
Figure 1
Positioning of the patient for minimally invasive access: the Zurich approach.
DOI: https://doi.org/10.4414/smw.2012.13715
Mitral valve (MV) reconstruction is the gold standard for the treatment of MV regurgitation (MR) today [1]. There is currently no reasonable alternative to surgery which is curative and results in the prevention of subsequent heart failure [2]. Mitral valve reconstruction has been demonstrated to be superior to MV replacement with improved post-operative survival rates and preserved left ventricular function [3, 4].
The MV is a complex structure based upon five different components: the mitral annulus, the anterior and posterior leaflet, the chordae tendinae, the papillary muscles and the left ventricle. A complex mechanism underlies the systolic and diastolic function of the MV. The two highest prevalence entities are primary (degenerative) and secondary (functional) MR. In primary MR, elongation or rupture of chordae tendineae and/or excessive tissue lead to repulsing or prolapsing of the leaflet into the atrium during systole with resulting insufficiency. Underlying pathologies are fibroelastic deficiency or myxomatous proliferation of the leaflet tissue as seen in Barlow’s disease. In secondary MR ischaemic or idiopathic cardiomyopathy leads to alterations in the ventricular geometry, the subvalvular apparatus and of the mitral annular geometry leading to MR. Therefore, the goal of a mitral valve repair procedure follows two fundamental principles: restore a sufficient surface of leaflet coaptation and correction of annular dilatation.
In daily clinical routine, a lack of adherence to guidelines addressing the timely referral of patients with indications for surgery can be observed. One reason might be that it is not possible to predict the success of MV repair. Additionally, current medical and surgical practice often seems to result in suboptimal care for the individual patient with degenerative mitral valve disease. A better implementation of education in regards of the guidelines is essential to advance the field. All cardiovascular specialists should have familiarity with the up-to-date standards in terms of MV disease differentiation, timing of intervention and surgical techniques in order to improve patient care.
Surgical intervention for chronic severe mitral valve regurgitation is usually triggered by the occurrence of symptoms, declining LV function, significant LV enlargement or the development of atrial fibrillation or severe pulmonary hypertension [5, 6]. Controversy exists about the timing of surgery in asymptomatic patients: Early surgical intervention before the onset of ventricular changes is preferred in several centres for patients with severe degenerative mitral valve disease. Others prefer a watchful waiting strategy. In the latest guidelines, the best evidence recommendation for asymptomatic patients is IC [5, 6]. This debate has put emphasis on the lack of predictability of mitral valve repair, despite broad consensus that this is the procedure of choice for patients undergoing surgical intervention.
A reason for early surgical correction of mitral valve regurgitation is the existing evidence that the majority of asymptomatic patients develop symptoms and/or other indications for surgery within 5–10 years of diagnosis.
Enriquez-Sarano et al. described high event rates in a series of asymptomatic patients with quantitatively graded severe degenerative mitral regurgitation (effective regurgitant orifice area ≥40 mm2). These results lead them to recommend prompt surgery in this patient cohort [7].
Rosenhek et al. published a series of 132 asymptomatic patients with severe degenerative mitral regurgitation. They applied a watchful waiting strategy and noted a good outcome. Within an 8-year interval, 45% of patients had an event (but not sudden death), with events occurring at a regular pace. Twenty four of 36 patients fulfilling indications for surgery became symptomatic. The rest required surgery because of asymptomatic LV dysfunction or enlargement, new onset atrial fibrillation or pulmonary hypertension [8]. Kang et al. published a study including 161 asymptomatic patients with severe degenerative mitral regurgitation who were subjected to early surgery, compared to 268 patients managed by watchful waiting over a nine year follow-up. A 99% cardiac event-free survival was observed in operated patients, compared with 85% in patients under a strategy of continued medical observation. Urgent surgery was proposed but refused by five of the six patients who died of congestive heart failure and one sudden death occurred in a patient who had become symptomatic. Three cases of sudden death occurred in asymptomatic patients [9]. Montant and colleagues investigated in a study the outcomes in patients with severe degenerative mitral regurgitation by semi-quantitative echocardiographic assessment, reporting on 67 patients managed with a conservative approach and 125 patients subjected to early mitral valve repair. Ten year survival was significantly lower in conservatively managed patients compared with those operated early [10].
