drug costs were CHF 24.86 million in 2009 and CHF 24.9 million in 2010 [37]. When we calculated the total drug costs by using the unpublished estimates provided by the health insurer, total drug costs in 2010 were about CHF 21.6 million (table 4) and CHF 22.7 million in 2011.
Due to the demographic chang
es in Switzerland favouring an older population and the European dementia prevalence rates, the number of Alzheimer’s dementia patients would steadily increase over the next five years. This would lead to an annual increase of medication costs of about CHF 0.6 to CHF 0.7 million between 2012 and 2016 assuming only mono treatment (fig. 2). Calculating the medication costs caused by the combination treatment with the French values [32], additional costs of around CHF 1.7 to CHF 1.9 million annually would occur (fig. 2).
Related to total health care costs of Alzheimer’s dementia, drug costs are very low. The ECOPLAN [25] study calculated total health care costs of dementia of about CHF 6.94 billion in 2009. Based on this calculation, Alzheimer’s dementia made up 60% and cost CHF 4.18 billion in the year 2009. With an annual increase in health care costs of 5%, Alzheimer’s dementia costs were CHF 4.6 billion in 2011 and would be CHF 4.83 billion in 2012, CHF 5.32 billion in 2014, and CHF 5.87 billion in 2016. Medication costs in 2016 would account for 0.5% of total health care costs of Alzheimer’s dementia and would be negligible (fig. 3).
The cost-effectiveness threshold used in the US varies between USD 50,000 and USD 100,000 per QALY [38]. NICE cost-effectiveness thresholds for the UK are set at GBP 20,000 to GBP 30,000 per QALY [39]. There is no official cost-effectiveness threshold in Switzerland, but CHF 100,000 per QALY is most often used.
The assumed median survival time over five cycles was the same for both therapies (mono and combination treatment) and represented 3.33 years. Patients treated with a cholinesterase inhibitor remained at home for 2.85 years, whereas patients treated with a cholinesterase inhibitor and memantine could stay at home 0.45 years longer compared to those treated with the mono treatment and were admitted to a nursing home for only 0.03 years. QALYs were calculated by multiplying the utility values for “home” and “nursing home” with the corresponding time spent either at home or in a nursing home. Compared to the mono treatment, the combination treatment generated a benefit of 0.12 QALYs (table 5).
Despite additional medication costs, the combination treatment saved costs of CHF 27,656 per patient over five years compared to the mono treatment from a health care system perspective. Savings of the combination treatment over five years from a societal perspective were substantially lower (CHF 4,780). The ICER’s of the combination treatment were dominant (more benefit, less costs) from both perspectives (table 5). The cost and benefit differences between the mono treatment and the combination treatment were even higher after seven years, favouring the combination treatment.
Additional medication costs over five years caused by the implementation of the reimbursed combination treatment would be approximately CHF 9.5 million. The cost-utility analysis showed that CHF 27,656 per patient over five years could be saved by using the combination treatment. Estimating that about 1,800 (approximately 18,000 are treated, 52% of those have an MMSE 10–20 and 19% receive combination treatment) patients would be treated with the combination treatment in the years 2012 to 2016, roughly CHF 50 million could be saved in care costs. Based on long term considerations, the combination strategy was the dominant strategy.
Almost all results of the sensitivity analysis showed the robustness of the base case findings. One exception was the reduction of direct health care costs to 80% of the base case assumptions. In this case, the combination treatment led to higher costs than the mono treatment, but only from a societal perspective.
