Carbon nanotubes: an insight into the mechanisms of their potential genotoxicity
DOI: https://doi.org/10.4414/smw.2012.13698
Damien
van Berlo, Martin J D
Clift, Catrin
Albrecht, Roel P F
Schins
Summary
After the health catastrophe resulting from the widespread use of asbestos which was once hailed as a new miracle material, the increasing use of carbon nanotubes (CNTs) has spawned major concern due to their similarities in terms of size, shape and poor solubility. Assessment of genotoxicity has shown that CNTs can damage DNAin vitro and in vivo. The genotoxic potential of different CNT samples varies considerably, however, with negative findings reported in a number of studies, probably due to the enormous heterogeneity of CNTs. The observed spectrum of genotoxic effects shows similarities with those reported for asbestos fibres. Mutagenicity has been found in vivobut in bacterial assays both asbestos and CNTs have mostly tested negative. An overview of key experimental observations on CNT-induced genotoxicity is presented in the first half of this review.
In the second part, the potential mechanisms of CNT-elicited genotoxicity are discussed. Whereas CNTs possess intrinsic ROS-scavenging properties they are capable of generating intracellular ROS upon interaction with cellular components, and can cause antioxidant depletion. These effects have been attributed to their Fenton-reactive metals content. In addition, CNTs can impair the functionality of the mitotic apparatus. A noteworthy feature is that frustrated phagocytosis, which is involved in asbestos-induced pathology, has been observed for specific CNTs as well. The involvement of other mechanisms generally implicated in particle toxicity, such as phagocyte activation and impairment of DNA repair, is largely unknown at present and needs further investigation.
List of abbreviations:
Aprt Adenine phosphoribosyltransferase
BER Base excision repair
CNTs Carbon nanotubes
DSB Double strand breaks
fpg Formamidopyrimidine glycosylase
hpgrt Hypoxanthine-guanine phosphoribosyltransferase
iNOS Inducible nitric oxide synthase
MDA Malondialdehyde
MW-CNTs Multi-walled carbon nanotubes
NER Nucleotide excision repair
OGG1 8-oxoguanine-DNA glycosylase
8-OHdG 8-hydroxydeoxyguanosine
PARP Poly(ADP-ribose) polymerase
PL-PEG Phospholipids with a polyethylene glycol moiety
RNS Reactive nitrogen species
ROS Reactive oxygen species
SOD Superoxide dismutase
SSB Single strand breaks
SW-CNTs Single-walled carbon nanotubes
TBARS Thiobarbituric acid reactive substances
TUNEL Terminal deoxynucleotidyl transferase dUTP nick end labeling
Introduction
Carbon nanotubes (CNTs)
In recent decades, considerable interest has focused on the development of novel nanomaterials that can be useful for a plethora of medical, industrial and consumer applications. Due to their physico-chemical, electrical, mechanical and thermal properties, carbon nanotubes (CNTs) can enhance a variety of consumer or industrial products, ranging from use in electronics or protective clothing to biomedical applications such as drug delivery or genetic modification of crops. CNTs feature a very large surface per weight ratio, which is useful in catalytic processes, a tensile strength that is many times higher than that of steel and excellent conduction of heat and electricity. They also possess good thermal stability and are resistant to many chemicals. However, due to their very small diameter inhalation of such materials is possible and they are thus capable of reaching the most distal areas of the pulmonary system, i.e. the alveolar sacs [1].
CNTs possess a robust graphene structure which is biopersistent. They are produced in several forms, the main distinction being the number of carbon atom layers; CNTs with one layer of carbon atoms are referred to as single-walled CNTs (SW-CNTs), while those with multiple layers are denominated multi-walled CNTs (MW-CNTs). Both of these forms can become aerosolised in occupational settings [2–5]. The diameter of SW-CNTs is generally 0.4 to 3 nm [6], but they readily form bundles held together by van der Waals forces called nano-ropes, which are commonly 20–30 nm in diameter [7] up to 500 nm [6]. MW-CNTs have a diameter that is more variable, ranging from roughly 5–100 nm, while MW-CNT nano-ropes can be 3μm in diameter [6]. To further improve their properties, MW-CNTs can be functionalised, which involves the addition of molecules to their carbon-based structure. CNTs can be as long as 18 cm [8], providing an incredible aspect ratio (i.e. the ratio of length to width). Clearly, nanotubes represent a highly heterogeneous hazard, with marked differences in size, rigidity, single- vs. multi-walled structures and possible functionalisation of their surface.
Due to their small size (providing a substantial atom-to-surface ratio), low biodegradability and fibrous structure (defined as an aspect ratio of at least 3:1), the important question arises whether the known severe health issues associated with exposure to another durable fibrous material (i.e. asbestos) might be relevant for CNT exposure.
Asbestos is a natural fibrous material that was once hailed as a new miracle material that was durable, easy to process and possessing remarkable flame-repellent properties. However, when these fibres become fractured, resulting in dimensions sufficiently small to be inhalable, they can cause severe pulmonary pathology, characterised by chronic inflammation, fibrosis and cancer – specifically mesothelioma.
Carcinogenesis is a multi-step process involving initial insult to the genome, compromised cellular defence mechanisms and proliferation. A first hint for the carcinogenic potential of CNTs was published in 2008. In heterozygous p53+/- mice, featuring reduced function of the crucial tumour suppressor protein p53, intraperitoneal injection of CNTs was reported to elicit mesothelioma formation [9]. However, the results from this study have been disputed since the investigators administered a very large dose (3 mg per mouse) of CNT aggregates that were several hundreds of μm in diameter. Fibres of these dimensions are not capable of reaching the alveoli upon inhalation and thus will not be taken up by macrophages, which is known to be of critical importance for asbestos-induced pathogenesis [10]. Moreover, the model used has not been validated to demonstrate applicability for fibre-induced mesothelioma [10]. In a follow-up to this study the authors addressed the first point of criticism related to dosimetry. Dose-dependent induction of mesothelioma was observed in the same p53+/- mouse strain upon intraperitoneal injection of MW-CNT at much lower doses of 3, 30 and 300 μg per mouse. At the two highest doses, most mice (17 and 19 out of 20) developed mesothelioma within a year [11]. In rats, with an in vivo model widely accepted for the evaluation of fibre pathogenesis and mesothelioma formation, increased development of mesothelioma has been observed after intraperitoneal injection of 1 or 10 mg MW-CNT. Effects were stronger for thin and rigid MW-CNTs compared to samples with a larger diameter [12]. However, another study involving intraperitoneal injection in rats has reported contrasting results. The effects of MW-CNT injection were evaluated after 2 years and compared to the positive control crocidolite asbestos. Even at a dose tenfold higher than that used for the asbestos sample, enhanced occurrence of mesothelioma was only found for asbestos [13]. The authors discuss the question whether this lack of effect might be due to the relatively short length of the CNTs used (<1 μm on average) and the absence of an inflammatory response, as well as the antioxidant capacity of the CNT sample. Due to contrasting observations on CNT carcinogenesis, this issue is at present unresolved.
Genotoxicity and mutations
Genotoxicity can be defined as damage to the genetic material encrypted within our DNA. When DNA integrity is compromised by a damaging agent, a number of scenarios are possible. These depend upon the type of damage, its extent and persistence [14]. Well-described forms of DNA damage include strand breakage, oxidative DNA damage and formation of bulky adducts. There are many agents with DNA-damaging potential. These include reactive oxygen and nitrogen species (ROS and RNS), aromatic compounds (e.g. benzo(a)pyrene), and also some types of particles and fibres (e.g. quartz, diesel engine exhaust particles, asbestos) (e.g. [15]). For particles (including fibres) specifically, genotoxicity has multiple mechanistic components. Primary genotoxicity is induced in the absence of an inflammatory response, while secondary genotoxicity is driven by the activation of inflammatory cells such as neutrophils and macrophages which can generate substantial amounts of reactive species in the so-called oxidative burst [15]. In addition, genotoxicity of particles can be direct and indirect. Direct genotoxicity can result from physical interactions of the particulate material with the genomic DNA. Indirect genotoxicity can result from increased ROS generation upon interaction with other cellular components (e.g. mitochondria, cell membrane) or can arise when particles elicit depletion of intracellular antioxidants. In this scenario ROS that are generated during physiological cellular processes (e.g. cellular respiration) can accumulate and damage the DNA.
Eukaryotic cells possess extensive defensive mechanisms to avert and counter damage to the genome. For instance, their antioxidant array repels the adverse action of penetrating or intracellular generated oxidants. Important antioxidants include glutathione, thioredoxin and superoxide dismutase (SOD). DNA repair enzymes restore damaged DNA to its original form, mainly via base excision repair (BER) and nucleotide excision repair (NER), while mismatch repair and direct repair via DNA alkytransferases are minor mechanisms. When the protective arsenal fails, a cell can undergo controlled cell death to prevent the fixation of mutations, which contributes to carcinogenesis. Alternatively, in seriously damaged cells necrotic cell death can occur, which is an uncontrolled process. When these mechanisms fail, fixation of the DNA mutation is a fact and the alteration is replicated upon cellular mitosis.
Various types of DNA mutations are known. Mutations of key loci within the genetic code are the molecular hallmark of cancer. These key genes can be divided into proto-oncogenes that stimulate cellular growth and proliferation (e.g. K-ras), and tumour suppressor genes that inhibit proliferation or are involved in DNA repair (e.g. p53). In addition to their dreaded role in carcinogenesis, DNA mutations can cause various pathologies and can modulate susceptibility to disease. Mutations can involve relatively small sequences, like single genes, or can occur on a larger scale. Examples of small-scale mutations are point mutations (i.e. transitions or transversions), in which one nucleotide is replaced by another; insertions, which add nucleotides to the genetic code; and deletions, which remove nucleotides from DNA.
Mutations can also affect chromosomal structures. Examples of clastogenic effects, affecting part(s) of a chromosome, are: amplifications, which augment the number of copies of chromosomal regions; chrosomal region deletions, which leads to the opposite and thus loss of information; chromosomal translocations, whereby sections of non-homologous chromosomes are interchanged; interstitial deletions, in which parts of a chromosome are removed, potentially creating fusion proteins from two previously unconnected sequences; chromosomal inversions, involving the rearrangement of segments within the same chromosome; and loss of heterozygosity, the loss of one allele of a gene for which the other allele was previously rendered inactive.
When chromosome segregation between daughter cells is disturbed during their mitosis, aneugenic effects can occur, resulting in alteration of the chromosome number. Aneuploidy can result from weakening or deactivation of mitotic checkpoints, attachment of the kinetochore (which bind chromosome centromeres to the microtubuli of the mitotic spindle to allow for segregation) to both mitotic spindles and formation of additional spindles or the loss of one.
Aim and contents
Although the (nano-) toxicological community has directed much attention towards possible adverse effects of CNTs, many questions are still unanswered due to the short time span available for toxicological assessment of this recently emerging potential hazard. The present review sets out to discuss whether CNTs may elicit genotoxicity, with a special focus on the underlying mechanisms. Assessment of genotoxicity is considered a useful tool for understanding of the potential carcinogenic risk of particles [16].