Several prognostic factors allowing risk stratification for asymptomatic patients have been proposed. High levels of brain natriuretic peptide (<105 pg/mL) were associated with an unfavourable outcome in a study by Pizzaro and colleagues [11]. Recently, Tribouilloy and colleagues have shown that a LV end-systolic diameter (LVED) ≥40 mm is independently associated with increased mortality under medical management and after surgery [12]. The LVESD is also a parameter that is used in the guidelines for recommendations for surgery. Recent European guidelines recommend referral for surgery when the LVESD is larger than 45 mm compared to North American guidelines with recommendations for surgery in LVED diameters larger than 40 mm [5, 6].
There is evidence, that early mitral valve repair is beneficial according to left ventricular function. A study by Suri and colleagues has shown superior recovery of left ventricular ejection fraction after early mitral valve repair in patients with an preoperative ejection fraction of ≥65% [13].
In accordance with the current guidelines, surgery is indicated in asymptomatic patients with LV dysfunction defined by an ejection fraction below 60% or a left ventricular end systolic diameter of 45 mm or more (Class I, Level C). Surgery is recommended to be considered in asymptomatic patients with preserved LV function, new onset of atrial fibrillation or pulmonary hypertension (Class IIa, Level C), preserved LV function, high likelihood of durable repair, low surgical risk and flail leaflet and increased LVESD (Class IIa, Level C) and maybe considered in asymptomatic patients with preserved LV function, high likelihood of durable repair, low surgical risk and left atrial dilatation and sinus rhythm or pulmonary hypertension on exercise (Class IIb, Level C) (table 1) [5].
| Table 1: Guideline recommendations for indications for surgery in severe primary mitral regurgitation. Adapted from: Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2012;33:2451–96 [5]. | ||
| Mitral valve repair should be the preferred technique when it is expected to be durable. | I | C |
| Surgery is indicated in sympotmatic patients with LVEF >30% and LVESD <55 mm. | I | B* |
| Surgery is indicated in asympotmatic patients with LV dysfunction (LVESD ≥45 mm and/or LVEF ≤60%). | I | C |
| Surgery should be considered in asymptomatic patients with preserved LV function and new onset of atrial fibrillation or pulmonary hypertension (systolic pulmonary pressure at rest >50 mm Hg). | IIa | C |
| Surgery should be considered in asymptomatic patients with preserved LV function, high likelihood of durable repair, low surgical risk and flail leaflet and LVESD ≥40 mm Hg. | IIa | C |
| Surgery should be considered in patients with severe LV dysfunction (LVEF <30% and/or LVESD >55 mm) refractory to medical therapy with high likelihood of durable repair and low comorbidity. | IIa | C |
| Surgery may be considered in patients with severe LV dysfunction (LVEF <30% and/or LVESD >55 mm) refractory to medical therapy with low likelihood of durable repair and low comorbidity. | IIb | C |
| Surgery may be considered in asymptomatic patients with preserved LV function, high likelihood of durable repair, low surgical risk and - Left atrial dilatation (volume index ≥60 ml/m2 BSA) and sinus rhythm, or - Pulmonary hypertension on exercise (SPAP ≥60 mm Hg at exercise). | IIb | C |
| BSA = body surface area; LV = left ventricle; LVEF = left ventricular ejection fraction; LVESD = left ventricular end-systolic diameter; SPAP = systolic pulmonary artery pressure; class = class of recommendation; level = level of evidence; * references supporting class I(A+B) and IIa+IIb (A+B) recommendations: [14, 15]. | ||
There are clear recommendations for surgery in current guidelines for patients with symptoms of left ventricular dysfunction [5, 6]. In contrast in daily clinical business there seems to be discordance to the guidelines. Several studies found that numerous patients with indications for surgery received no operation. Mirabel and colleagues found that 49% of patients with symptomatic mitral valve regurgitation from the Euro Heart Survey were not referred for surgery due to advanced age, co-morbidities and an decreased ejection fraction [16]. In the same patient cohort, Detaint and colleagues later identified 101 patients with severe mitral regurgitation. They found that 29% have not received intervention although they had no relevant co-morbidities and fulfilled guideline recommendation criteria for surgery [17]. They considered the relatively low level of evidence as the underlying cause for denial of referral.