| Table 2: Calculated prevalence of dementia and Alzheimer’s dementia in Switzerland in 2011. | ||||||||
| Age | Prevalence rates | Swiss demographics 2011 | Number of dementia cases | 60% Alzheimer’s dementia | ||||
| Men | Women | Men | Women | Men | Women | Total | ||
| 30–64 | 0.07% | 0.07% | 1,967,826 | 1,950,722 | 1,377 | 1,366 | 2,743 | 1,646 |
| 65–69 | 2.20% | 1.10% | 201,790 | 212,638 | 4,439 | 2,339 | 6,778 | 4,067 |
| 70–74 | 4.60% | 3.90% | 145,210 | 168,391 | 6,680 | 6,567 | 13,247 | 7,948 |
| 75–79 | 5.00% | 6.70% | 113,483 | 146,974 | 5,674 | 9,847 | 15,521 | 9,313 |
| 80–84 | 12.10% | 13.50% | 77,510 | 121,330 | 9,379 | 16,380 | 25,759 | 15,455 |
| 85–89 | 18.50% | 22.80% | 41,712 | 82,945 | 7,717 | 18,911 | 26,628 | 15,977 |
| 90+ | 31.90% | 34.10% | 18,709 | 49,198 | 5,968 | 16,777 | 22,745 | 13,647 |
| Total | 2,566,240 | 2,732,198 | 41,234 | 72,187 | 113,421 | 68,053 | ||
| Table 3: Calculated prevalence of dementia and Alzheimer’s dementia in Switzerland for the years 2012, 2014, and 2016. | ||||||
| Age | Swiss population 2012 | Dementia patients 2012 | Swiss population 2014 | Dementia patients 2014 | Swiss population 2016 | Dementia patients 2016 |
| 30–64 | 3,943,097 | 2,760 | 3,980,696 | 2,786 | 4,017,528 | 2,812 |
| 65–69 | 424,348 | 6,945 | 436,079 | 7,142 | 438,196 | 7,182 |
| 70–74 | 327,734 | 13,852 | 358,642 | 15,173 | 387,813 | 16,423 |
| 75–79 | 262,244 | 15,614 | 269,857 | 16,041 | 282,396 | 16,763 |
| 80–84 | 203,170 | 26,304 | 210,140 | 27,175 | 216,897 | 28,024 |
| 85–89 | 127,188 | 27,149 | 133,498 | 28,440 | 140,397 | 29,840 |
| 90+ | 71,392 | 23,907 | 78,891 | 26,408 | 86,915 | 29,081 |
| Total | 5,359,173 | 116,531 | 5,467,803 | 123,165 | 5,570,142 | 130,125 |
| 60% Alzheimer’s dementia patients | 69,919 | 73,899 | 78,075 | |||
| Table 4: Total drug costs in 2010 based on the estimates from Helsana. | |||||
| Trade name | Substance | Frequency* | Number of treated patients | Average annual costs per patient [CHF]* | Total costs [CHF] |
| Aricept® | Donepezil (ChE-I) | 42% | 6,785 | 1,494 | 10,136,790 |
| Reminyl® | Galantamin (ChE-I) | 22% | 3,553 | 1,505 | 5,347,265 |
| Exelon® | Rivastigmin (ChE-I) | 13% | 2,100 | 1,139 | 2,391,900 |
| Axura®, Ebixa® | Memantine | 23% | 3,714 | 994 | 3,691,716 |
| Total (drug costs in the year 2010) | 21,567,671 | ||||
| *data provided by Helsana. | |||||
| Table 5: Incremental costs and cost-utility result over 5 years (base-case). | ||||||||
| Survival (years) | Time at home | Time in nursing home | QALYs | Health care system | Society | |||
| Costs | ICER | Costs | ICER | |||||
| ChE-I | 3.33 | 2.85 | 0.48 | 1.87 | 67,394 | 211,638 | ||
| ChE-I & M | 3.33 | 3.3 | 0.03 | 1.99 | 39,738 | 206,857 | ||
| Difference | +0.45 | –0.45 | +0.12 | –27,656 | Dominant | –4,780 | Dominant | |
This study calculated the budget impact and the cost-effectiveness of the combination treatment of a cholinesterase inhibitor and memantine in Alzheimer’s dementia in Switzerland assuming reimbursement to have started in 2012. Additional medication costs due to the combination treatment from the years 2012 to 2016 were CHF 9.5 million and were very low in relation to total health care costs caused by the disease. The cost utility analysis showed that the combination treatment saved care costs of about CHF 50 million between the years 2012 to 2016. Despite causing additional drug costs of approximately CHF 10 million from 2012 to 2016, the combination treatment would generate net savings of CHF 40 million. The sensitivity analysis confirmed the robustness of the results.
Strength and limitations of the cost of illness study on which our calculations were mainly based have previously been described in the original publication [14]. However, there were some limitations that were specific to this analysis. These are mainly related to the limited availability of country-specific data, which is a substantial and common problem associated with health economic evaluations.
Using average annual drug costs per patient provided by Helsana led to lower estimated total medication costs than the calculations of IMS Health. This difference can be explained by the different types of cost data: insurer data only include medication costs without additional patient co-payments whereas IMS Health includes both. Therefore, the insurer costs represent true medication costs for Alzheimer’s dementia from a healthcare payer perspective. This strengthens the results of the study, because we are calculating the budget impact of the combination treatment for the health care system.
