Impaired left ventricular function as a predictive factor for mid-term survival in octogenarians after primary coronary artery bypass surgery
DOI: https://doi.org/10.4414/smw.2012.13704
Denis
Berdajs, Sotirios
Marinakis, Ulf
Kessler, Mirza
Muradbegovic, Enrico
Ferrari, Ludwig K
von Segesser
Summary
BACKGROUND: The impact of preoperative impaired left ventricular ejection fraction (EF) in octogenarians following coronary bypass surgery on short-term survival was evaluated in this study.
METHODS: A total of 147 octogenarians (mean age 82.1 ± 1.9 years) with coronary artery diseases underwent elective coronary artery bypass graft between January 2000 and December 2009. Patients were stratified into: Group I (n = 59) with EF >50%, Group II (n = 59) with 50% > EF >30% and in Group III (n = 29) with 30% > EF.
RESULTS: There was no difference among the three groups regarding incidence of COPD, renal failure, congestive heart failure, diabetes, and preoperative cerebrovascular events. Postoperative atrial fibrillation was the sole independent predictive factor for in-hospital mortality (odds ratio (OR), 18.1); this was 8.5% in Group I, 15.3% in Group II and 10.3% in Group III. Independent predictive factors for mortality during follow up were: decrease of EF during follow-up for more that 5% (OR, 5.2), usage of left internal mammary artery as free graft (OR, 18.1), and EF in follow-up lower than 40% (OR, 4.8).
CONCLUSIONS: The results herein suggest acceptable in-hospital as well short-term mortality in octogenarians with impaired EF following coronary artery bypass grafting (CABG) and are comparable to recent literature where the mortality of younger patients was up to 15% and short-term mortality up to 40%, respectively. Accordingly, we can also state that in an octogenarian cohort with impaired EF, CABG is a viable treatment with acceptable mortality.
Introduction
In the last several decades the number of the patients with impaired left ventricular ejection fraction (EF) due to ischemic cardiomyopathy has increased. Although improvements in medical therapies and surgical techniques have been achieved, the management of patients with coronary artery disease (CAD) and low EF is still challenging. Optional treatment modalities for this patient cohort include medical therapy, surgical revascularisation and heart transplantation. Medical treatment per se is because of limited long-term survival and is not an optimal treatment solution. Mortality in patients with impaired EF receiving only medical treatment over 5 years ranges between 20% and 30%, and rose up to 50% at 10 years [1, 2]. In contrast, heart transplantation as a treatment modality for ischemic cardiomyopathy offers excellent survival of 65.6% over 5 years, however, only 10% of patients being listed to the transplantation may receive a corresponding organ, because of a shortage of donor organs [3]. Coronary artery bypass grafting (CABG) has shown to be superior to medical therapy alone for low-EF patients with 5-year mortality of 10% and about 20% mortality after 7 years [1, 3, 4]. However we have to be aware that early, as well as long-term, morbidity and mortality for patients with impaired EF following CABG is significantly elevated compared to patients with normal EF [1]. Many require an early postoperative period, prolonged inotropic support, prolonged intubation and longer hospitalisation. It is true that recently, improvement in operative techniques and peri-operative management has decreased peri-operative as well postoperative mortality and morbidity in this patient population.
In recent literature little is known about the results of successful CABG in octogenarians with impaired left ventricular EF. Since life expectancy in western countries is constantly increasing, and we believe it is just a matter of time until this patient group will be regularly addressed to the surgery. The aim of this retrospective study was to evaluate intra-operative and mid-term follow up results after primary CABG in patients with moderate to severe impaired preoperative left ventricular function being treated in the period between January 2000 and December 2009.
Patients and methods
Between January 2000 and December 2009, 88 octogenarians with impaired left ventricular EF who underwent elective primary CABG at the University Hospital in Lausanne, Switzerland, were enrolled in this retrospective study, assessing data collected in a prospective continuous way. Local Medico-Ethical Review Committees approved the study. As a control group, 59 octogenarians who also underwent primary CABG with normal left ventricular ejection fraction were included. Exclusion criteria were recent cardiogenic shock, emergency CABG, off-pump CABG, and concomitant procedure such as a valve replacement and/or reconstruction. Preoperative patient characteristics are summarised in table 1. A EuroScore II validation for each group was calculated. Congestive heart failure was defined according to actual guidelines of ESC [5].