In section II, findings on CNT-induced genotoxicity in experimental in vivo and in vitro systems are presented. Available data are summarised in tables 1 and 2 for SW-CNTs and MW-CNTs respectively. A discussion on the similarity to the known genotoxic and carcinogenic hazard asbestos and an overview of studies comparatively evaluating the genotoxicity of CNTs and asbestos will be found in section III. Subsequently, in section IV, hypothetical mechanisms of CNT-associated genotoxicity are discussed. The proposed mechanisms are visualised in figure 1.
|
Table 1:SW-CNT-induced genotoxic effects. |
|
Endpoint
|
Cells
|
Reference
|
| DNA strand breakage (comet assay) |
Normal and malignant human mesothelial cells
Primary mouse embryo fibroblast cells
BEAS-2B human bronchial epithelial cells
RAW264.7 mouse macrophages
V79 Chinese hamster lung fibroblasts
Leukocytes from Swiss-Webster mice |
Pacurari et al., 2008 [20]
Yang et al., 2009 [29]
Lindberg et al., 2009* [21]
Di Giorgio et al., 2011 [43]
Kisin et al., 2011 [30]
Patlolla et al., 2010b [34] |
| DNA base oxidation |
FE1 MutaTM Mouse lung epithelial cells
RAW264.7 mouse macrophages |
Jacobsen et al., 2008 [38]
Migliore et al., 2010 [39] |
| Micronuclei |
RAW264.7 mouse macrophages
HDMEC normal human dermal fibroblasts
BEAS-2B human bronchial epithelial cells
BEAS-2B human bronchial epithelial cells
RAW264.7 mouse macrophages
V79 Chinese hamster lung fibroblasts
Bone marrow cells from Swiss-Webster mice |
Di Giorgio et al., 2011 [43]
Cveticanin et al., 2010 [28]
Lindberg et al., 2009 [21]
Manshian et al., 2012 [42]
Migliore et al., 2010 [39]
Kisin et al., 2011 [30]
Patlolla et al., 2010b [34] |
| Chromosomal aberrations |
RAW264.7 mouse macrophages
BEAS-2B human bronchial epithelial cells
Human airway epithelial cells
Bone marrow cells from Swiss-Webster mice |
Di Giorgio et al., 2011 [43]
Sargent et al., 2012 [50]
Sargent et al., 2009; 2012 [49, 50]
Patlolla et al., 2010b [34] |
| Formation of γH2AX foci |
Normal and malignant human mesothelial cells
HDMEC normal human dermal fibroblasts |
Pacurari et al., 2008 [20]
Cveticanin et al., 2010 [28] |
| Mutant frequencies |
BEAS-2B human bronchial epithelial cells
Lung tissue from C57BL/6 mice |
Manshian et al., 2012 [42]
Shvedova et al., 2008 [55] |
| * The SW-CNT sample in this study had a purity of about 50%, the remaining fraction contained other nanotubes. |
|
Table 2:MW-CNT-induced genotoxic effects. |
|
Endpoint
|
Cells/tissue
|
Reference
|
| DNA strand breakage (comet assay) |
A549 human lung epithelial cells
A549 human lung epithelial cells
A549 human lung epithelial cells
A549 human lung epithelial cells
RAW264.7 mouse macrophages
NHDF normal human dermal fibroblasts
Primary rat lung epithelial cells
Lung tissue from ICR mice |
Karlsson et al., 2008 [22]
Yamashita et al., 2010 [23]
Ursini et al., 2012 [24]
Cavallo et al., 2012 [25]
Di Giorgio et al., 2011 [43]
Patlolla et al., 2010a [26, 27]
Kim et al., 2012 [36]
Kato et al., 2012 [35] |
| DNA base oxidation |
RAW264.7 mouse macrophages
Lung tissue from ICR mice |
Migliore et al., 2010 [39]
Kato et al., 2012 [35] |
| Micronuclei |
A549 human lung epithelial cells
RAW264.7 mouse macrophages
MCF-7 human lung epithelial cells
Rat type II lung epithelial cells
RLE rat lung epithelial cells
HDMEC normal human dermal fibroblasts
RAW264.7 mouse macrophages |
Kato et al., 2012 [35]
Di Giorgio et al., 2011 [43]
Muller et al., 2008b [47]
Muller et al., 2008b [47]
Muller et al., 2008a [46]
Cveticanin et al., 2010 [28]
Migliore et al., 2010 [39] |
| Chromosomal aberrations |
RAW264.7 mouse macrophages
CHO AA8 Chinese hamster ovary cells |
Di Giorgio et al., 2011 [43]
Kato et al., 2012 [35] |
| Formation of gH2AX foci |
HDMEC normal human dermal fibroblasts
HUVEC human umbilical vein endothelial cells
Mouse embryonic stem cells |
Cveticanin et al., 2010 [28]
Guo et al., 2011 [31]
Zhu et al., 2007 [32] |
| Mutant frequencies |
Mouse embryonic stem cells (Aprt)
Lung tissue from gpt delta transgenic mice |
Zhu et al., 2007 [32]
Kato et al., 2012 [35] |
Investigations on CNT genotoxicity
DNA strand breakage
Breakage of the DNA backbone can occur due to the direct action of reactive species such as hydroxyl radicals (HO•) [17]. However, strand breaks can also be elicited by adduct formation and subsequent DNA repair enzymes (e.g. endonucleases) that transiently cleave the DNA. In addition, DNA strand breakage is one of the features of apoptosis [18].
Both single and double DNA strand breakage can occur. Whereas single strand breaks (SSB) are generally reparable, double strand breaks (DSB) are not. SSB (and alkali labile sites which are converted into SSB) are usually measured by the comet assay in its alkaline variant. Higher levels of SSB increase the ability of DNA to migrate out of nucleoids of lysed cells during electrophoresis. The pH-neutral version of the comet assay detects DSB. This type of DNA strand breakage can also be assessed by measuring H2AX phosphorylation, which results in the formation of γ-H2AX foci that are considered a marker for DSB. Activation of the poly(ADP-ribose) polymerase (PARP) enzyme is considered another indicator of strand breakage because of its role in DNA repair [19].
Effects have been evaluated in several cell types:
Mesothelial cells: importantly, Pacurari et al. explored the effects of SW-CNTs in normal and malignant mesothelial cells, the target cell for mesothelioma development. They found enhanced DNA strand breakage in both cell types, assessed by comet assay and by measurement of histone H2AX phosphorylation and activation of poly(ADP-ribose) polymerase 1 (PARP-1) [20].
Lung epithelial cells: a CNT sample consisting of SW- and MW-CNTs elicited significant, dose-dependent DNA strand breakage measured by the comet assay in BEAS-2B human bronchial epithelial cells. Effects were observed at all concentrations (1–100 μg/cm2) and time points (24h, 48h and 72h) tested. Graphite nanofibres (which are of similar length but are roughly 100–200 times as thick) showed far lower genotoxic potency in the same assay [21]. In A549 lung epithelial type II cells (human carcinoma cell line), MW-CNT treatment elicited enhanced DNA strand breakage determined by the comet assay [22]. Enhanced DNA strand breakage was induced by MW-CNTs in A549 cells [23–25], especially by nanofibres that were relatively thick and long [23]. SW-CNTs elicited smaller effects [23].
Fibroblasts: in normal human dermal fibroblasts, both normal and functionalised MW-CNTs elicited enhanced DNA strand breakage [26, 27]. The occurrence of H2AX foci was induced in human fibroblasts by normal SW-CNTs as well as amide-functionalised SW-CNTs [28]. Comparative evaluation of several particle types (including CNT, carbon black, zinc oxide [ZnO] and silica [SiO2]) in primary mouse embryo fibroblasts, showed that CNTs were the most potent inducers of DNA strand breakage [29]. In V79 lung fibroblasts, SW-CNT treatment elicited enhanced DNA strand breakage [30].
Endothelial cells: treatment of human umbilical vein endothelial cells with MW-CNTs resulted in enhanced formation of γH2AX foci [31].
Mouse embryonic stem cells: enhanced expresson of the double strand break repair protein Rad51 and increased H2AX phosphorylation were found after treatment with MW-CNTs [32].
In vivo: In bronchoalveolar lavage cells obtained from apolipoprotein E deficient mice exposed to SW-CNTs via intratracheal instillation, a statistically significant induction of DNA strand breakage was detected compared to the effect in control mice [33]. In a more recent study, genotoxic effects of functionalised and non-functionalised MW-CNTs were evaluated in murine bone-marrow cells after repeated intraperitoneal injection. Significant induction of DNA strand breakage was observed in leukocytes. Also, the purified functionalised MW-CNTs demonstrated stronger genotoxic potential than non-functionalised MW-CNTs [34].
In lung tissue obtained from mice exposed to a single dose of MW-CNTs (0.05 or 0.2 mg per animal), dose-dependent enhanced DNA strand breakage was measured by comet assay [35]. Significant induction of DNA strand breakage was also observed in primary lung cells isolated from rats which were exposed to aerosolized MW-CNTs (0.94 mg/m3) for 5 days, 6 h per day. Cells were analysed using the comet assay either directly after exposure ended or one month post-exposure [36].
Oxidative DNA damage
The induction of oxidative stress is considered a main drive behind particle exposure-associated genotoxicity (e.g. [37]) The prime example of an oxidative DNA lesion is 8-hydroxydeoxyguanosine (8-OHdG), which can for instance be detected using high performance liquid chromatography, gas chromatography coupled mass spectrometry. A more recent alternative to these highly sensitive methods is the comet assay plus additional treatment with the enzyme formamidopyrimidine glycosylase (fpg), which recognises 8-OHdG and subsequently incises the DNA backbone.
In vitro: SW-CNT treatment induced oxidative DNA damage in the FE1-MutaTMMouse lung epithelial cell line, but no direct strand breakage was detected [38]. In SW-CNT- and MW-CNT-treated RAW 246.7 cells, enhanced oxidation of purines and pyrimidines was observed by endonuclease III- or fpg-modified comet assay. However, whereas purines were found to be oxidised at treatment concentrations as low as 1 μg/ml, pyrimidine oxidation was measured only after treatment with 100 μg/ml SW-CNTs [39]. No oxidative DNA damage, assessed using the fpg-modified comet assay, was detected in A549 cells after treatment with either pristine [24, 25] or OH-functionalised MW-CNT [24].
In vivo: in rats, oral administration of SW-CNTs (0.064 and 0.64 mg/kg b.w.) has been reported to lead to augmented 8-OHdG formation in the lung and liver [40]. Enhanced formation of 8-OHdG and heptanone etheno-deoxyribonucleosides, indicative of oxidative DNA damage, was found in lungs of MW-CNT-exposed ICR mice [35].
Clastogenic and aneugenic effects
Clastogenic effects arise when part(s) of a chromosome is/are deleted, added or rearranged. Commonly, chromosomal damage is assessed using the chromosomal aberration assay or by the micronucleus test, which respectively evaluate structural chromosomal alterations using a microscope and the formation of micronuclei, which can be formed when a part of a chromosome is broken off. Both methods are also used for evaluation of aneugenic effects which result from insults inducing numerical modification of chromosomes. Aneuploidy can be measured using the chromosomal aberration test by the numerical assessment of stained chromosomes. In the micronucleus test it can be identified by fluorescent in situ hybridisation with probes targeting the chromosomal centromeres. The latter method allows discrimination of micronuclei containing a complete chromosome (i.e. containing a centromere) or merely chromosomal fragments (i.e. without centromere).