Toledano and colleagues surveyed Canadian cardiologists and found that nearly 40% of those indicated that they would wait with the referral of an asymptomatic patient with severe mitral valve regurgitation for surgery until the ejection fraction fell below 40% or symptoms occur. The decrease in LVEF was considered as clearly underestimated by the cardiologists in this study [18]. In conclusion, it has been demonstrated that referral for surgery in patients with symptomatic MR is not in accordance with the guidelines in up to 50%. The underlying causes are multi-factorial. An underestimation of the decrease of the LVEF has been considered as factor as well as the underuse of current guidelines due to lower level of evidence. Additionally, referral of patients has been demonstrated to be more in accordance with the guidelines when physicians that have graduated later are the referring person. These data show that there is need for continued medical education of practicing clinicians to increase familiarity with current guidelines.
The classical access for mitral valve repair is full median sternotomy. Besides smaller accesses such as partial sternotomy, the right lateral thoracotomy (fig. 1) is used in many expert centres today. In regards of mortality, excellent short and long-term results are described [19–21]. Patients benefit from better cosmetic results and less blood transfusion has been reported in some series. On the other hand this may come at an expense of increased cardiopulmonary bypass time and longer cross-clamp and procedure times [22].

Figure 1
Positioning of the patient for minimally invasive access: the Zurich approach.
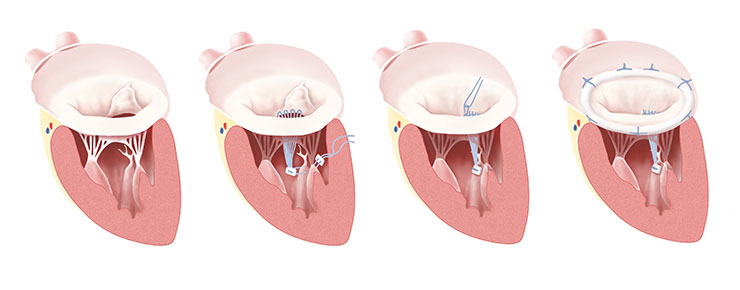
Figure 2
Gore-Tex loop technique and ring annuloplasty; Fibroelastic deficiency with prolapse of the anterior leaflet; Gore-Tex loops are constructed, and the apparatus is attached to the fibrous tip of the papillary muscle. Individual loops are attached to the prolapsing segment margin and completed repair after ring annuloplasty.

Figure 3
Measurement of the length for the artificial chords. implanted chord and the complete neo-chord as available from the manufacturer.
A modification is the endoscopic technique also performed through a thoracotomy but without the use of a thoracic retractor and without direct vision [23]. In specialised centres, the mitral valve is repaired totally endoscopically with the use of the da Vinci system [24].
A consensus statement of the International Society for Minimally Invasive Cardiothoracic Surgery (ISMICS) gives an evidence class IIb recommendation for minimally invasive surgery in patients with mitral valve disease. This recommendation is based on comparable short and long term mortality, in hospital morbidity, reduced sternal complications, blood transfusion, atrial fibrillation and reduced ventilation time, intensive care unit stay and length of hospital stay standing against an increased risk of stroke, aortic dissection, phrenic nerve lesion and prolonged cross clamp, cardiopulmonary bypass and procedure time [25].
Taking all factors into calculation it seems that the minimally invasive or port-access approach is at least as good and safe as the conventional sternotomy approach but there is still a lack of prospective, randomized controlled trials with adequate patient numbers and follow up times to determine the balance of benefits and risks.
The only contraindication for minimally invasive access is severe calcification of the annulus. Debridement of calcified tissue is required to restore a good mobility to leaflet tissue and to ensure an adequate surface of coaptation. However special instruments are necessary that are not available for minimally invasive techniques and access is very limited. Severe complications such as ventricle rupture can occur that cannot be managed so well through the minimally invasive access.
Three principle goals of MV repair were introduced by Carpentier: Stabilisation of the annulus with the retention of an adequately sized mitral orifice, restoration of physiological leaflet motion and recreation of a sufficient line of coaptation. The first technique to reach this was the so-called “French correction” introduced in 1983.
A resection of redundant tissue, chordal transfer, chordal shortening and remodelling annuloplasty were parts of the operation described by Carpentier and colleagues [26]. This ‘classical’ repair technique has been demonstrated to result in excellent outcome with a high durability and high freedom from reoperation rate [1, 20, 21, 27, 28].