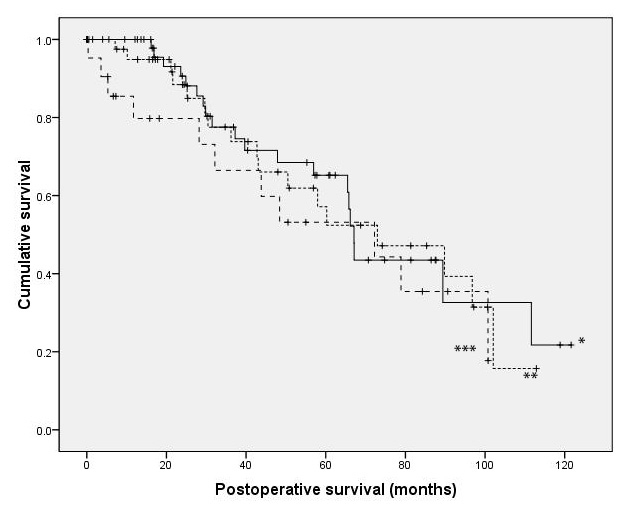
Figure 1
Kaplan Meier analysis of survival between patients with EF >50% (*), 50%> EF >30% and 30% >EF showed no difference over 40 months. Median survival time (CI 95) was 67.1 months (62.2–69.0) in group I, 73 months (37.1–108.9) in group II and 72.2 months (25.0–119.5) in group III (p >0.05 for all the groups).
Primary endpoints were: in-hospital mortality as well mortality during follow up. Secondary endpoints were evolution of LVEF during follow up and incidence of major cardiovascular events (myocardial ischemia and neurological events).
Preoperative evaluation of left ventricular function
In all patients (n = 147), left ventricular EF was investigated by preoperative transesophageal echocardiography. For this, the 4-apical chambers method was used. In 59 patients left ventricular EF was >50% (Group I), in 59 EF was <50% and >30% (Group II), and in 29 patients EF was <30% (Group III).
Surgical technique
All patients underwent isolated CABG and a similar surgical technique was used in all cases. After sternotomy, harvesting of the internal mammary artery and saphenous vein was performed, cardiopulmonary bypass (CPB) was established by cannulation of ascending aorta and right atrium. Surgery was performed in normothermia. Crystaloid (St. Thomas) cardioplegia was intermittently given through the ascending aorta. Complete revascularisation was achieved.
Follow up
Follow up was performed by telephone calls to the patients, referred cardiologist and personal physicians. The information regarding the patient’s general wellbeing was obtained from patients. For detailed information of non-heart related events, personal physicians were contacted. For all cardiac-related events the referred cardiologists were addressed. In follow up, major cardiovascular events such as myocardial ischemia (new onset of angina, myocardial infarction), stroke, need for coroangiography with or without intervention, and evolution of left ventricular ejection fraction were registered.
Statistical analysis
Statistical analyses were performed using SPSS software, version 18.0 (SPSS, Chicago, IL, USA). Data were tested for normality and equal distribution via the Kolmogorov-Smirnov test and were assessed for skewness and kurtosis. Data was presented as mean and standard deviation (SD) for continuous variables, or as percent frequencies for categorical variables, unless otherwise specified.
ANOVA with pairwise post-hoc tests (Bonferroni, and Dunnet-T3) were applied to compare continuous variables between patient groups with different preoperative LVEF (table 1 and 2). Chi-square test or Fisher’s exact test were used for categorical variables.