The patent of donepezil (Aricept®) developed by Pfizer expired in May 2012 [40]. In Germany, a generic of Aricept®, donepezilhydrochlorid developed by Pfizer is available [41] at a lower price than Aricept®. Several generics of donepezil are available in Switzerland, e.g. Donepezil Actavis® or Donepezil Helvepharm® [42] at lower costs than Aricept®. As the price of donepezil will decrease and generics of galantamin, rivastigmin and memantine with decreased prices might follow in the future, the budget impact of the treatment with donepezil alone or in combination with memantine will be lower and the decreased prices could also have an effect on the cost-effectiveness of the combination treatment. Comparing memantine alone instead of cholinesterase inhibitors alone to combination treatment could decrease the cost benefit of the combination treatment, because memantine alone could have a greater health benefit than cholinesterase inhibitors alone.
The budget impact of the combination treatment was calculated based on the assumption that 25% of all Alzheimer dementia patients receive treatment with antidementia drugs. Despite demographic changes the total amount of prescribed antidementia drugs has remained unchanged over the last years in Switzerland. Thus, percentage wise less Alzheimer dementia patients will be treated with antidementia drugs due to the increasing older population and increasing prevalence of Alzheimer dementia in the next years. The very low increase in antidementia drug costs from 2009 to 2010 according to the calculation of IMS Health [37] supports this hypothesis. If this remains the case in the following years, we would be overestimating the budget impact of the combination treatment. Of course, one should aim to increase the percentage of diagnosed Alzheimer dementia patients to improve treatment coverage and quality of life.
As we did not know how many patients were treated with the combination treatment in Switzerland, we had to use empirical values from France. In this study [32], 77% of all patients were treated compared to 25% in Switzerland and approximately 19% received the combination treatment. The overall treatment rate in France was much higher than in Switzerland, as for several years the French government has recognised Alzheimer disease as being a major national health issue. Therefore, our assumption that 19% would receive the combination treatment in Switzerland is likely to be an optimistic estimate of the real combination treatment rate. Not all patients are suitable for prescription of the combination treatment and only those patients already receiving treatment with antidementia drugs may possibly be suitable for the combination treatment. Patients, who initially receive a cholinesterase inhibitor and are subsequently switched to memantine according to their indication, will most likely not receive the combination treatment. If our assumption of the percentage of Alzheimer dementia patients receiving antidementia treatment with the combination treatment in Switzerland is an overestimate, the budget impact of the combination treatment will also be lower.
Due to the lack of Swiss specific and actual data we had to rely on all input parameters in the cost-effectiveness model used by Touchon et al. [24], except for the costs which were derived from Swiss sources. Probability of time to death calculated by Touchon et al. [24] based on the median survival of 4.5 years from onset of dementia [36] was maintained and was constant over the time horizon of the model and the same for both treatment groups, i.e. survival was not age dependent. The clinical parameters used were mainly based on the observational, non-randomised, American study by Lopez et al. [10] which could have biased the results of our cost-utility analysis. Differences in cultural, societal and economic characteristics between American and Swiss patients, e.g. regarding insurance coverage, nursing home placement, access to healthcare, drug reimbursement, etc. might influence time to nursing home placement and therefore, the main benefit included in the model (delay of 8.9 months to nursing home placement with combination treatment compared to mono treatment) could be different for Swiss patients. However, utility values, transition probabilities into a nursing home or death, and direct and indirect costs were varied in the sensitivity analysis to show the impact of the different model input parameters on the outcomes of the model. All results of the variations in the sensitivity analysis performed from a health care perspective confirmed the results of our base case analysis. There was only one case (care costs lowered to 80% of the original values) when the combination treatment was no more cost saving compared to the mono treatment from a societal perspective.
Despite these limitations and according to our knowledge, this is the first attempt to calculate the potential budget impact and cost-effectiveness of the combination treatment of a cholinesterase inhibitor and memantine in Alzheimer dementia patients in a Swiss setting assuming reimbursement to have started in 2012.
All other costs than medication costs were conservatively measured. Therefore, this study might have underestimated the real total costs of Alzheimer’s dementia in Switzerland. By using optimistic foreign estimations of the percentage of Alzheimer dementia patients treated with the combination treatment, the additional medication costs are likely to be overestimated. Studies evaluating the clinical benefit of the combination treatment compared to the mono treatment showed additional benefit in Alzheimer dementia patients receiving both a cholinesterase inhibitor and memantine. The combination treatment is routinely used and reimbursed in several European countries, but not in Switzerland. The encouraging findings in this study may help decision makers to consider reimbursement of the combination treatment in Switzerland and in general, help to improve treatment coverage in Alzheimer dementia patients.
Acknowledgement:We thank the insurer Helsana (Pius Gyger and Oliver Reich) for providing us with frequency and cost data on cholinesterase inhibitor and memantine use in Switzerland.