In a recent study, induction of genotoxicity by MW-CNTs and chrysotile asbestos was comparatively evaluated using Chinese hamster lung cells. Both samples were internalised upon treatment. Similar genotoxic effects and potential were found; both samples elicited enhanced numbers of bi- and multi-nucleated cells and aneuploidy. However, only the asbestos fibres induced marginal micronucleus formation [41]. In BEAS-2B cells a mixture of SW-CNTs and MW-CNTs induced MN formation at concentrations of 10 μg/cm2 and over, with 100 μg/cm2 representing the highest concentration tested [21]. Another study reported a significant dose- and time-dependent induction of micronuclei in BEAS-2B cells for three SW-CNT samples with differing aspect ratios, in the absence of cytotoxicity [42]. A significant induction of micronuclei by MW-CNTs was observed in A549 cells, while enhanced sister chromatid exchanges were found in CHO AA8 cells [35]. Enhanced micronucleus formation was also observed in RAW 246.7 murine macrophages treated with SW-CNTs and MW-CNTs, even at low doses (0.1 and 1 μg/ml) [39]. In RAW 264.7 murine macrophages, enhanced chromosomal aberrations were detected upon treatment with SW-CNTs and MW-CNTs [43]. Using a human fibroblast cell line as well as human lymphocytes, enhanced micronucleus formation was observed after treatment with MW-CNTs, SW-CNTs and amide-functionalised SW-CNTs [28]. In another study, enhanced induction of micronucleus formation was also observed in lung fibroblasts after SW-CNT treatment [30]. However, baytubes (agglomerates of MW-CNTs) did not elicit chromosomal aberrations at concentrations of 2.5–10 μg/ml in the same V79 cell line, in presence or absence of S9 mix [44]. No chromosomal damage, measured by the sister chromatid exchange and micronucleus tests, was found in human lymphocytes [45].
Exposing MW-CNTs to high temperatures (600 or 2400 °C) to modify their structure (annealing of defects) and metal content, was shown to reduce their ability to elicit micronucleus formation in rat lung epithelial cells. However, grinding of heated samples restores their genotoxic potential, which suggests that this potential is driven by their structural defects [46].
In vivo, a significant, dose-dependent increase of micronucleus formation was observed in lung epithelial cells obtained from rats after 3 days of intratracheal instillation of 0.5 or 2 mg MW-CNTs [47]. After intraperitoneal injection, enhanced induction of structural chromosomal aberrations and micronucleus formation was detected in leukocytes obtained from Swiss-Webster mice. Stronger effects were found for functionalised MW-CNTs [34].
The ability of CNT to trigger aneugenic effects has been explored in several studies: both centromere-positive and -negative micronuclei were induced in lung epithelial cells treated with MW-CNTs, indicating both clastogenic and aneugenic effects [47]. These observations have resulted in the hypothesis that CNTs are capable of disturbing mitotic spindle formation [48]. Similar findings were obtained using RAW 264.7 murine macrophages by both SW-CNTs and MW-CNTs; enhanced breakage, decondensation and numerical alteration of chromosomes were reported [43]. Sargent et al. found numerical chromosome changes, fragmented centrosomes, multiple mitotic spindle poles and anaphase bridges in SW-CNT-treated human airway epithelial cells [49]. Moreover, they showed the presence of SW-CNTs within the cell nuclei, interacting with the centrosomes and mitotic spindle using confocal microscopy [49]. To investigate potential effects in an exposure scenario estimated to be realistic for CNTs, BEAS-2B and primary human lung epithelial cells were treated with low concentrations of SW-CNT equivalent to a 20-week inhalation exposure at the OSHA (Occupational Safety and Health Administration) Permissible Exposure Limit of 5 mg/m3. Doses of 0.024 μg/cm2 and over (24–72 h treatment) elicited chromosome fragmentation, mitotic spindle disruption and aneuploidy. At the lower treatment concentrations these findings were in the absence of cytotoxicity [50]. The potential of CNTs to associate with the cellular mitotic machinery is hypothesised to depend on their similarity to cellular microtubuli; they are robust and possess similar dimensions, especially when they form nano-ropes [7].
Gene mutations
Mutations can be detected using bacterial systems (e.g., Salmonella typhimurium in the Ames test), in cell lines or in tissues from animals. In particular, a number of genetically modified in vitro and in vivo models have been developed to facilitate detections of mutations (e.g., Big Blue® rats or FE1-MutaTMMouse cells).
Bacterial assays: In Ames tests using five different bacterial strains, no mutagenicity was found in the presence or absence of metabolic activators after treatment with two MW-CNT samples [51] or with SW-CNT [52]. In another study, two MW-CNT samples with high (diameter 10–15 nm, length 10 μm) and low aspect ratio (diameter 10–15 nm, length 150 nm) did not induce mutagenicity in the Ames test [53]. Similarly, using three different bacterial strains, MW-CNTs did not show mutagenicity, either using the CNT sample alone or with the additional presence of the metabolic activator S9 [54]. At concentrations up to 5,000 μg/plate, no mutagenicity was detected in the Ames test following treatment with baytubes either with or without S9 mix [44].
In vitro(mammalian cells): Treatment of mouse embryonic stem cells with MW-CNTs has been reported to result in a 2-fold induction of the adenine phosphoribosyltransferase (aprt) mutation frequency compared to non-treated cells [32]. Hprt mutations were observed in SW-CNT-treated BEAS-2B cells, at doses of 25 ug/ml or higher and only for the sample with medium aspect ratio (length of 1–3 μm) [42]. In contrast, in Chinese hamster lung cells, no hypoxanthine-guanine phosphoribosyltransferase (hpgrt) mutagenicity was detected after MW-CNT treatment [41]. Nor was enhancement of chromosome aberrations detected in Chinese hamster ovary cells (CHO-k1) treated with two MW-CNT samples featuring a high and low aspect ratio [53]. After long-term treatment (24 days) of FE1-MutaTMMouse cells with SW-CNTs, no increased mutant frequency was measured in the CLL gene, contrasting with earlier observations using carbon black and diesel engine exhaust particles [38].
In vivo: Also, a 4-day (5 h/day) inhalation exposure to 5mg/m3 SW-CNTs resulted in enhanced pulmonary mutation of the proto-oncogene K-ras in C57BL/6 mice [55]. No induction of micronucleus formation compared to control animals was detected in bone marrow cells from ICR mice exposed to MW-CNT (two samples, high and low aspect ratio) by intraperitoneal injection [53]. In the lungs of MW-CNT-exposed gpt delta transgenic mice, enhanced gpt mutation frequencies were found [35]. After oral exposure of rats to SW-CNTs or MW-CNTs, no enhanced urinary mutagenicity was detected using the Ames test [45].
Comparison to asbestos
When attempting to perform risk assessment for the potential genotoxic hazard posed by CNTs, available data on asbestos, a known genotoxic, mutagenic and carcinogenic fibre, might prove to be particularly useful. Exposure to asbestos fibers can lead to the development of malignant tumours of the lung and the mesothelium of the pleura, peritoneum and pericardium (mesothelioma). Properties involved in their bioactivity and toxicity are their dimensions, aspect ratio, surface reactivity, crystallinity, chemical composition and transition metal content [6].
Six different silicates are classified as asbestos: the serpentine mineral chrysotile and the amphibole minerals actinolite, amosite, anthophyllite, crocidolite and tremolite. All of them are characterised by a fibrous structure, and all are known carcinogens for humans. The serpentine fibre chrysotile has the smallest diameter, ranging from 25 to 100 nm, while the other (amphibole) fibres possess a diameter that ranges from 100 to 200 nm [56]. The existing fibre paradigm considering the pathogenic potential of high aspect ratio particles, such as asbestos, has identified thin, long and biopersistent as the most toxic. It has been suggested that this paradigm is applicable to carbon nanotubes as well (e.g. [57]): asbestos shares a number of properties with CNTs; their dimensions and shape are comparable, especially considering the fact that CNTs tend to form nanorope bundles, increasing their diameter to values similar to asbestos. Also, like asbestos, CNTs are considered to be biopersistent. Lower clearance from and thus higher retention in the pleura has been shown for long CNTs compared to shorter fibres, in mice instilled with CNTs directly into the pleura [58]. CNTs are highly durable and poorly soluble [59], and have been observed to frustrate macrophage clearance [60].
The genotoxic potency of asbestos is well known, although the mechanisms involved are still incompletely understood. In asbestos-exposed workers significantly increased oxidised pyrimidines and chromosomal aberrations were detected compared with non-exposed controls [61]. Augmented formation of 8-nitroguanine, a mutagenic DNA lesion associated with chronic inflammation, has been measured in asbestos-exposed mice [62]. In rats, induction of DNA strand breaks, mutations and 8-OHdG has been observed [63, 64]. Enhanced mutation frequencies have also been reported in transgenic mice [65].
In vitro a range of genotoxic effects have been observed, including oxidative DNA damage [66, 67], DNA strand breakage [68–73], clastogenic effects [74–76], aneugenic effects [76, 77] and mutations [78, 79].
As has been observed in several experiments involving assessment of CNT mutagenicity in bacterial systems (summarised earlier), many negative results have been reported for asbestos using these systems (e.g. [64, 67, 78, 80]). This is in stark contrast to observations described following experimentation with other tests. Vital differences between the cell membrane of mammalian cells and bacteria, resulting in different uptake mechanisms, have been suggested as crucially limiting the applicability of bacterial assays for toxicological assessment of nanomaterials and nanoparticles (e.g., [80, 81]). In particular the use of bacteria-based tests for nanoparticle-associated mutagenicity is disputed and further study is necessary.
Some genotoxicity studies have included both asbestos and CNTs for comparative evaluation. In Chinese hamster lung (CHL/IU) cells treated with CNTs or asbestos, very similar findings on genotoxicity were reported. Both types of fibre elicited enhanced formation of polyploidy and an increased number of multi-nucleated cells while structural chromosome aberrations and hprt mutagenicity were not induced. The one exception to these highly similar responses was the slight induction of micronuclei that was observed only in asbestos-treated cells [41]. In V79 Chinese hamster lung fibroblasts, both crocidolite asbestos and SW-CNTs elicited DNA strand breakage and micronucleus induction [30].
Taken together, the observed similarity between asbestos and CNTs in terms of physicochemical properties and their responses in in vitro genotoxicity tests, warrants mechanistic investigation into CNT-elicited genotoxicity. The following section will provide an overview and discussion of current knowledge on potentially involved mechanisms, including a comparison with asbestos.
Potential mechanisms of CNT-associated genotoxicity
Intrinsic ROS generation
Generation of ROS has been considered as a crucial driving mechanism of toxic effects attributed to particles and fibres including asbestos (e.g. [15, 16, 82, 83]). Many types of particle possess intrinsic ROS-generating potential, which has been linked to a number of characteristics such as transition metal content (e.g., [82]), surface area (e.g. [84]), carbon-centered radicals (e.g. [85]) and the presence of polycyclic aromatic hydrocarbons and/or quinones (e.g. [86]). Mechanisms of intrinsic particulate ROS generation include Fenton chemistry (linked to the presence of transition metals) [87] and quinone cycling of organics [88]. ROS are generally extremely short-lived, but some species (such as hydrogen peroxide [H2O2] and superoxide [O2•-]) have been found to be capable of negotiating cellular membranes [89] and after extracellular production can induce effects within the cell. The most reactive of all is the hydroxyl radical (•OH), that almost instantly reacts with the first molecule it encounters after it is generated. Stable species such as H2O2 can enter cells, potentially reaching the genomic material within the nucleus, where they might form •OH radicals via Fenton chemical processes.
CNT properties potentially involved in their ROS-generating potency include metals, which can be present as contamination/impurities or deliberately added as catalysts, as an example of CNT functionalisation. A broad range of metal impurities, both qualitative and quantitative, have been measured in CNT samples [90].
In addition, they possess a large surface area per unit of mass and might contain carbon-centred radicals due to their graphene structure, which can be introduced via functionalisation to improve reactivity. However, findings on ROS generation show that in acellular environments both MW-CNTs [91, 92] and SW-CNTs [93] exert quenching activity. In fact they have been proposed as novel antioxidants [94]. Interestingly, the toxic potential of CNTs has been linked to the presence of structural defects, which are reported to be a possible driving force of their scavenging activity [95]. Thus, intrinsic ROS generating properties could be inversely related to genotoxic effects, a concept that is presently not understood.