Logistic stepwise regression analysis was used to model the event probability of first mortality during hospitalisation and second mortality during follow-up. Included were variables showing a significant correlation with the above-named dependent variables: (1): For mortality during hospitalisation: Left main stem coronary disease, diabetes, Left IMA pump duration longer than 100 min intra-aortic balloon pump re-operation for bleeding postoperative (postop.) severe heart failure, postop. myocardial infarction, postop. atrial fibrillation, postop sepsis; and (2) for mortality during follow- up: Hypercholesterolemia preop. renal failure usage of the left internal mammary artery as free graft, a decrease of the ejection fraction (EF) of more than 5% during follow-up (FU), EF below 40% at FU. For a binary model, the continuous variables pump duration, EF decrease during FU, and EF at FU were transformed into categorical variables using receiving operator characteristics analysis for an optimal cut-off.
Survival was assessed via Kaplan Meier curves among patients with different preoperative EF, as well as in patients with the newly defined EF-variables correlated with mortality (EF decrease more than 5% during follow-up, EF below 40% at FU). We also compared survival between EF groups using a Cox proportional hazards model.
Two-sided tests were also used throughout the analysis and pvalues <0.05 were considered significant.
|
Table 1: Preoperative parameters of octogenarians being addressed to coronary bypass surgery. |
|
Parameter preop.
|
Patients group
EF >50%
mean (SD)
Group I
|
Patients group
30% <EF <50%
Group II
|
Patients group
EF ≤30%Groupe III
|
p
high
vs.
mid
|
p
high
vs.
low
|
p
mid
vs.
low
|
| No. of patients |
59 |
59 |
29 |
|
|
|
| Gender (female) |
18/59 (30.5%) |
14/59
(23.7%) |
10/29
(34.5%) |
0.535 |
0.809 |
0.316 |
| Age (years) |
81.9 ± 1.8
(range 80–88) |
81.8 ± 1.8
(range 80–88) |
82.7 ± 2.3
(range 80.5–87) |
1.000 |
0.209 |
1.000 |
| Preop EF |
62.7 ± 6.2
N = 59 |
45.3 ± 4.2
N = 59 |
24.6 ± 4.6
N = 29 |
<0.001 |
<0.001 |
<0.001 |
| Smoker |
14/59 (23.7%) |
18/59
(30.5%) |
5/29
(17.2%) |
0.535 |
0.588 |
0.208 |
| COPD |
0/59 (0%) |
1/59
(1.7%) |
1/29
(3.4%) |
1.000 |
0.330 |
1.000 |
| Hypercholesteremia
(>240 mg/dL) |
34/59 (57.6%) |
30/59
(50.8%) |
15/29
(51.7%) |
0.580 |
0.652 |
1.000 |
| Renal failure