The paradigm of ‘respect rather than resect’ tissue emerged in recent years. Use of polytetrafluoroethylene (PTFE) neochordae was introduced to support fixation of the free edge of prolapsing segments and ‘displacing’ abnormal excess tissue into the ventricle to ensure a good surface of coaptation instead of resection of leaflet tissue [29]. A variation of this technique is the ‘loop technique’ where the PTFE is anchored into the tip of the papillary muscle and ‘loops’ are attached to the free edge mimicking a chordal fan (fig. 2) [30, 31]. The neochordae are anchored on the anterior or posterior papillary muscle in their fibrous portion. It’s important that crossing the midline or individual native chordae is avoided in order to prevent excess traction on the leaflet margin. In the case of significant excess posterior leaflet height, the neochordae are made short enough to displace the prolapsing segment into the left ventricle, to ensure a large surface of coaptation for the anterior leaflet while preventing anterior leaflet displacement in the outflow tract. Exact measurement is necessary before implantation. The relevant length is measured from the tip of the papillary muscle to intended coaptation line (fig. 3). No or limited leaflet resection in combination with PTFE loops is now a preferred technique in our and many other centres.
In all mitral valve reconstructions a prosthetic ring or band annuloplasty is used to restore the normal circumference and shape of the mitral valve to match the available leaflet tissue [32]. The fibrous skeleton of the heart and long-standing regurgitation associated with ventricular and atrial enlargement leads to dilatation of the mitral annulus, particularly along the posterior aspect of the valve. To have an idea of the appropriate ring size, measurement of the surface of the anterior leaflet with a seizer is used to estimate the appropriate ring size for the amount of leaflet tissue. This may have important implications for percutaneous techniques that primarily attempt to address prolapse by attaching opposing leaflets without concomitantly changing the shape of the annulus.
For patients with secondary MR, undersized mitral annuloplasty is the current surgical gold standard. This strategy tempts to reshape the mitral annulus to a more anatomically correct form, thus leading to increased leaflet coaptation and a ompetent MV. Important for this operation is the implantation of a complete rigid annuloplasty ring, rather than an open flexible band [33, 34]. Undersized annuloplasty has been associated with left ventricular reverse remodelling and improvement of symptoms in the majority of patients, but recurrent MR occurs more frequently than in patients with degenerative disease. Recently developed strategies such as the ‘ring and string’ concept, secondary chordal cutting, septal-lateral banding and posterior leaflet extension have been suggested as additional techniques that may minimise the risk of recurrent MR [35, 36].
Numerous additional repair techniques such as the edge-to-edge repair, papillary muscle shortening, leaflet reduction plasty and others have been developed, affording the surgeon a wide armamentarium of approaches [37–40]
Today, several evolving technologies are arising to overcome challenges such as the need to perform the operation on the arrested heart under non-physiological conditions. The estimation of the result of the reconstruction is difficult. Due to this the outcome of a MV repair is strongly dependent to the caseload of the performing centres and the experience of the surgeons [41, 42]. New devices were introduced lately focusing on overcoming these pitfalls and intending to improve operative results and provide techniques to avoid the CPB. These devices have features to adjust the diameter or length of annuloplasty rings or neochordae, respectively. Animal experiments showed promising results and clinical trials are running at present [43, 44].
Modern cardiac surgery programmes with a high volume number have achieved very high and durable MV repair rates with minimal perioperative mortality and long-term outcomes that are comparable with the general population.
Braunberger and colleagues described their very long term results with follow up periods of more than 20 years. 162 patients were included, mainly operated due to degenerative MV disease (90%) or endocarditis (10%). The classical French correction technique was used. After three months the MV reoperation rate was below two percent. Within the 20 years follow-up only seven patients had to undergo repeat MV operation. The survival rate was around 50% comparable to the survival rate of the normal population with that age structure [27]. Seeburger and colleagues described the results of 1,339 patients who had undergone minimally invasive mitral valve repair in a time period of eight years. Success rate was almost 100%. The five year Kaplan-Meier estimation for freedom from MV reoperation was 96.3%. Thirty day mortality was 2.4% and the five year survival was 82.6% [21]. The same group investigated the outcome of MV repair focusing on different underlying degenerative forms. Around half of the patients were treated for posterior leaflet prolapse. The other half of patients had to undergo surgery for anterior or bileaflet prolapse. The repair rate was 94%. Freedom from reoperation rate at five years was 95.6%. The thirty days mortality was 1.8% and the five-year survival was 87.3% in this patient cohort. No significant differences in outcome and duration of the repair were observed in regards of the different underlying prolapse [1].