1 Jalbert JJ, Daiello LA, Lapane KL. Dementia of the Alzheimer type. Epidemiol Rev. 2008;30:15–34.
2 Harvey HJ, Rossor MN, Skelton-Robinson M, Garralda E. Young onset dementia: epidemiology, clinical symptoms, family burden, support, and outcome. Dementia Research Group. Imperial College of Science, Technology and Medicine. London: 1998.
3 Hofman A, Rocca WA, Brayne C, Breteler MMB, Clarke M, Cooper B, et al. The prevalence of dementia in Europe: a collaborative study of 1980–1990 findings. Int J Epidemiol. 1991;20(3):736.
4 Wimo A and Prince M. World Alzheimer Report 2010: the global economic impact of dementia. Alzheimer’s Disease International. 2010.
5 Volz A, Monsch AU, Zahno A, Wettstein A, Stähelin HB, Grünig R. Was kostete die Schweiz die Alzheimer-Krankheit 1998? Eine präliminäre Analyse. Praxis. 2000;89:803–11.
6 Sadik K and Wilcock G. The increasing burden of Alzheimer disease. Alzheimer Dis Assoc Disord. 2003;17:S75–S79.
7 Wimo A, Winblad B, Jönsson L. The worldwide societal costs of dementia: Estimates for 2009. Alzheimer’s & Dementia. 2010;6:98–103.
8 Gauthier S, Molinuevo JL, Lemming O. Effects of memantine in patients with moderate to severe Alzheimer’s disease receiving stable doses of donepezil: a meta-analysis. Poster presented at the 10th International Conference on Alzheimer’s & Parkinson’s Diseases, 2011
9 Atri A, Shaughnessy LW, Locascio JJ, and Growdon JH. Long-term course and effectiveness of combination treatment in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22(3):209–21.
10 Lopez OL, Becker JT, Wahed AS, Saxton J, Sweet RA, Wolk DA, et al. Long-term effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009;80:600–7.
11 Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil. JAMA. 2004;291(3):317–24.
12 Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med. 2012;366:893–903.
13 Farrimond LE, Roberts E, McShane R. Memantine and cholinesterase inhibitor combination therapy for Alzheimer’s disease: a systematic review. BMJ Open. 2012;2(3):e000917.
14 Kraft E, Marti M, Werner S, Sommer H. Cost of dementia in Switzerland. Swiss Med Wkly. 2010;140:w13093
15 Rive B, Aarsland D, Grishchenko M, Cochran J, Lamure M, Toumi M. Cost-effectiveness of memantine in moderate and severe Alzheimer’s disease in Norway. Int J Geriatr Psychiatry. 2011. Epub ahead of print. doi: 10.1002/gps.2755.
16 Hoogveldt B, Rive B, Severens J, Maman K, Guilhaume C. Cost-effectiveness of memantine for moderate-to-severe Alzheimer’s disease in the Netherlands. Neuropsychiatr Dis Treat. 2011;7:313–7.
17 Rive B, Grishchenko M, Guilhaume-Goulant C, Katona C, Livingston G, Lamure M, et al. Cost effectiveness of memantine in Alzheimer’s disease in the UK. J Med Econ. 2010;13(2):371–80.
18 Gagnon M, Rive B, Hux M, Guilhaume C. Cost-effectiveness of memantine compared with standard care in moderate-to-severe Alzheimer disease in Canada. Can J Psychiatry. 2007;52(8):519–26.
19 François C, Sintonen H, Sulkava R, Rive B. Cost-effectiveness of memantine in moderately severe to severe Alzheimer’s disease: A Markov model in Finland. Clin Drug Investig. 2004;24(7):373–84.
20 Antonanzas F, Rive B, Badenas JM, Gomez-Lus S, Guilhaume C. Cost-effectiveness of memantine in community-based Alzheimer’s disease patients: an adaption in Spain. Eur J Health Econ. 2006;7:137–44.
21 Jones RW, McCrone P, Guilhaume C. Cost-effectiveness of memantine in Alzheimer’s disease: an analysis based on a probabilistic Markov model from a UK perspective. Drugs Aging. 2004;21(9):607–20.
22 Jönsson L. Cost-effectiveness of memantine for moderate to severe Alzheimer’s disease in Sweden. Am J Geriatr Pharmacother. 2005; 3(2):77–86.
23 Lachaine J, Beauchemin C, Legault M, Bineau S. Economic evaluation of the impact of memantine on time to nursing home admission in the treatment of Alzheimer disease. Can J Psychiatry. 2011;56(10):596–604.