(Creatinin clerance <30 ml/min) |
13/59 (22.0%) |
16/59
(27.1%) |
6/29
(20.7%) |
0.335 |
1.000 |
0.606 |
| Peripheral arterial disease |
10/59 (16.9%) |
22/59
(37.3%) |
9/29
(31.0%) |
0.022 |
0.170 |
0.640 |
| Cerebrovascular event |
20/59 (16.9%) |
19/59
(16.1%) |
6/29
(20.7%) |
1.000 |
0.226 |
0.320 |
| Prior myocardial infarction |
24/59 (40.7%) |
29/59
(49.2%) |
20/29
(69.0%) |
0.459 |
0.023 |
0.110 |
| Hypertension |
43/59 (72.9%) |
42/59
(71.2%) |
24/29
(82.8%) |
1.000 |
0.427 |
0.301 |
| Congestive heart failure |
14/59 (23.7%) |
11/59
(18.6%) |
4/29
(13.8%) |
0.326 |
0.401 |
0.765 |
| EuroSCORE
(Logistic) (%) |
13.9 ± 0.2 |
19.4 ± 0.1 |
20.2 ± 01 |
0.002 |
0.01 |
0.6 |
| Diabetes |
9/59
(15.3%) |
12/59
(20.3%) |
9/29
(31.0%) |
0.631 |
0.098 |
0.296 |
|
NYHA
|
|
|
|
0.978 |
0.240 |
0.324 |
| NYHA II |
6/29 (20.7%) |
5/26
(19.2%) |
1/9
(11.1%) |
|
|
|
| NYHA III |
18/29 (62.1%) |
16/26
(61.5%) |
4/9
(44.4%) |
|
|
|
| NYHA IV |
5/29
(17.2%) |
5/26
(19.2%) |
4/9
(44.4%) |
|
|
|
| ON Pump |
49/59
(83.1%) |
50/59
(84.7%) |
27/29
(93.1%) |
1.000 |
0.323 |
0.326 |
| IABP |
4/58
(6.9%) |
7/57
(12.3%) |
7/29
(24.1%) |
0.361 |
0.037 |
0.217 |
| COPD = chronic obstructive pulmonary disease; NYHA = New York Heart Association. |
Results
Mean age in Group I was 81.9 ± 1.8 years (range 80–88), 81.8 ± 1.8 years (80–88) in Group II, and 82.7 ± 2.3 years (80–87) in Group III. The preoperative characteristics of patients in all three groups are shown in table 1. Mean ejection fraction in Group I was 62.7 ± 6.2%, in Group II 45.3 ± 4.2%, and in Group III it was 24.6 ± 4.6%.
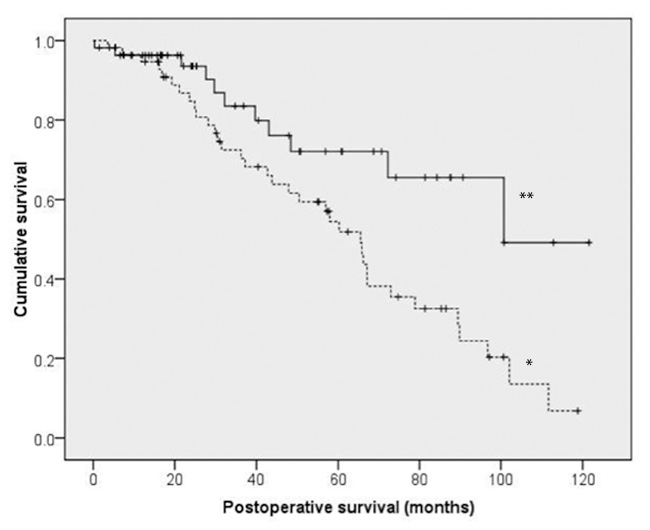
Figure 2
Kaplan-Meier analysis to compare survival between patients with differences between entry- and follow-up ejection fractions (EF) below 5% (*) and equal or above 5% (**). Median survival (CI95) was significantly different between patients with difference of EF below 5% (100.7 months [74.6–104.8]) and equal or above 5% (65.5 months [55.4–75.6], p <0.01).
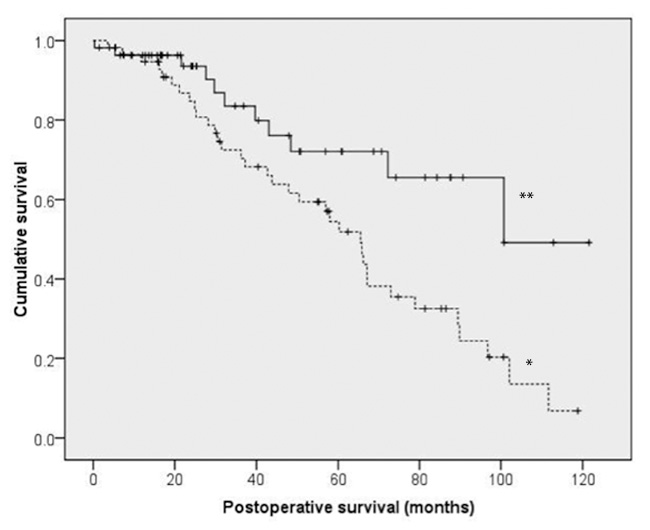
Figure 3
Kaplan- Meier analysis to compare survival between patients with follow-up ejection fractions (EF) equal or above 40% (**) and below 40% (*). Survival was significantly different between patients with follow-up EF equal or above 40% (median (CI95) (89.4 months [60.2–118.6]) and EF below 40% (50.5 months [30.7–70.3], p <0.01).