Although there are clear similarities between asbestos fibres and CNTs, considering their dimensions, biopersistence and aspect ratio, there appears to be a marked difference considering their ROS-generating potential. Asbestos fibres have been reported to induce ROS generation (e.g., [64]) and intrinsic ROS-generating potential is considered to be implicated in their genotoxicity [83].
ROS generation on cellular activation
The interaction of CNTs with cellular components or constituents, such as cell membranes or mitochondria, can result in the formation of radicals, as has been described for other particles. For instance, the interaction with the bi-lipid membrane can give rise to lipid peroxidation products, which are known to be capable of eliciting intracellular oxidative stress.
MW-CNTs have shown very high ROS-generating potential in Jurkat lymphocytic cells and A549 lung epithelial cells [96]. In MH-S murine macrophages enhanced ROS formation measured by DCF-DA fluorescence was only induced by the MW-CNT sample with the smaller diameter of two samples tested (diameter 9.4 and 70 nm, length <5 μm for both) [92]. Another study adopted a more extensive approach in characterising ROS formed by CNTs in vitro, employing mesothelial cells. In suspensions of these cells treated with SW-CNTs, enhanced formation of •OH radicals was measured using electron paramagnetic resonance which was inhibited by addition of the antioxidant catalase or the metal chelator deferoxamine. In addition, the authors showed that the formation of O2•- and H2O2, measured by specific dyes, was increased in CNT-treated cells; these effects could be abrogated by pre-treatment with superoxide dismutase (SOD) or catalase [20]. The effectiveness of deferoxamine in reducing •OH formation shows the involvement of transition metals. Moreover, the induction of DNA strand breaks by SW-CNT treatment was diminished by co-incubation with catalase, SOD or deferoxamine, causally linking DNA damage to ROS formation. Guo et al. confirmed these findings in HUVEC cells; MW-CNT treatment elicited enhanced ROS generation, measured by DCFH-DA assay [31]. When cells were previously treated with the glutathione precursor N-acetyl cysteine, ROS generation as well as the formation of gamma-H2AX foci was markedly reduced, showing the involvement of ROS in induction of DSB by MW-CNTs [31]. The ability of SW-CNTs to elicit intracellular ROS formation has been attributed in another study to iron traces, since the acid-treated sample did not elicit ROS formation in contrast to the untreated sample [97].
A similar mode of action has been described for asbestos; ROS formation is considered to be heavily involved in its ability to damage DNA, while the presence of iron is known to be involved in its ability to generate ROS as well [98, 99].
a. Interaction with mitochondria:the importance of mitochondrial interactions in particle-induced adverse effects has been shown for ultrafine environmental particulate matter and asbestos [86, 100]. Also, inhibition of the mitochondrial respiratory chain abolished quartz-induced oxidative DNA damage in epithelial cells, suggesting that oxidative DNA damage in quartz-exposed cells results from mitochondria-derived ROS [101]. A likely candidate for this is the relatively stable oxidant H2O2, formed by spontaneous or enzyme-mediated dismutation of superoxide anions leaking from the respiratory chain. A similar mechanism of genotoxicity may also occur for asbestos fibres for which oxidative stress responses were found to depend on the mitochondrial respiratory chain [100, 102].
Several findings report mitochondrial localisation, damage and ROS generation in response to CNT exposure. SW-CNTs functionalised with phospholipids with a polyethylene glycol moiety (PL-PEG), a modification used for medicinal applications, were shown to localise predominantly in the mitochondria of tumour and healthy cells [103]. Mitochondrial localisation of SW-CNTs was also reported in a second study; this translocation induced collapse of mitochondrial membrane potential, which leads to exaggerated ROS production [104]. In rat lung epithelial cells, MW-CNT treatment induced collapse of mitochondrial membrane integrity, mitochondrial apoptotic factor and cytochrome C release into the cytosol, while it reduced cellular ATP content [105]. Treatment of the NR8383 and A549 cell lines, representing alveolar macrophages and type II pneumocytes respectively, with SW-CNTs and MW-CNTs resulted in a dose- and time-dependent decrease in mitochondrial membrane potential. In contrast, acid-treated SW-CNTs for which residual metal traces were eliminated showed no effect [97].
Contradicting these observations, similar superoxide formation was observed in the mitochondria of SW-CNT-treated HeLa cells and controls, measured by MitoSOXTMRed [106]. In lung epithelial cells, ROS formation was induced by SW-CNT treatment. Application of the mitochondrial inhibitor rotenone did not modify this effect, showing that other sources were involved [107]. In A549 cells ROS formation induced by MW-CNTs was found to be independent of mitochondrial activity measured by MTT assay [108].
b. Lipid peroxidation:when cellular membranes which consist of a lipid bilayer suffer an oxidative insult, lipid peroxidation can occur. This is a chain reaction in which mutagenic compounds such as malondialdehyde (MDA) and 4-hydroxynenal can be formed [109].
Lipid peroxidation has been observed in vitro and in vivo after CNT exposure. Increased thiobarbituric acid reactive substances (TBARS), which are low-molecular-weight products formed during the decomposition of lipid peroxidation products, were detected in HEK293 kidney cells upon treatment with MW-CNTs [110]. Guo et al. found enhanced MDA formation in MW-CNT-treated HUVEC cells [31]. MW-CNT treatment of A549 human alveolar epithelial cells resulted in enhanced MDA production [108].
MDA levels were significantly increased in rat blood after intraperitoneal injection of DNA functionalised SW-CNTs [111]. After MW-CNT exposure via intratracheal instillation, enhanced MDA levels were measured in the blood [112] and bronchoalveolar lavage fluid [113] of rats. Higher MDA levels were also measured in the blood of mice exposed for 3 months to SW-CNTs intravenously [114].
In summary, SW-CNTs and MW-CNTs can induce the intracellular formation of ROS and this has been linked to their genotoxic potential. Intracellular ROS generation has been attributed in a number of studies to the presence of transition metals in the CNT sample. Offering a further mechanistic explanation for the induction of intracellular ROS, CNTs were shown to be able to translocate to the mitochondria and impact on their function, thereby eliciting exaggerated ROS formation. Once more, their transition metal content was found to be associated with perturbation of mitochondrial function. It should be noted however, that several studies report negative findings concerning CNT-induced effects on the mitochondria, and that this might depend on the specific CNT sample or the cell type used. Another mechanism that might explain intracellular ROS generation is lipid peroxidation, which has been observed in vitro as well as in murine models.
Depletion of antioxidants
Intracellular antioxidant defences can be enzymatic, such as SOD and catalase, and non-enzymatic, like glutathione and thioredoxin. When they are compromised, the potential of subsequently generated free radicals to cause oxidative insult to the genome is increased [115]. A number of findings have described the potential of CNTs to dent the extensive cellular antioxidant armour, described below per cell type/experimental system.
Lung epithelial cells: A dose- and time-dependent depletion of intracellular antioxidants was observed in rat lung epithelial cells treated with MW-CNTs at concentrations of 0.5 up to 10 μg/ml [116]. Catalase and glutathione activity were found to be decreased in MW-CNT-treated A549 human type II epithelial cells [108]. SW-CNT treatment was also found to elicit reduced levels of glutathione as well as SOD-1 and -2 in rat lung epithelial cells [107].
Macrophages:After treatment of RAW 246.7 macrophages with two SW-CNT samples with a high (26 wt.%) and a low (2 wt.%) iron content, a significant reduction in intracellular glutathione was detected for the former sample only. Moreover, catalase partially inhibited the effect of the iron-rich CNTs, confirming the role of iron in the modulation of the intracellular redox state [117]. Another study reported enhanced glutathione depletion in MH-S murine macrophages after treatment with an MW-CNT sample with a small diameter (9.4 nm), while a sample with a diameter of 70 nm tested negative in this assay [92].
Other cell types: Treatment with two MW-CNT samples elicited glutathione depletion in HEK human kidney cells [118]. SW-CNTs caused antioxidant depletion in human epidermal keratinocytes [119]. MW-CNT treatment modified glutathione peroxidase levels and enzyme activity of SOD in HUVEC cells [31]. Treatment of human embryonic kidney cells (HEK293) with MW-CNTs resulted in decreased intracellular glutathione levels [110].
In vivo: In Balb/c mice, seven-day inhalation exposure to SW-CNTs and MW-CNTs elicited significant decreases in the pulmonary levels of glutathione, SOD and catalase [120]. Glutathione depletion was also found in the lungs of SW-CNT-exposed C57Bl/6 mice [121]. In mice receiving a diet deficient in vitamin E, SW-CNT treatment elicited enhanced depletion of pulmonary antioxidants compared to control mice put on the same diet [122]. The induction of antioxidant depletion by CNT exposure may extend to extrapulmonary organs, as was shown by the lower levels of glutathione and SOD, the reduced activity of catalase and the lower total antioxidant response in the serum of rats intratracheally instilled with MW-CNTs [112]. Another clue to potential extrapulmonary effects was delivered by the finding that the antioxidant capacity in rat blood, measured as hydrogen donating capacity, was sharply decreased after intraperitoneal administration of functionalised SW-CNTs [111]. When mice were exposed for 3 months to SW-CNTs administered intravenously, decreasing glutathione concentrations were observed in their blood [114].
Summarising the above, antioxidant depletion has been observed after CNT exposure in several in vitro and in vivo systems. One study showed that the iron content of SW-CNTs, known to be responsible for oxidant generation via Haber-Weiss chemistry, plays a role in this adverse effect. Whether this finding can be extrapolated to other cell systems remains to be established, as does its relevance for the in vivo situation. Noteworthy are the findings reported in two studies concerning antioxidant depletion in blood after exposure via intratracheal instillation and intraperitoneal injection to MW-CNTs and SW-CNTs respectively.
Bystander effects
Bystander effects occur when a response (either positive or negative) to, for instance, a drug or environmental factor is passed on to another cell via the intercellular milieu or gap junctional communication. These effects are well described in relation to radiation exposure, have been observed in response to aging and cancer [123] and have also been discussed in relation to particles. Bhabra et al. [124] found that 29.5 nm chromium-cobalt nanoparticles caused damage to mitochondria in a placental cell line used as a surrogate cellular barrier. This resulted in indirect, ATP-induced DNA damage in the fibroblast cell line grown below the BeWo barrier cells, despite the absence of physical contact with the CoCr nanoparticles [124]. In a more recent study, CoCr nanoparticles induced DSB and chromosomal aberrations (predominantly tetraploidy) in human fibroblasts across the BeWo cellular barrier, while ceramic nanoparticles caused SSB in the same system [125]. Intercellular genotoxicity is also considered as a potential pathogenic mechanism for asbestos [126], but so far no investigations of potential bystander effects of CNTs have been published.
Inhibition of DNA repair
Inhibition of DNA repair has been described as a main mechanism for the carcinogenicity of certain metals (e.g. arsenic, lead and nickel) in humans [127]. It is possible that CNTs affect DNA repair, thus perhaps leading to (facilitation) of genotoxic processes. Activation of p53, higher expression of 8-oxoguanine-DNA glycosylase (OGG1) and Rad 51 were detected in mouse embryonic cells treated with MW-CNTs, indicative of modulation of DNA repair. One of the tumour suppressing functions of p53 is the activation of DNA repair proteins, while OGG1 is involved in the base excision repair of oxidative DNA lesions such as 8-OhdG, and Rad51 represents an essential protein in DNA repair by homologous recombination [32]. In renal epithelial cells, SW-CNT treatment induced the protein expression of p53 and p21 [128]. Similarly, activation of p53 and p21 was observed in response to MW-CNTs in lung epithelial cells [105]. Expression of p53 was also upregulated in the lungs of mice instilled with SW-CNTs up to 28 days post-exposure [129]. SW-CNTs were found to activate cleaved PARP, known to facilitate DNA repair, on its activation by DNA strand breaks, in human mesothelial cells [20]. Protein expression of the DNA mismatch repair protein Msh2 was modified in MW-CNT-treated U937 human monoblastic leukaemia cells [130]. In J774.1 murine macrophages, PARP was not activated by MW-CNTs [131].