24 Touchon J, Lachaine J, Beauchemin C, Crochard A, Rive B, Bineau S. Memantine delays the admission of Alzheimer’s disease patients to nursing home: cost-effectiveness analysis in the French setting. Poster presented at the 13th ISPOR Annual European Congress, 2010.
25 ECOPLAN. Kosten der Demenz in der Schweiz – Schlussbericht. Studie im Auftrag der Schweizerischen Alzheimervereinigung. Bern: 2010.
26 Bundesamt für Statistik. Ständige Wohnbevölkerung 2011. Available from: http://www.bfs.admin.ch/bfs/portal/de/index/themen/01/02/blank/key/alter/nach_geschlecht.html Access at: 20 October 2011
27 Bundesamt für Statistik. Zukünftige Bevölkerungsentwicklung der Schweiz in den Jahren 2012-2016. Available from: http://www.bfs.admin.ch/bfs/portal/de/index/themen/01/03.html Access at: 20 October 2011
28 Schweizerische Alzheimervereinigung. Zahlen zur Demenz – Kosten der Demenz in der Schweiz: Update 2009. Available from: http://www.alz.ch/d/data/data_883.pdf Access at: 3 October 2011.
29 CSS Gruppe. FaktenBlatt Gesundheitspolitik – Fragen und Antworten zur Kosten- und Prämienentwicklung im Gesundheitswesen. Available from: https://www.css.ch/media/de/ documents/ueber_uns/themen_im_fokus/faktenblatt_d_kostenentwicklung.pdf Access at: 17 November 2011
30 Schweizerische Alzheimervereinigung. Demenz und Gesellschaft – Fakten zur Demenz. Available from: http://www.alz.ch/d/data/data_781.pdf Access at: 20 October 2011
31 IMS Health. Antidementia drugs in Europe and USA – percentage of population treated with antidementia drugs from 2005–2009 [Graphic].
32 Tifratene K, Le Duff F, Pradier C, Quetel R, Robert P. Usage des medicaments anti-Alzheimer en France: une analyse des pratiques à partir de la Banque Nationale de données Alzheimer (BNA). La Revue de gériatrie. 2011;36(8):557–65.
33 Haute Autorité de Santé – Médicaments: EBIXA Available from: http://www.has-sante.fr/portail/jcms/c_1117850/ebixa?xtmc=Ebixa&xtcr=1 Access at: 6December 2011
34 Ward A, Caro JJ, Getsios D, Ishak K, O’Brien J, Bullock R; AHEAD Study Group. Assessment of health economics in Alzheimer’s disease (AHEAD): treatment with galantamine in the UK. Int J Geriatr Psychiatry. 2003;18(8):740–7.
35 Neumann PJ, Kuntz KM, Leon J, Araki SS, Hermann RC, Hsu MA, et al. Health utilities in Alzheimer’s disease: a cross-sectional study of patients and caregivers. Med Care. 1999;37(1):27–32.
36 Helmer C, Joly P, Commenges D, Dartigues J-F. Mortality with dementia: results from a French prospective community-based cohort. Am J Epidemiol 2001;154:642–8.
37 Bundesamt für Statistik. Apotheken/SD-Ärzte Index Schweiz. Available from: http://www.obsan.admin.ch/bfs/obsan/de/index/04/04/blank/blank/sdaindex/01.html Access at: 17 November 2011
38 WHO. Cost-effectiveness thresholds. Available from: http://www.who.int/choice/costs /CER_thresholds/en/index.html Access at: 6 December 2011
39 NICE. Measuring effectiveness and cost-effectiveness: the QALY. Available from: http://www.nice.org.uk/newsroom/features/measuringeffectivenessandcosteffectivenesstheqaly.jsp Access at: 6 December 2011
40 PharmaWiki – Medikamente und Gesundheit: Donepezil. Available from: http://www.pharmawiki.ch/wiki/index.php?wiki=Donepezil Access at: 30April 2012.
41 Mein Gesundheitszentrum – Alzheimer: Aricept. Available from: http://www.meingesundheitszentrum.de/aricept-alzheimer/ Access at: 30April 2012.
42 Arzneimittel-Kompendium der Schweiz® – Donepezil. Available from: www.kompendium.ch Access at: 7August 2012.
Financial support:This research was supported by an unrestricted educational grant from Lundbeck (Schweiz) AG. R. W. Kressig received lecturer and advisory board fees from Janssen-CILAG, Lundbeck, Merz, Novartis, and Pfizer. No other potential conflict of interest relevant to this article was reported.