The prevalence of COPD, active smokers, impaired renal function cerebrovascular events and hypertension did not show any significant difference among the three groups (table 1). The frequency of preoperative myocardial infarction was significantly higher in Group III compared to Group I (p = 0.023). Diabetes as well congestive heart failure were equally represented in all three groups. Logistics EuroScore II was significantly higher in Group II and Group III compared to the normal EF group (table 1).
Overall distribution of coronary artery vessel disease distribution within the 3 groups showed no significant difference. Frequencies of triple vessel disease with left main trunk obstruction was higher in Group II compared to Group I and Group III (30.5% vs. 15.3% and 6.9%). In contrast, isolated left main trunk obstruction was less frequent in Group III with 17.2% compared to Group I and II with 33.9% and 37.3%, respectively.
Operation details
Mean CPB time in Group I was 86.9 ± 40.1 min, 85.9 ± 26.7 min in Group II, and 98.5 ± 40.4 min in Group III. Cross-clamp time in Group I was 52.9 ± 20.5 min, 62.6 ± 19.4 min in Group II, and 65.6 ± 21.6 min in Group III.
All procedures were performed on CPB by using a single cross-clamp technique. Regarding cardiopulmonary bypass time, cross clamp time, number of CABGs, usage of or right internal mammary artery and number of veins showed no significant difference in the three groups. Usage of left internal thoracic artery was significantly lower in Group III (n = 21, 72.4%) as compared to Group II (n = 55, 93.2%), and I (n = 57, 96.6%) (p = 0.002 and p = 0.017, respectively).
Perioperative complications
Mean hospitalisation time was not significantly different between the three groups taking 12.4 ± 4.3 days in Group I versus 14.5 ± 16.5 days, and 12.5 ± 5.2 days in Group II and III, respectively. The incidences of postoperative cerebrovascular event, postoperative mediastinitis reoperations because of bleeding, and myocardial infarction were not significantly higher in the low EF group compared to moderate impaired EF group and normal EF group. However, renal failure in Group III was registered for 41.4% (n = 12) cases and was significantly higher compared to 11.9% in Group I (n = 7), and 16.9% in Group II (p = 0.003 and 0.019, respectively).
In-hospital mortality was 10.3% in Group III, 15.3% in Group II, and 8.5% in Group I (Group I vs. Group II p = 0.394, Group I vs. Group III p = 1.0, and Group II vs. Group III p = 0.744).
Cardiac cause of death was registered in 3 patients in Group III (10.3%), in 8 and 4 in Group II and I (13.6% vs. 6.8%), respectively.
Follow-up
Mean follow-up time was 41.3 ± 31.0 months in Group I (range 1–122 months), 45.0 ± 30.5 months in Group II (range 4–113 months) and 39.4 ± 35.0 months in Group III (range 2–101 months). As shown in table 2, mortality during follow up was not significantly different between the three groups (35.1%, 36.0% and 45.8% in group I, II and III, respectively). The Kaplan-Meier curve did not show any significant difference in long-term survival between the three groups (fig. 1) with a mean time of follow up of 41.3 ± 31.0 months in Group I, 45.0 ± 30.5 in Group II, and 39.4 ± 35.0 in Group III (p = 1.0 for all groups). Cox proportional hazards model gave similar conclusions and so the results have not been presented.
The incidence of major cardiovascular events was as follows. Cardiac cause of death was registered in 21.4%, 11.6%, and 6.9% of cases in Group I, II, and III, respectively (p <0.001). Incidence of stroke was 14.8% in Group I, 14.0% in Group II, and 7.7% in Group III. Myocardial ischemia was registered as follows in 7.7% of cases in Group III, in 10% of cases in Group II, and in 12.9% of cases in Group I. The incidence of anastomosis stenosis was lower in Group II compared to group I and III (4.0% vs. 9.2% and 11.5%, respectively) (table 2).