Although the implications of the observed mRNA expression, protein expression and/or protein activity changes in these studies for CNT genotoxicity remain to be elucidated, they can be considered as potential markers of DNA damage. A potential adverse effect of CNTs on DNA repair remains to be demonstrated. At present one can only speculate that the expression changes and activation observed in various in vitro models could be due to a feedback loop which is activated in response to disturbed DNA repair.
For asbestos, a recent study has evaluated its effect on PARP1 expression and activity. While elevated PARP1 expression was detected in asbestos-exposed subjects and mesothelioma patients, its activity was relatively low in pleural biopsies and in mesothelioma biopsies. This finding led to the hypothesis that asbestos inhibits PARP1 activity, contributing to mesothelioma development [132].
Activation of inflammatory cells
To eliminate pathogens, professional phagocytes such as neutrophils and macrophages have the capability to release large amounts of toxic reactive species in the respiratory or oxidative burst. Depending on their type and dose, inhaled particles can also induce the phagocytic oxidative burst. For fibres specifically, frustrated phagocytosis can occur when their length is larger than 20 μm and the fibres are rigid, resulting in prolonged ROS generation by phagocytes. This has been shown for asbestos (e.g. [133]) and may also be applicable to CNTs. In addition to a direct DNA-damaging effect, some ROS, such as hypochlorous acid (HOCl) and nitric oxide (NO•), potently inhibit DNA repair [134–136]. HOCl is formed by the conversion of H2O2, catalysed by neutrophilic myeloperoxidase. Pulmonary inflammation (featuring the influx of inflammatory cells such as neutrophils and macrophages in the acute phase) in response to CNT exposure has been observed in rats (e.g. [137]) and mice (e.g. [129]). Currently, however, data are lacking on the potential of CNTs to stimulate the phagocytic oxidative burst, which is the driving factor for secondary genotoxicity. To the best of our knowledge no observations on extracellular ROS generation after CNT treatment have been reported in the literature. Enhanced intracellular ROS generation has been observed in innate immune cells (e.g. [43, 117, 121, 138, 139], but it still needs to be established whether this represents the oxidative burst or the upregulation of cellular signalling, for which small amounts of ROS are needed.
Also, reactive nitrogen species (RNS) could be involved in CNT-induced pathogenic effects. The main source of RNS in the lung is the pulmonary macrophage, of which most reside within the alveoli. For asbestos, the involvement of nitric oxide in its toxicity has been shown despite some conflicting data [140]. Formation of peroxynitrite (ONOO-), a reactive product formed on reaction of O2•- and nitric oxide (NO•), has been reported to be the mechanism of DNA damage caused by some nanoparticles (TiO2 and C60 fullerenes) [141]. Upon pharyngeal aspiration of MW-CNT, expression of the inducible nitric oxide synthase (iNOS) gene was affected in mice at both time points assessed, i.e. 7 days and 56 days post-exposure [142]. However, treatment of rat alveolar macrophage cell line NR8383 with SW-CNTs and two MW-CNT samples did not result in enhanced NO• release [97]. In RAW 246.7 cells treated with SW-CNT, production of NO• was also not induced [121]. The same group subsequently compared effects of an iron-rich and iron-stripped SW-CNT sample in the same cell line and found no increased intracellular production of NO• [117]. A more recent study using peritoneal macrophages treated with SW-CNTs confirmed these findings [143].
Inflammatory effects of CNTs do of course drive secondary genotoxicity and are logically expected to contribute to this mode of action. However, findings on the induction of pro-inflammatory signalling and release of pro-inflammatory factors by CNTs are not within the scope of the current manuscript.
Summing up of conclusions
The hypothesised mechanisms of CNT-induced genotoxicity are shown in figure 1. SW-CNTs and MW-CNTs have displayed genotoxic potential in a number of in vivo and in vitro systems. In vitro, oxidative DNA damage, DNA strand breakage (single and double), clastogenic and aneugenic effects were observed, while positive findings in (bacterial) gene mutation tests were not reported. Peritoneal exposure in rodents was found to induce various markers of genotoxicity in bone marrow cells and leukocytes [34]. A few other studies report pulmonary genotoxicity upon inhalation or instillation exposure [33, 35, 40, 47, 55]. With SW-CNT, in vitrogenotoxicity has been also observed in mesothelial cells [20]. However, interpretation of these data is difficult at present; CNT translocation has been observed in rodents, but it is unclear whether they translocate to the pleura on deposition in the human lung and to what extent [60]. Moreover, relevant human exposure levels cannot at present be accurately predicted. There is thus a pressing need for estimation of the (biologically effective) dose affecting the lung. When this is established, in vitro studies may be designed using CNT concentration ranges that mimic realistically achievable target doses in vivo. Genotoxic effects observed in vitro at very high treatment concentrations may not be applicable to the in vivo situation. An indication of the potential genotoxicity of SW-CNT in a realistic exposure scenario is provided by a recent study in which BEAS-2B and primary human lung epithelial cells were treated by a dose roughly equivalent to 20-week inhalation exposure of a worker. In this study, genotoxic effects were observed at the estimated realistic dose which translated to treatment with 0.024 μg/cm2 SW-CNTs in vitro [50].
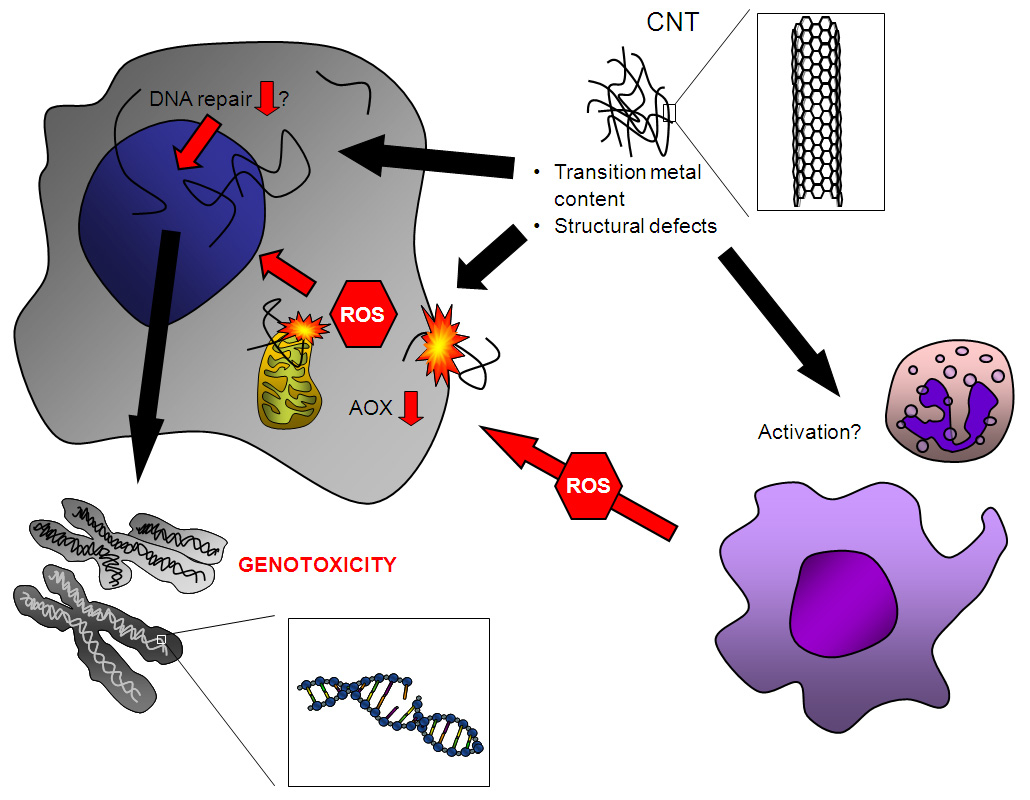
Figure 1
Proposed mechanisms of CNT genotoxicity. The presence of structural defects and transition metals has been implicated in their genotoxic potential. CNTs can induce ROS formation by interaction with cellular components, such as mitochondria and the cell membrane, and are capable of compromising intracellular antioxidant (AOX) defences. Contamination with transition metals may also contribute to oxidative DNA damage induction. In addition, CNT have been reported to disturb the mitotic apparatus, which could explain aneugenic effects as observed in several studies. Whether CNT are capable of inhibiting DNA repair is currently unknown. Secondary genotoxic effects may result from ROS that are generated from recruited phagocytes during CNT-induced inflammation and/or activation of these cells. Further research is needed to unravel the relevance of each of the above postulated mechanisms that should take into account realistic exposure scenarios.
As discussed in this review, there are several similarities to asbestos genotoxicity studies; for many of the endpoints similar findings have been reported for these mineral fibres as well, including their propensity to test negatively in bacterial mutagenicity assays. For particles in general, the relevance of bacterial test methods has been questioned [81]. Of specific interest is the fact that, exactly like asbestos [144, 145], CNTs have been reported to interact with mitotic spindle components, leading to aneuploidy [7, 49, 50]. This has been hypothesised as being due to their similarity to spindle components, i.e. tubulin fibres [7]. The “frustrated phagocytosis” phenomenon, characterised by impaired clearance and exaggerated ROS production, is considered highly important for asbestos-induced pathogenesis [146]. Long and rigid CNTs have been described as mimicking the notorious asbestos fibres in this regard; frustrated phagocytosis was observed for rigid CNT samples longer than 20μm [60].
For both CNTs and asbestos, the presence of transition metals has been shown to be involved in adverse effects. However, unlike the propensity of asbestos fibres to generate ROS intrinsically, CNTs exhibit an antioxidant potential in an acellular environment [91–94]. Moreover, the presence of structural defects, considered a driving force for their scavenging activity, has been linked to their toxicity [95]. However, CNT have been shown to be capable of causing ROS generation within cells, probably by physical interaction with and/or activation of cellular constituents. These findings match observations in asbestos-exposed test systems. Although translocation to mitochondria and disturbance of mitochondrial function have been observed in CNT-treated cells [103–105], inhibition experiments with rotenone have shown that other cellular sources of ROS are also involved [107]. In both in vivo and in vitro experiments, lipid peroxidation has been observed, representing a possible cause of oxidative DNA damage (e.g. [31, 108, 114, 115]). It is also possible that a compromised intracellular antioxidant status, shown in several studies upon CNT exposure (e.g. [92, 108, 118]) contributes to oxidative stress and oxidative DNA damage within CNT-treated cells. In vivo, antioxidant depletion has been observed as well [111, 114, 120–122].
The potential role of further mechanisms shown to be relevant for particle-elicited genotoxicity, such as bystander effects and modulation of DNA repair, remains to be demonstrated. Also, the relevance of secondary genotoxicity arising through phagocyte recruitment and/or activation is largely unknown. Using macrophages, enhanced ROS or RNS production was not measured after CNT treatment [97, 117, 143]. However, neutrophils represent the main source of ROS within the inflamed lung, and experiments using these phagocytes are required to answer questions regarding their contribution.
The hypothesised mechanisms of CNT-induced genotoxicity are shown in figure 1:
It is important to stress that both in vitro and in vivo negative results were obtained for various endpoints. The likely explanation for this is the extreme heterogeneity of CNTs: marked differences exist in length, rigidity, structure (i.e. multi-walled vs. single-walled) and in addition, a plethora of modifications have been implemented to achieve functionalisation (improvement of properties for various applications), with many more under development.