Slightly different results were described by David and colleagues. More than 700 patients were included with MR due to prolapsing of anterior, posterior or bileaflet prolapse. The distribution of the location of the prolapse was similar to the work of Seeburger and colleagues described above. In contrast the results for the repair of anterior leaflet prolapse and bileaflet prolapse were worse than for posterior leaflet prolapse. The freedom from reoperation rate was 96% for posterior leaflet repair compared to 94% of both leaflets repair and 88% of anterior leaflet repair. The follow-up period was 12 years and the survival at this time was 75% [20].
These superb results can also be achieved through a minimal invasive technique leading to a better cosmetic result, a decreased incidence of respiratory failure, decreased post-operative pain and a faster recovery [22, 45]. For robotic supported MV surgery no large randomized trial has been published to compare totally endoscopic MV repair to port-access surgery. Chitwood and colleagues presented their results of 300 mitral valve repairs performed with the da Vinci system and reported safe performance of the procedure as well as low short and midterm mortality. 30-day mortality was below 1%, late mortality was 2%. There was no conversion to sternotomy, but to conventional port access surgery (in 9 of 309 intended-to-treat patients) due to technical problems with the da Vinci system or the need for mitral valve replacement. The conclusion of this paper was that the technique is safe and feasible but longer time follow up is necessary [26].
The outcome of patients undergoing MV repair for secondary MR is dependent to the underlying cause of cardiomyopathy and the concomitant procedure. Gummert and colleagues described a 30-day mortality rate of 6.1% and a 5-year survival of 66% in patients with dilated and ischaemic cardiomyopathy. Tricuspid valve repair and atrial fibrillation ablation and atrial size reduction were the only accepted concomitant procedure [46]. Bax and colleagues reported their results for 51 patients undergoing coronary revascularisation and parallel restrictive MV repair in ischaemic cardiomyopathy. Their early mortality rate was 5.6% and the 2-year survival was 84%. One patient had to undergo reoperation for recurrent MR. 2-year echocardiographic follow-up showed non or mild MR in all patients as well as a decrease in left ventricular end systolic and end diastolic dimensions [47]. Additionally, differences in outcome in regards of the use of a complete or a partial annuloplasty ring have been observed by Kwon and colleagues. In a retrospective study of 479 patients that had undergone MV repair due to secondary MR, they found a greater freedom from recurrent MR in the 209 patients where a complete ring was used compared to 270 patients treated with a partial ring. A difference in survival during the follow-up could not be detected [48].
Current surgical mitral valve repair offers a highly effective and safe treatment for patients with MR, even in those patients who require reoperative procedures. Access is either gained through a full sternotomy or through minimally invasive access such as right sided thoracotomy or partially and/or totally endoscopically with robot systems. The minimally invasive technique shows similar reconstructive results as open repair. However, the cosmetic results lead to a higher patient satisfaction and less blood transfusion. In experienced centres it is the standard approach.
The first repair technique introduced by Carpentier in 1983 was the “French correction” that included leaflet-resection, chordal replacement and annuloplasty with ring devices. Starting from there a continuous evolution of the techniques can be observed. To date, neo-chord implantation additionally to annuloplasty is a preferred technique in many centres to treat primary mitral valve insufficiency despite the fact that there is no clear advantage in regards of mortality or durability of the repair compared to resection of prolapsing segments. An explanation might be that implantation of chords is more straightforward especially when the operation is performed minimally-invasive and video-assisted. Additionally this technique permits implantation of larger annuloplasty-rings and a larger area of coaptation can be achieved [49]. Results show that the improvement of the techniques has led to better outcomes in regards to mortality and to a decrease of reoperation rates compared to the initial techniques. Different underlying pathologies are better addressed by introduction of these new techniques. MR still demonstrates a high potential for innovative techniques. Adjustable devices were lately introduced to the market and off-pump techniques are under preclinical and clinical investigation.