Ejection fraction at follow up was 56.5 ± 11.3, 41.9 ± 11.8, and 34.1 ± 11.3 in Group I, II, and III respectively. Comparing the EF measured at follow up to the preoperative values, EF increased in group I by 6.3 ± 11.2, 3.6 ± 11.4 in Group II, and in Group III it decreased by –2.1 ± 11.5 (table 2).
Predictive factors for mortality
The results of the last steps of stepwise logistic regression analysis for in- hospital mortality and mortality during follow-up are demonstrated in table 3. Parameters with an OR of 0 were omitted (Re-operation for bleeding, postop. severe heart failure, postop. myocardial infarction) Postoperative atrial fibrillation was shown to be an independent predictive factor for in-hospital mortality (Odds ratio (OR) 21.4 (95% confidence interval (CI): 1.6–294.5; p = 0.022). Usage of left internal mammary artery as a free graft was an independent predictive factor for mortality during follow up (OR 18.1 (CI95%: 1.5–216.2; p = 0.021)). Furthermore the decrease of EF for more than 5% during follow up (OR 5.2 (CI95%: 1.7–13.8; p <0.001)) (fig. 2) and ejection fraction below 40% at follow-up (OR 4.8 CI95%: 1.7–13.1; p <0.01) were also registered as independent predictive factors for mortality during follow up (fig. 3).
|
Table 2: Shows follow up results. |
| |
Patients group
EF >50%
mean (SD)
|
Patients group
30% <EF <50%
|
Patients group
EF <30%
|
p
high
vs.
mid
|
p
high
vs.
low
|
p
mid
vs.
low
|
| Follow up completed |
54/54
(100%) |
44/50
(88%) |
23/26
(88.5%) |
0.093 |
1.000 |
0.170 |
| In hospital mortality |
5/59
(8.5%) |
9/59
(15.3%) |
3/29
(10.3%) |
0.394 |
1.000 |
0.744 |
| Died during follow up |
19/54
(35.1%) |
18/50
(36.0%) |
11/26
(45.8%) |
1.000 |
0.454 |
0.454 |
| Neurological complication |
8/54
(14.8%) |
7/50
(14.0%) |
2/26
(7.7%) |
1.000 |
0.271 |
0.408 |
| Incidence of myocardial ischemia |
7/54
(12.9%) |
5/50
(10%) |
2/26
(7.7%) |
0.750 |
1.000 |
1.000 |
| Coro-angiography with
angioplasty |
5/54
(9.2%) |
3/50
(6.0%) |
3/26
(11.5%) |
1.000 |
0.684 |
0.406 |
| CABG stenosis in coroangipgraphy |
5/54
(9.2%) |
2/50
(4.0%) |
3/26
(11.5%) |
0.291 |
1.000 |
0.320 |
| EF at follow up |
56.5 ± 11.3
N = 53 |
41.9 ± 11.8
N = 42 |
34.1 ± 11.3
N = 25 |
0.000 |
0.000 |
0.024 |
| EF entry minus EF follow up |
6.2 ± 11.2
N = 53 |
3.6 ± 11.4
N = 42 |
–2.1 ± 11.5
N = 25 |
0.775 |
0.009 |
0.155 |
| CABG = coronary artery bypass graft; EF = ejection fraction. |
|
Table 3: Predictive potential for mortality during hospitalization and during follow up according to binary stepwise regression analysis). |
| Mortality during hospitalisation |
(n = 17) |
|
|
|
| Variable |
n |
OR |
CI95% |
p
|
| Left main stem cor. disease |
47 |
0.08 |
0.0–3.2 |
0.18 |
| Diabetes |
30 |
1.0 |
0.0–32.4 |
0.99 |
| Left IMA |
135 |
0.9 |
0.0–310 |
0.89 |
| Pump duration >100 min |
37 |
0.1 |
0.0–3.7 |
0.05 |
| Intra-aortic balloon pump |
18 |
2.0 |
0.0–453 |
0.81 |
| Postop. atrial fibrillation |
69 |
21.4 |
1.6–295 |
0.02 |
| Mortality during follow-up (n = 49) |
|
|
|
|
| Variable |
n |
OR |
CI95% |
p
|
| Hypercholesterolemia |
74 |
0.4 |
0.2–1.1 |
0.08 |
| Preop. renal failure |
33 |
2.8 |
0.9–8.6 |
0.6 |
| Left IMA as free graft |
5 |
18.1 |
1.5–216.2 |
0.02 |
| EF-decrease >5% during FU |
59 |
5.2 |
1.7–13.8 |
<0.001 |
| EF <40% at FU |
33 |
4.8 |
1.7–13.1 |
<0.01 |
| OR = odds ratio; CI95% = 95% confidence interval; Postop. = postoperative, cor. = coronary; IMA = internal mammary artery; EF = ejection fraction; FU = follow-up. |
Discussion