Several factors limit the unifying interpretation of genotoxic studies with CNT. As mentioned before, a great variety of CNT types can be (and are being) generated, possessing markedly different physico-chemical properties. Charting those of their characteristics that are relevant for toxicological hazard and risk assessment should be a major focus of future research. Currently available genotoxicity studies have employed different dispersion protocols; therefore careful characterisation of their physico-chemical properties in treatment medium is a pre-requisite for meaningful research [147]. Moreover, CNTs, like other types of nanomaterials, feature various physico-chemical properties distinct from soluble chemicals, which can interfere with assay reagents or measuring equipment in toxicity assays designed to evaluate toxic properties of chemical compounds [81, 148].
In conclusion, the currently available literature indicates that exposure to carbon nanotubes could represent a genotoxic hazard. However, the inconsistent findings reported among different studies provide clear evidence that this hazard may be highly variable in dependence of their heterogeneous physicochemical properties. However, as the toxic responses to CNT may also depend on the choice of dispersing agents and/or culture medium composition, no solid statement can be made at present regarding the actual hazard. The challenge for future research will be to address genotoxic effects of CNT in controlled experimental settings and selection of an appropriate dose range on the basis of available human exposure data. Such testing should also take into account an extensive characterisation of the CNT under the applied assay conditions, and ideally, aim for the identification of underlying mechanism of actions.
References
1 Gehr P, Muhlfeld C, Rothen-Rutishauser B, Blank F, editors. Particle-lung interactions, second edition. New York: Informa Healthcare USA, Inc.; 2009.
2 Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, Castranova V. Exposure to carbon nanotube material: aerosol release during the handling of unrefined single-walled carbon nanotube material. J Toxicol Environ Health A. 2004;67(1):87–107.
3 Aitken RJ, Chaudhry MQ, Boxall AB, Hull M. Manufacture and use of nanomaterials: current status in the UK and global trends. Occup Med (Lond). 2006;56(5):300–6.
4 Han JH, Lee EJ, Lee JH, So KP, Lee YH, Bae GN, et al. Monitoring multiwalled carbon nanotube exposure in carbon nanotube research facility. Inhal Toxicol. 2008;20(8):741–9.
5 Yeganeh B, Kull CM, Hull MS, Marr LC. Characterization of airborne particles during production of carbonaceous nanomaterials. Environ Sci Technol. 2008;42(12):4600–6.
6 Sanchez VC, Pietruska JR, Miselis NR, Hurt RH, Kane AB. Biopersistence and potential adverse health impacts of fibrous nanomaterials: what have we learned from asbestos? Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(5):511–29.
7 Sargent LM, Reynolds SH, Castranova V. Potential pulmonary effects of engineered carbon nanotubes: in vitro genotoxic effects. Nanotoxicology. 2010;4:396–408.
8 Wang X, Li Q, Xie J, Jin Z, Wang J, Li Y, Jiang K, Fan S. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009;9(9):3137–41.
9 Takagi A, Hirose A, Nishimura T, Fukumori N, Ogata A, Ohashi N, et al. Induction of mesothelioma in p53+/- mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 2008;33(1):105–16.
10 Donaldson K, Stone V, Seaton A, Tran L, Aitken R, Poland C. Re: Induction of mesothelioma in p53+/- mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 2008;33(3):385; author reply 386–8.
11 Takagi A, Hirose A, Futakuchi M, Tsuda H, Kanno J. Dose-dependent mesothelioma induction by intraperitoneal administration of multi-wall carbon nanotubes in p53 heterozygous mice. Cancer Sci. 2012;103(8):1440–4.
12 Nagai H, Okazaki Y, Chew SH, Misawa N, Yamashita Y, Akatsuka S, et al. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci U S A. 2011;108(49):E1330–8.
13 Muller J, Delos M, Panin N, Rabolli V, Huaux F, Lison D. Absence of carcinogenic response to multiwall carbon nanotubes in a 2-year bioassay in the peritoneal cavity of the rat. Toxicol Sci. 2009;110(2):442–8.
14 Schins RP, Hei TK. Genotoxic Effects of Particles. In: Particle toxicology (Eds: Donaldson K, Borm P). Boca Raton, CRC Press/Taylor & Francis Group; 2006:285–98.
15 Schins RP, Knaapen AM. Genotoxicity of poorly soluble particles. Inhal Toxicol. 2007;19(Suppl 1):189–98.
16 Greim H, Borm P, Schins R, Donaldson K, Driscoll K, Hartwig A, et al. Toxicity of fibers and particles. Report of the workshop held in Munich, Germany, 26–27 October 2000. Inhal Toxicol. 2001;13(9):737–54.
17 Eastman A, Barry MA. The origins of DNA breaks: a consequence of DNA damage, DNA repair, or apoptosis? Cancer Invest. 1992;10(3):229–40.
18 Stewart BW. Mechanisms of apoptosis: integration of genetic, biochemical, and cellular indicators. J Natl Cancer Inst. 1994;86(17):1286–96.
19 Althaus FR, Lawrence SD, Sattler GL, Pitot HC. ADP-ribosyltransferase activity in cultured hepatocytes. Interactions with DNA repair. J Biol Chem. 1982;257(10):5528–35.
20 Pacurari M, Yin XJ, Zhao J, Ding M, Leonard SS, Schwegler-Berry D, Ducatman BS, Sbarra D, Hoover MD, Castranova V, Vallyathan V. Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-kappaB, and Akt in normal and malignant human mesothelial cells. Environ Health Perspect. 2008;116(9):1211–7.
21 Lindberg HK, Falck GC, Suhonen S, Vippola M, Vanhala E, Catalán J, et al. Genotoxicity of nanomaterials: DNA damage and micronuclei induced by carbon nanotubes and graphite nanofibres in human bronchial epithelial cells in vitro. Toxicol Lett. 2009;186(3):166–73.
22 Karlsson HL, Cronholm P, Gustafsson J, Möller L. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol. 2008;21(9):1726–32.
23 Yamashita K, Yoshioka Y, Higashisaka K, Morishita Y, Yoshida T, Fujimura M, et al. Carbon nanotubes elicit DNA damage and inflammatory response relative to their size and shape. Inflammation. 2010;33(4):276–80.
24 Ursini CL, Cavallo D, Fresegna AM, Ciervo A, Maiello R, Buresti G, et al. Comparative cyto-genotoxicity assessment of functionalized and pristine multiwalled carbon nanotubes on human lung epithelial cells. Toxicol In Vitro. 2012;26(6):831–40.
25 Cavallo D, Fanizza C, Ursini CL, Casciardi S, Paba E, Ciervo A, et al. Multi-walled carbon nanotubes induce cytotoxicity and genotoxicity in human lung epithelial cells. J Appl Toxicol. 2012;32(6):454–64.
26 Patlolla A, Knighten B, Tchounwou P. Multi-walled carbon nanotubes induce cytotoxicity, genotoxicity and apoptosis in normal human dermal fibroblast cells. Ethn Dis. 2010;20(1 Suppl 1):S1–65–72.
27 Patlolla A, Patlolla B, Tchounwou P. Evaluation of cell viability, DNA damage, and cell death in normal human dermal fibroblast cells induced by functionalized multiwalled carbon nanotube. Mol Cell Biochem. 2010;338(1-2):225–32.
28 Cveticanin J, Joksic G, Leskovac A, Petrovic S, Sobot AV, Neskovic O. Using carbon nanotubes to induce micronuclei and double strand breaks of the DNA in human cells. Nanotechnology. 2010;21(1):015102.
29 Yang H, Liu C, Yang D, Zhang H, Xi Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol. 2009;29(1):69–78.
30 Kisin ER, Murray AR, Sargent L, Lowry D, Chirila M, Siegrist KJ, et al. Genotoxicity of carbon nanofibers: are they potentially more or less dangerous than carbon nanotubes or asbestos? Toxicol Appl Pharmacol. 2011;252(1):1–10.
31 Guo YY, Zhang J, Zheng YF, Yang J, Zhu XQ. Cytotoxic and genotoxic effects of multi-wall carbon nanotubes on human umbilical vein endothelial cells in vitro. Mutat Res. 2011;721(2):184–91.
32 Zhu L, Chang DW, Dai L, Hong Y. DNA damage induced by multiwalled carbon nanotubes in mouse embryonic stem cells. Nano Lett. 2007;7(12):3592–7.
33 Jacobsen NR, Møller P, Jensen KA, Vogel U, Ladefoged O, Loft S, Wallin H. Lung inflammation and genotoxicity following pulmonary exposure to nanoparticles in ApoE-/- mice. Part Fibre Toxicol. 2009;6:2.
34 Patlolla A, Hussain SM, Schlager JJ, Patlolla S, Tchounwou PB. Comparative study of the clastogenicity of functionalized and nonfunctionalized multiwalled carbon nanotubes in bone marrow cells of Swiss-Webster mice. Environ Toxicol. 2010;25(6):608–21.
35 Kato T, Totsuka Y, Ishino K, Matsumoto Y, Tada Y, Nakae D, et al. Genotoxicity of multi-walled carbon nanotubes in both in vitro and in vivo assay systems. Nanotoxicology. 2012 Apr 4. [Epub ahead of print]
36 Kim JS, Sung JH, Song KS, Lee JH, Kim SM, Lee GH, et al. Persistent DNA damage measured by Comet assay of Sprague-Dawley rat lung cells after five days of inhalation exposure and 1 month post-exposure to dispersed multi-wall carbon nanotubes (MWCNTs) generated by new MWCNT aerosol generation system. Toxicol Sci. 2012;128(2):439–48.
37 Schins RP. Mechanisms of genotoxicity of particles and fibers. Inhal Toxicol. 2002;14(1):57–78.
38 Jacobsen NR, Pojana G, White P, Møller P, Cohn CA, Korsholm KS, et al. Genotoxicity, cytotoxicity, and reactive oxygen species induced by single-walled carbon nanotubes and C(60) fullerenes in the FE1-MutatmTMMouse lung epithelial cells. Environ Mol Mutagen. 2008;49(6):476–87.
39 Migliore L, Saracino D, Bonelli A, Colognato R, D’Errico MR, Magrini A, et al. Carbon nanotubes induce oxidative DNA damage in RAW 264.7 cells. Environ Mol Mutagen. 2010;51(4):294–303.
40 Folkmann JK, Risom L, Jacobsen NR, Wallin H, Loft S, Møller P. Oxidatively damaged DNA in rats exposed by oral gavage to C60 fullerenes and single-walled carbon nanotubes. Environ Health Perspect. 2009;117(5):703–8.
41 Asakura M, Sasaki T, Sugiyama T, Takaya M, Koda S, Nagano K, et al. Genotoxicity and cytotoxicity of multi-wall carbon nanotubes in cultured Chinese hamster lung cells in comparison with chrysotile A fibers. J Occup Health. 2010;52(3):155–66.
42 Manshian BB, Jenkins GJ, Williams PM, Wright C, Barron AR, Brown AP, et al. Single-walled carbon nanotubes: differential genotoxic potential associated with physico-chemical properties. Nanotoxicology. 2012 Jan 20. [Epub ahead of print]
43 Di Giorgio ML, Bucchianico SD, Ragnelli AM, Aimola P, Santucci S, Poma A. Effects of single and multi walled carbon nanotubes on macrophages: cyto and genotoxicity and electron microscopy. Mutat Res. 2011;722(1):20–31.
44 Wirnitzer U, Herbold B, Voetz M, Ragot J. Studies on the in vitro genotoxicity of baytubes, agglomerates of engineered multi-walled carbon-nanotubes (MWCNT). Toxicol Lett. 2009;186(3):160–5.