The timing for surgical intervention is still a matter of discussion. The occurrence of symptoms should be a trigger for referring a patient according to the guidelines. Interestingly, it seems that the referral for surgery is often not in accordance with the guidelines in patients with severe MR. Explanations might be the relatively low evidence levels of the guidelines or underestimations of factors that are clear triggers for the referral. In asymptomatic patients the decision finding process is even more difficult. Different parameters such as left ventricular end-diastolic dimension and a decrease of left ventricular function are indicators for the referral to surgery. However there is still controversy about the preference of a watchful waiting strategy and early surgery. The evidence-level of the guidelines seems to leave a confidence gap resulting in uncertainty about the timing for referral. This shows that there is need for continued efforts in investigating the outcomes of mitral valve surgery in different patient cohorts and improve medical education of practicing clinicians to increase familiarity with current guidelines.
In conclusion, the results of mitral valve repair are excellent and it is widely accepted that a reconstruction is preferable to a valve replacement. Surgery can result in a complete correction of the MR and normalisation of valve morphology and thus represents the only curative treatment strategy for patients with mitral valve regurgitation. It therefore represents the current gold standard for the treatment of mitral valve regurgitation.
1 Seeburger J, Borger MA, Doll N, Walther T, Passage J, Falk V, et al. Comparison of outcomes of minimally invasive mitral valve surgery for posterior, anterior and bileaflet prolapse. Eur J Cardiothorac Surg. 2009;36:532–8.
2 Carabello BA. The current therapy for mitral regurgitation. J Am Coll Cardiol. 2008;52:319–26.
3 Jokinen JJ, Hippeläinen MJ, Pitkänen OA, Hartikainen JEK. Mitral valve replacement versus repair: propensity-adjusted survival and quality-of-life analysis. Ann Thorac Surg. 2007;84:451–8.
4 Ryan WH, Brinkman WT, Dewey TM, Mack MJ, Prince SL, Herbert MA. Mitral valve surgery: comparison of outcomes in matched sternotomy and port access groups. J Heart Valve Dis. 2010;19:51–8– discussion 59.
5 Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2012;33:2451–96.
6 Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anaesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1–142.
7 Enriquez-Sarano M, Avierinos J-FO, Messika-Zeitoun D, Détaint D, Capps M, Nkomo V, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–83.
8 Rosenhek R, Rader F, Klaar U, Gabriel H, Krejc M, Kalbeck D, et al. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation. 2006;113:2238–44.
9 Kang D-H, Kim JH, Rim JH, Kim M-J, Yun S-C, Song J-M, et al. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation. 2009;119:797–804.
10 Montant P, Chenot F, Robert A, Vancraeynest D, Pasquet A, Gerber B, et al. Long-term survival in asymptomatic patients with severe degenerative mitral regurgitation: a propensity score-based comparison between an early surgical strategy and a conservative treatment approach. J Thorac Cardiovasc Surg. 2009;138:1339–48.
11 Pizarro R, Bazzino OO, Oberti PF, Falconi M, Achilli F, Arias A, et al. Prospective validation of the prognostic usefulness of brain natriuretic peptide in asymptomatic patients with chronic severe mitral regurgitation. J Am Coll Cardiol. 2009;54:1099–106.
12 Tribouilloy C, Grigioni F, Avierinos J-F, Barbieri A, Rusinaru D, Szymanski C, et al. Survival implication of left ventricular end-systolic diameter in mitral regurgitation due to flail leaflets a long-term follow-up multicenter study. J Am Coll Cardiol. 2009;54:1961–8.
13 Suri RM, Schaff HV, Dearani JA, Sundt TM, Daly RC, Mullany CJ, et al. Recovery of left ventricular function after surgical correction of mitral regurgitation caused by leaflet prolapse. J Thorac Cardiovas Surg. 2009;137:1071–6.
14 Haan CD, Cabral CI, Conetta DA, Coombs LP, Edwards FH. Selecting patients with mitral regurgitation and left ventricular dysfunction for isolated mitral valve surgery. Ann Thorac Surg. 2004;78:820–5.