45 Szendi K, Varga C. Lack of genotoxicity of carbon nanotubes in a pilot study. Anticancer Res. 2008;28(1A):349–52.
46 Muller J, Huaux F, Fonseca A, Nagy JB, Moreau N, Delos M, et al. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: toxicological aspects. Chem Res Toxicol. 2008;21(9):1698–705.
47 Muller J, Decordier I, Hoet PH, Lombaert N, Thomassen L, Huaux F, et al. Clastogenic and aneugenic effects of multi-wall carbon nanotubes in epithelial cells. Carcinogenesis. 2008;29(2):427–33.
48 Gonzalez L, Lison D, Kirsch-Volders M. Genotoxicity of nanomaterials: a critical review. Nanotoxicology 2008;2:252–73.
49 Sargent LM, Shvedova AA, Hubbs AF, Salisbury JL, Benkovic SA, Kashon ML, et al. Induction of aneuploidy by single-walled carbon nanotubes. Environ Mol Mutagen. 2009;50(8):708–17.
50 Sargent LM, Hubbs AF, Young SH, Kashon ML, Dinu CZ, Salisbury JL, et al. Single-walled carbon nanotube-induced mitotic disruption. Mutat Res. 2012;745(1-2):28–37.
51 Ema M, Imamura T, Suzuki H, Kobayashi N, Naya M, Nakanishi J. Evaluation of genotoxicity of multi-walled carbon nanotubes in a battery of in vitro and in vivo assays. Regul Toxicol Pharmacol. 2012;63(2):188–95.
52 Naya M, Kobayashi N, Mizuno K, Matsumoto K, Ema M, Nakanishi J. Evaluation of the genotoxic potential of single-wall carbon nanotubes by using a battery of in vitro and in vivo genotoxicity assays. Regul Toxicol Pharmacol. 2011;61(2):192–8.
53 Kim JS, Lee K, Lee YH, Cho HS, Kim KH, Choi KH, et al. Aspect ratio has no effect on genotoxicity of multi-wall carbon nanotubes. Arch Toxicol. 2011;85(7):775–86.
54 Di Sotto A, Chiaretti M, Carru GA, Bellucci S, Mazzanti G. Multi-walled carbon nanotubes: lack of mutagenic activity in the bacterial reverse mutation assay. Toxicol Lett. 2009;184(3):192–7.
55 Shvedova AA, Kisin E, Murray AR, Johnson VJ, Gorelik O, Arepalli S, et al. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress, and mutagenesis. Am J Physiol Lung Cell Mol Physiol. 2008;295(4):L552–65.
56 Bernstein D, Castranova V, Donaldson K, Fubini B, Hadley J, Hesterberg T, et al.; ILSI Risk Science Institute Working Group. Testing of fibrous particles: short-term assays and strategies. Inhal Toxicol. 2005;17(10):497–537.
57 Donaldson K, Murphy FA, Schinwald A, Duffin R, Poland CA. Identifying the pulmonary hazard of high aspect ratio nanoparticles to enable their safety-by-design. Nanomedicine (Lond). 2011;6(1):143–56.
58 Murphy FA, Poland CA, Duffin R, Al-Jamal KT, Ali-Boucetta H, Nunes A, et al. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am J Pathol. 2011;178(6):2587–600.
59 Osmond-McLeod MJ, Poland CA, Murphy F, Waddington L, Morris H, Hawkins SC, et al. Durability and inflammogenic impact of carbon nanotubes compared with asbestos fibres. Part Fibre Toxicol. 2011;8:15.
60 Donaldson K, Poland CA. Nanotoxicology: new insights into nanotubes. Nat Nanotechnol. 2009;4(11):708–10.
61 Dusinská M, Collins A, Kazimírová A, Barancoková M, Harrington V, Volkovová K, et al. Genotoxic effects of asbestos in humans. Mutat Res. 2004;553(1-2):91–102.
62 Hiraku Y, Kawanishi S, Ichinose T, Murata M. The role of iNOS-mediated DNA damage in infection- and asbestos-induced carcinogenesis. Ann N Y Acad Sci. 2010;1203:15–22.
63 Jung M, Davis WP, Taatjes DJ, Churg A, Mossman BT. Asbestos and cigarette smoke cause increased DNA strand breaks and necrosis in bronchiolar epithelial cells in vivo. Free Radic Biol Med. 2000;28(8):1295–9.
64 Unfried K, Schürkes C, Abel J. Distinct spectrum of mutations induced by crocidolite asbestos: clue for 8-hydroxydeoxyguanosine-dependent mutagenesis in vivo. Cancer Res. 2002;62(1):99–104.
65 Rihn B, Coulais C, Kauffer E, Bottin MC, Martin P, Yvon F, et al. Inhaled crocidolite mutagenicity in lung DNA. Environ Health Perspect. 2000;108(4):341–6.
66 Chao CC, Park SH, Aust AE. Participation of nitric oxide and iron in the oxidation of DNA in asbestos-treated human lung epithelial cells. Arch Biochem Biophys. 1996;326(1):152–7.
67 Xu A, Wu LJ, Santella RM, Hei TK. Role of oxyradicals in mutagenicity and DNA damage induced by crocidolite asbestos in mammalian cells. Cancer Res. 1999;59(23):5922–6.
68 Levresse V, Renier A, Levy F, Broaddus VC, Jaurand M. DNA breakage in asbestos-treated normal and transformed (TSV40) rat pleural mesothelial cells. Mutagenesis. 2000;15(3):239–44.
69 Liu W, Ernst JD, Broaddus VC. Phagocytosis of crocidolite asbestos induces oxidative stress, DNA damage, and apoptosis in mesothelial cells. Am J Respir Cell Mol Biol. 2000;23(3):371–8.
70 Okayasu R, Takahashi S, Yamada S, Hei TK, Ullrich RL. Asbestos and DNA double strand breaks. Cancer Res. 1999;59(2):298–300.
71 Msiska Z, Pacurari M, Mishra A, Leonard SS, Castranova V, Vallyathan V. DNA double-strand breaks by asbestos, silica, and titanium dioxide: possible biomarker of carcinogenic potential? Am J Respir Cell Mol Biol. 2010;43(2):210–9.
72 Dong HY, Buard A, Lévy F, Renier A, Laval F, Jaurand MC. Synthesis of poly(ADP-ribose) in asbestos treated rat pleural mesothelial cells in culture. Mutat Res. 1995;331(2):197–204.
73 Nygren J, Suhonen S, Norppa H, Linnainmaa K. DNA damage in bronchial epithelial and mesothelial cells with and without associated crocidolite asbestos fibers. Environ Mol Mutagen. 2004;44(5):477–82.
74 Yegles M, Saint-Etienne L, Renier A, Janson X, Jaurand MC. Induction of metaphase and anaphase/telophase abnormalities by asbestos fibers in rat pleural mesothelial cells in vitro. Am J Respir Cell Mol Biol. 1993;9(2):186–91.
75 Srivastava RK, Lohani M, Pant AB, Rahman Q. Cyto-genotoxicity of amphibole asbestos fibers in cultured human lung epithelial cell line: role of surface iron. Toxicol Ind Health. 2010;26(9):575–82.
76 Pietruska JR, Johnston T, Zhitkovich A, Kane AB. XRCC1 deficiency sensitizes human lung epithelial cells to genotoxicity by crocidolite asbestos and libby amphibole. Environ Health Perspect. 2010;118(12):1707–13.
77 Gibson DP, Aardema MJ, Kerckaert GA, Carr GJ, Brauninger RM, LeBoeuf RA. Detection of aneuploidy-inducing carcinogens in the Syrian hamster embryo (SHE) cell transformation assay. Mutat Res. 1995;343(1):7–24.
78 Park SH, Aust AE. Participation of iron and nitric oxide in the mutagenicity of asbestos in hgprt-, gpt+ Chinese hamster V79 cells. Cancer Res. 1998;58(6):1144–8.
79 Keysar SB, Fox MH. Kinetics of CHO A L mutant expression after treatment with gamma radiation, EMS, and asbestos. Cytometry A. 2009;75(5):412–9.
80 Jaurand MC, Renier A, Daubriac J. Mesothelioma: Do asbestos and carbon nanotubes pose the same health risk? Part Fibre Toxicol. 2009;6:16.
81 Stone V, Johnston H, Schins RP Development of in vitro systems for nanotoxicology: methodological considerations. Crit Rev Toxicol. 2009;39(7):613–26.
82 Lund LG, Aust AE. Iron-catalyzed reactions may be responsible for the biochemical and biological effects of asbestos. Biofactors. 1991;3(2):83–9.
83 Jaurand MC. Mechanisms of fiber-induced genotoxicity. Environ Health Perspect. 1997;105 Suppl 5:1073–84.
84 Donaldson K, Stone V. Current hypotheses on the mechanisms of toxicity of ultrafine particles. Ann Ist Super Sanita. 2003;39(3):405–10.
85 Huang X, Zalma R, Pezerat H. Chemical reactivity of the carbon-centered free radicals and ferrous iron in coals: role of bioavailable Fe2+ in coal workers pneumoconiosis. Free Radic Res. 1999;30(6):439–51.
86 Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111(4):455–60.
87 Donaldson K, Brown DM, Mitchell C, Dineva M, Beswick PH, Gilmour P, MacNee W. Free radical activity of PM10: iron-mediated generation of hydroxyl radicals. Environ Health Perspect. 1997;105 Suppl 5:1285–9.
88 Squadrito GL, Cueto R, Dellinger B, Pryor WA. Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter. Free Radic Biol Med. 2001;31(9):1132–8.
89 Qi S, den Hartog GJ, Bast A. Superoxide radicals increase transforming growth factor-beta1 and collagen release from human lung fibroblasts via cellular influx through chloride channels. Toxicol Appl Pharmacol. 2009;237(1):111–8.
90 Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, Alexander A. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci. 2006;92(1):5–22.
91 Fenoglio I, Tomatis M, Lison D, Muller J, Fonseca A, Nagy JB, Fubini B. Reactivity of carbon nanotubes: free radical generation or scavenging activity? Free Radic Biol Med. 2006;40(7):1227–33.
92 Fenoglio I, Aldieri E, Gazzano E, Cesano F, Colonna M, Scarano D, Mazzucco G, Attanasio A, Yakoub Y, Lison D, Fubini B. Thickness of multiwalled carbon nanotubes affects their lung toxicity. Chem Res Toxicol. 2012 Jan 13;25(1):74–82.
93 Lucente-Schultz RM, Moore VC, Leonard AD, Price BK, Kosynkin DV, Lu M, Partha R, Conyers JL, Tour JM. Antioxidant single-walled carbon nanotubes. J Am Chem Soc. 2009;131(11):3934–41.
94 Galano A. Carbon nanotubes: promising agents against free radicals. Nanoscale. 2010;2(3):373–80.
95 Fenoglio I, Greco G, Tomatis M, Muller J, Raymundo-Piñero E, Béguin F, et al. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: physicochemical aspects. Chem Res Toxicol. 2008;21(9):1690–7.
96 Thurnherr T, Brandenberger C, Fischer K, Diener L, Manser P, Maeder-Althaus X, et al. A comparison of acute and long-term effects of industrial multiwalled carbon nanotubes on human lung and immune cells in vitro. Toxicol Lett. 2011;200(3):176–86.
97 Pulskamp K, Diabaté S, Krug HF. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol Lett. 2007;168(1):58–74.
98 Poser I, Rahman Q, Lohani M, Yadav S, Becker HH, Weiss DG, Schiffmann D, Dopp E. Modulation of genotoxic effects in asbestos-exposed primary human mesothelial cells by radical scavengers, metal chelators and a glutathione precursor. Mutat Res. 2004;559(1-2):19–27.
99 Kamp DW. Asbestos-induced lung diseases: an update. Transl Res. 2009;153(4):143–52.
100 Kamp DW, Panduri V, Weitzman SA, Chandel N. Asbestos-induced alveolar epithelial cell apoptosis: role of mitochondrial dysfunction caused by iron-derived free radicals. Mol Cell Biochem. 2002;234–235(1-2):153–60.
101 Li H, Haberzettl P, Albrecht C, Höhr D, Knaapen AM, Borm PJ, Schins RP. Inhibition of the mitochondrial respiratory chain function abrogates quartz induced DNA damage in lung epithelial cells. Mutat Res. 2007;617(1-2):46–57.
102 Driscoll KE, Carter JM, Howard BW, Hassenbein D, Janssen YM, Mossman BT. Crocidolite activates NF-kappa B and MIP-2 gene expression in rat alveolar epithelial cells. Role of mitochondrial-derived oxidants. Environ Health Perspect. 1998;106(Suppl 5):1171–4.
103 Zhou F, Xing D, Wu B, Wu S, Ou Z, Chen WR. New insights of transmembranal mechanism and subcellular localization of noncovalently modified single-walled carbon nanotubes. Nano Lett. 2010;10(5):1677–81.
104 Yang Z, Zhang Y, Yang Y, Sun L, Han D, Li H, Wang C. Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating Alzheimer disease. Nanomedicine. 2010;6(3):427–41.
105 Ravichandran P, Baluchamy S, Sadanandan B, Gopikrishnan R, Biradar S, Ramesh V, et al. Multiwalled carbon nanotubes activate NF-κB and AP-1 signaling pathways to induce apoptosis in rat lung epithelial cells. Apoptosis. 2010;15(12):1507–16.
106 Yehia HN, Draper RK, Mikoryak C, Walker EK, Bajaj P, Musselman IH, Daigrepont MC, Dieckmann GR, Pantano P. Single-walled carbon nanotube interactions with HeLa cells. J Nanobiotechnology. 2007;5:8.
107 Sharma CS, Sarkar S, Periyakaruppan A, Barr J, Wise K, Thomas R, Wilson BL, Ramesh GT. Single-walled carbon nanotubes induce oxidative stress in rat lung epithelial cells. J Nanosci Nanotechnol. 2007;7(7):2466–72.
108 Srivastava RK, Pant AB, Kashyap MP, Kumar V, Lohani M, Jonas L, Rahman Q. Multi-walled carbon nanotubes induce oxidative stress and apoptosis in human lung cancer cell line-A549. Nanotoxicology. 2011;5:195–207.
109 Knaapen AM, Borm PJ, Albrecht C, Schins RP. Inhaled particles and lung cancer. Part A: Mechanisms. Int J Cancer. 2004;109(6):799–809.
110 Rama Narsimha Reddy A, Narsimha Reddy Y, Himabindu V, Rama Krishna D. Induction of oxidative stress and cytotoxicity by carbon nanomaterials is dependent on physical properties. Toxicol Ind Health. 2011;27(1):3–10.
111 Clichici S, Mocan T, Filip A, Biris A, Simon S, Daicoviciu D, et al. Blood oxidative stress generation after intraperitoneal administration of functionalized single-walled carbon nanotubes in rats. Acta Physiol Hung. 2011;98(2):231–41.
112 Reddy AR, Rao MV, Krishna DR, Himabindu V, Reddy YN. Evaluation of oxidative stress and anti-oxidant status in rat serum following exposure of carbon nanotubes. Regul Toxicol Pharmacol. 2011;59(2):251–7.
113 Reddy AR, Reddy YN, Krishna DR, Himabindu V. Pulmonary toxicity assessment of multiwalled carbon nanotubes in rats following intratracheal instillation. Environ Toxicol. 2010b [Epub ahead of print]
114 Yang ST, Wang X, Jia G, Gu Y, Wang T, Nie H, et al. Long-term accumulation and low toxicity of single-walled carbon nanotubes in intravenously exposed mice. Toxicol Lett. 2008;181(3):182–9.
115 Donaldson K, Poland CA, Schins RP. Possible genotoxic mechanisms of nanoparticles: criteria for improved test strategies. Nanotoxicology. 2010;4:414–20.
116 Ravichandran P, Periyakaruppan A, Sadanandan B, Ramesh V, Hall JC, Jejelowo O, et al. Induction of apoptosis in rat lung epithelial cells by multiwalled carbon nanotubes. J Biochem Mol Toxicol. 2009;23(5):333–44.
117 Kagan VE, Tyurina YY, Tyurin VA, Konduru NV, Potapovich AI, Osipov AN, Kisin ER, Schwegler-Berry D, Mercer R, Castranova V, Shvedova AA. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: role of iron. Toxicol Lett. 2006;165(1):88–100.
118 Reddy AR, Reddy YN, Krishna DR, Himabindu V. Multi wall carbon nanotubes induce oxidative stress and cytotoxicity in human embryonic kidney (HEK293) cells. Toxicology. 2010;272(1-3):11–6.
119 Shvedova AA, Castranova V, Kisin ER, Schwegler-Berry D, Murray AR, Gandelsman VZ, et al. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health A. 2003;66(20):1909–26.
120 Ravichandran P, Baluchamy S, Gopikrishnan R, Biradar S, Ramesh V, Goornavar V, et al. Pulmonary biocompatibility assessment of inhaled single-wall and multiwall carbon nanotubes in BALB/c mice. J Biol Chem. 2011;286(34):29725–33.
121 Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289(5):L698–708.
122 Shvedova AA, Kisin ER, Murray AR, Gorelik O, Arepalli S, Castranova V, et al. Vitamin E deficiency enhances pulmonary inflammatory response and oxidative stress induced by single-walled carbon nanotubes in C57BL/6 mice. Toxicol Appl Pharmacol. 2007;221(3):339–48.
123 Dickey JS, Baird BJ, Redon CE, Sokolov MV, Sedelnikova OA, Bonner WM. Intercellular communication of cellular stress monitored by {gamma}-H2AX induction. Carcinogenesis. 2009;30(10):1686–95.
124 Bhabra G, Sood A, Fisher B, Cartwright L, Saunders M, Evans WH, et al. Nanoparticles can cause DNA damage across a cellular barrier. Nat Nanotechnol. 2009;4(12):876–83.
125 Parry MC, Bhabra G, Sood A, Machado F, Cartwright L, Saunders M, et al. Thresholds for indirect DNA damage across cellular barriers for orthopaedic biomaterials. Biomaterials. 2010;31(16):4477–83.
126 Emerit I, Jaurand MC, Saint-Etienne L, Levy A. Formation of a clastogenic factor by asbestos-treated rat pleural mesothelial cells. Agents Actions. 1991;34(3-4):410–5.
127 Beyersmann D, Hartwig A. Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch Toxicol. 2008;82(8):493–512.
128 Nam CW, Kang SJ, Kang YK, Kwak MK. Cell growth inhibition and apoptosis by SDS-solubilized single-walled carbon nanotubes in normal rat kidney epithelial cells. Arch Pharm Res. 2011;34(4):661–9.
129 Park EJ, Roh J, Kim SN, Kang MS, Han YA, Kim Y, et al. A single intratracheal instillation of single-walled carbon nanotubes induced early lung fibrosis and subchronic tissue damage in mice. Arch Toxicol. 2011;85(9):1121–31.
130 Haniu H, Matsuda Y, Takeuchi K, Kim YA, Hayashi T, Endo M. Proteomics-based safety evaluation of multi-walled carbon nanotubes. Toxicol Appl Pharmacol. 2010;242(3):256–62.
131 Hirano S, Kanno S, Furuyama A. Multi-walled carbon nanotubes injure the plasma membrane of macrophages. Toxicol Appl Pharmacol. 2008;232(2):244–51.
132 Tomasetti M, Amati M, Nocchi L, Saccucci F, Strafella E, Staffolani S, et al. Asbestos exposure affects poly(ADP-ribose) polymerase-1 activity: role in asbestos-induced carcinogenesis. Mutagenesis. 2011;26(5):585–91.
133 Moolgavkar SH, Brown RC, Turim J. Biopersistence, fiber length, and cancer risk assessment for inhaled fibers. Inhal Toxicol. 2001;13(9):755–72.
134 Güngör N, Godschalk RW, Pachen DM, Van Schooten FJ, Knaapen AM. Activated neutrophils inhibit nucleotide excision repair in human pulmonary epithelial cells: role of myeloperoxidase. FASEB J. 2007;21(10):2359–67.
135 Pero RW, Sheng Y, Olsson A, Bryngelsson C, Lund-Pero M. Hypochlorous acid/N-chloramines are naturally produced DNA repair inhibitors. Carcinogenesis. 1996;17(1):13–8.
136 Jaiswal M, LaRusso NF, Shapiro RA, Billiar TR, Gores GJ. Nitric oxide-mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology. 2001;120(1):190–9.
137 Wako K, Kotani Y, Hirose A, Doi T, Hamada S. Effects of preparation methods for multi-wall carbon nanotube (MWCNT) suspensions on MWCNT induced rat pulmonary toxicity. J Toxicol Sci. 2010;35(4):437–46.
138 Rothen-Rutishauser B, Brown DM, Piallier-Boyles M, Kinloch IA, Windle AH, Gehr P, Stone V. Relating the physicochemical characteristics and dispersion of multiwalled carbon nanotubes in different suspension media to their oxidative reactivity in vitro and inflammation in vivo. Nanotoxicology. 2010;4(3):331–42.
139 Müller L, Riediker M, Wick P, Mohr M, Gehr P, Rothen-Rutishauser B. Oxidative stress and inflammation response after nanoparticle exposure: differences between human lung cell monocultures and an advanced three-dimensional model of the human epithelial airways. J R Soc Interface. 2010;7(Suppl 1):S27–40.
140 Zeidler PC, Castranova V. Role of nitric oxide in pathological responses of the lung to exposure to environmental/occupational agents. Redox Rep. 2004;9(1):7–18.
141 Xu A, Chai Y, Nohmi T, Hei TK. Genotoxic responses to titanium dioxide nanoparticles and fullerene in gpt delta transgenic MEF cells. Part Fibre Toxicol. 2009;6:3.
142 Pacurari M, Qian Y, Porter DW, Wolfarth M, Wan Y, Luo D, Ding M, Castranova V, Guo NL. Multi-walled carbon nanotube-induced gene expression in the mouse lung: Association with lung pathology. Toxicol Appl Pharmacol. 2011;255(1):18–31.
143 Deng X, Xiong D, Wang Y, Chen W, Luan Q, Zhang H, Jiao Z, Wu M. Water soluble multi-walled carbon nanotubes enhance peritoneal macrophage activity in vivo. J Nanosci Nanotechnol. 2010;10(12):8663–9.
144 MacCorkle RA, Slattery SD, Nash DR, Brinkley BR. Intracellular protein binding to asbestos induces aneuploidy in human lung fibroblasts. Cell Motil Cytoskeleton. 2006;63(10):646–57.
145 Dopp E, Saedler J, Stopper H, Weiss DG, Schiffmann D. Mitotic disturbances and micronucleus induction in Syrian hamster embryo fibroblast cells caused by asbestos fibers. Environ Health Perspect. 1995;103(3):268–71.
146 Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 2010;7:5.
147 Bouwmeester H, Lynch I, Marvin HJ, Dawson KA, Berges M, Braguer D, et al. Minimal analytical characterization of engineered nanomaterials needed for hazard assessment in biological matrices. Nanotoxicology. 2011;5(1):1–11.
148 Kroll A, Pillukat MH, Hahn D, Schnekenburger J. Current in vitro methods in nanoparticle risk assessment: limitations and challenges. Eur J Pharm Biopharm. 2009;72(2):370–7.