15 Enriquez-Sarano M, Tajik AJ, Schaff HV, Orszulak Ta, McGoon MD, Bailey KR, Frye RL. Echocardiographic prediction of left ventricular function after correction of mitral regurgitation: results and clinical implications. J Am Coll Cardiol. 1994;24:1536–43.
16 Mirabel M, Iung B, Baron G, Messika-Zeitoun D, Détaint D, Vanoverschelde JL, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007;28:1358–65.
17 Détaint D, Iung B, Lepage L, Messika-Zeitoun D, Baron G, Tornos P, et al. Management of asymptomatic patients with severe non-ischaemic mitral regurgitation. Are practices consistent with guidelines? Eur J Cardiothorac Surg. 2008;34:937–42.
18 Toledano K, Rudski LG, Huynh T, Béïque F, Sampalis J, Morin J-F. Mitral regurgitation: determinants of referral for cardiac surgery by Canadian cardiologists. Can J Cardiol. 2007;23:209–14.
19 Gammie JS, Bartlett ST, Griffith BP. Small-incision mitral valve repair: safe, durable, and approaching perfection. Ann Surg. 2009; 250:409–15.
20 David TE, Ivanov J, Armstrong S, Christie D, Rakowski H. A comparison of outcomes of mitral valve repair for degenerative disease with posterior, anterior, and bileaflet prolapse. J Thorac Cardiovasc Surg. 2005;130:1242–9.
21 Seeburger J, Borger MA, Falk V, Kuntze T, Czesla M, Walther T, et al. Minimal invasive mitral valve repair for mitral regurgitation: results of 1339 consecutive patients. Eur J Cardiothorac Surg. 2008;34:760–5.
22 Cheng DCH, Martin J, Lal A, Diegeler A, Folliguet TA, Nifong LW, et al. Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations. (Phila) 2011;6:84–103.
23 Vanermen H, Farhat F, Wellens F, De Geest R, Degrieck I, Van Praet F, et al. Minimally invasive video-assisted mitral valve surgery: from Port-Access towards a totally endoscopic procedure. J Card Surg. 2000;15:51–60.
24 Chitwood WR, Rodriguez E, Chu MWA, Hassan A, Ferguson TB, Vos PW, et al. Robotic mitral valve repairs in 300 patients: a single-centre experience. J Thorac Cardiovasc Surg. 2008;136:436–41.
25 Falk V, Cheng DCH, Martin J, Diegeler A, Folliguet TA, Nifong LW, et al. Minimally invasive versus open mitral valve surgery: a consensus statement of the international society of minimally invasive coronary surgery (ISMICS) 2010. Innovations (Phila). 2011;6:66–76.
26 Carpentier A. Cardiac valve surgery – the “French correction”. J Thorac Cardiovasc Surg. 1983;86:323–37.
27 Braunberger E, Deloche A, Berrebi A, Abdallah F, Celestin JA, Meimoun P, et al. Very long-term results (more than 20 years) of valve repair with carpentier’s techniques in nonrheumatic mitral valve insufficiency. Circulation. 2001;104:I8–11.
28 Modi P, Rodriguez E, Hargrove WC III, Hassan A, Szeto WY, Chitwood WR Jr. Minimally invasive video-assisted mitral valve surgery: A 12-year, 2-center experience in 1178 patients. J Thorac Cardiovasc Surg. 2009;137:1481–7.
29 Perier P, Hohenberger W, Lakew F, Batz G, Urbanski P, Zacher M, et al. Toward a new paradigm for the reconstruction of posterior leaflet prolapse: midterm results of the “respect rather than resect” approach. ATS. 2008; 86:718–25.
30 Oppell von UO, Mohr FW. Chordal replacement for both minimally invasive and conventional mitral valve surgery using premeasured Gore-Tex loops. ATS. 2000;70:2166–8.
31 Falk V, Seeburger J, Czesla M, Borger MA, Willige J, Kuntze T, et al. How does the use of polytetrafluoroethylene neochordae for posterior mitral valve prolapse (loop technique) compare with leaflet resection? A prospective randomized trial. J Thorac Cardiovasc Surg. 2008;136:1205–discussion 1205–6.
32 Rausch MK, Bothe W, Kvitting J-PE, Swanson JC, Miller DC, Kuhl E. Mitral valve annuloplasty: a quantitative clinical and mechanical comparison of different annuloplasty devices. Ann Biomed Eng. 2012;40:750–61.
33 Bolling SF, Pagani FD, Deeb GM, Bach DS. Intermediate-term outcome of mitral reconstruction in cardiomyopathy. J Thorac Cardiovasc Surg. 1998;115:381–6– discussion 387–8.
34 Maisano F, Caldarola A, Blasio A, De Bonis M, La Canna G, Alfieri O. Midterm results of edge-to-edge mitral valve repair without annuloplasty. J Thorac Cardiovasc Surgery. 2003;126:1987–97.
35 Langer F, Schäfers H-J. RING plus STRING: papillary muscle repositioning as an adjunctive repair technique for ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2007;133:247–9.
36 Borger MA, Murphy PM, Alam A, Fazel S, Maganti M, Armstrong S, et al. Initial results of the chordal-cutting operation for ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2007;133:1483–92.
37 Maisano F, Torracca L, Oppizzi M, Stefano PL, D’Addario G, La Canna G, et al. The edge-to-edge technique: a simplified method to correct mitral insufficiency. Eur J Cardiothorac Surg. 1998;13:240–5; discussion 245–6.
38 Dreyfus GD, Bahrami T, Alayle N, Mihealainu S, Dubois C, De Lentdecker P. Repair of anterior leaflet prolapse by papillary muscle repositioning: a new surgical option. ATS. 2001;71:1464–70.
39 Rankin JS, Sharma MK, Teague SM, McLaughlin VW, Johnston TS, McRae AT. A new approach to mitral valve repair for rheumatic disease: preliminary study. J Heart Valve Dis. 2008;17:614–9.
40 Chu MWA, Gersch KA, Rodriguez E, Nifong LW, Chitwood WR. Robotic “haircut” mitral valve repair: posterior leaflet-plasty. Ann Thorac Surg. 2008; 85:1460–2.
41 Bridgewater B, Hooper T, Munsch C, Hunter S, Oppell von U, Livesey S, et al. Mitral repair best practice: proposed standards. Heart. 2006;92:939–44.
42 Vassileva CM, Boley T, Markwell S, Hazelrigg S. Impact of hospital annual mitral procedural volume on mitral valve repair rates and mortality. J Heart Valve Dis. 2012;21:41–7.
43 Maisano F, Vanermen H, Seeburger J, Mack M, Falk V, Denti P, et al. Direct access transcatheter mitral annuloplasty with a sutureless and adjustable device: preclinical experience. Eur J Cardiothorac Surg. 2012;42:524–9
44 Seeburger J, Leontjev S, Neumuth M, Noack T, Höbartner M, Misfeld M, et al. Trans-apical beating-heart implantation of neo-chordae to mitral valve leaflets: results of an acute animal study. Eur J Cardiothorac Surg. 2012;41:173–6; discussion 176
45 Holzhey DM, Shi W, Borger MA, Seeburger J, Garbade J, Pfannmüller B, et al. Minimally invasive versus sternotomy approach for mitral valve surgery in patients greater than 70 years old: a propensity-matched comparison. Ann Thorac Surg. 2011;91:401–5.
46 Gummert JF, Rahmel A, Bucerius J, Onnasch J, Doll N, Walther T, et al. Mitral valve repair in patients with end stage cardiomyopathy: who benefits? Eur J Cardiothorac Surg. 2003;23:1017–22– discussion 1022.
47 Bax JJ, Braun J, Somer ST, Klautz R, Holman ER, Versteegh MIM, et al. Restrictive annuloplasty and coronary revascularization in ischemic mitral regurgitation results in reverse left ventricular remodelling. Circulation. 2004;110:II103–8.
48 Kwon MH, Lee LS, Cevasco M, Couper GS, Shekar PS, Cohn LH, et al. Recurrence of mitral regurgitation after partial versus complete mitral valve ring annuloplasty for functional mitral regurgitation. J Thorac Cardiovasc Surg. Published online first: 23 August 2012. doi:10.1016/j.jtcvs.2012.07.049
49 Lange R, Guenther T, Noebauer C, Kiefer B, Eichinger W, Voss B, et al. Chordal replacement versus quadrangular resection for repair of isolated posterior mitral leaflet prolapse. Ann Thorac Surg. 2010;89:1163–70 – discussion 1170.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.