Patients with coronary artery disease and advanced ventricular dysfunction have poor prognoses with medical treatment alone. According to the recent literature, 5-year survival in patients with tree-vessel disease and impaired left ventricular EF after onset of medical treatment ranges between 20% and 30% [4, 6].
In contrast, following surgical revascularisation 5-year survival is at 65% and is superior compared to results of a conservative treatment modality [6].
Following CABG as independent predictive factors that may negatively influence short as well as long-term survival, apart from left ventricular function, age was identified as the most frequently cited risk factor [7–11].
Indeed the peri-operative mortality in the low EF patients cohort [6] following CABG ranges between 3.8% and 10.5%, and is about 4 times higher than mortality registered in a normal EF patient group [8, 9]. The same tendency may be registered for the mid-term result where the 5-year mortality, varying from report to report, shows a large variability, ranging between 17% and 44% [6, 8, 9]. To date, however, to our best knowledge, no report has been published stressing the mid-term results of CABG in octogenarians with impaired left ventricular function. Weighting both risk factors in a single-outcome cohort would provide important information for decision-making prior to surgery, angioplasty and conservative therapy. In modern times, as we are facing growing life expectancy, this is becoming an important issue to consider.
According to aforementioned reports it would be not surprising that the addition of risk factors such as advanced age and impaired EF would tremendously elevate perioperative as well mid-term mortalities.
In this study we evaluated perioperative as well mid-term mortality along with the incidence of major cardiovascular events in octogenarians with ischemic cardiomyopathy following CABG. In-hospital mortality in the low EF patient group was 10.3% and 15.3% in the moderate EF group. This is comparable to results in other recent literature [6, 11], where the mean age of patients was younger by almost two decades and in-hospital mortality ranged between 4% and 10%. The same may be stated for a mean follow-up time of about 40 months, where 45.8% mortality was registered in the low EF group, 36.0% mortality in moderate EF, and 35.1% mortality in normal EF group (fig. 1). Low and moderate EF group mortality are comparable to the results of STICH trail [12] and meta-analysis of Kunadian where 5-year mortality in low EF was reported to be slightly under 40%. Note that both reports cited a mean age of the population about two decades younger. The logistic EuroScore II in all three groups was >12% [13], although for the high-risk cohort reported herein, our results were lower compared to rates in recent literature [6, 8, 9]. This in some degree may be due to very meticulous surgical techniques such as usage of the single-cross clamp technique in elder patients, a no-touch technique for coronary graft harvesting, and last but not least superb postoperative management in ICU for these high-risk patients. On the other hand, we noticed that age in our cohort was not a predictive factor for either short- or mid-term mortality following CABG in an impaired EF patient population.
To a greater extent than patient age, a reduction of EF of more than 5% in follow up and EF inferior to 40% during the follow up were directly correlated to mortality (fig. 2 and 3). Left EF decreases under 5% during a median of 40 months was a predictive factor for mortality. Patient survival compared to those who improved in their function for more than 5% was significantly different. This decrease of EF may be explained due to a silent ischemia [14] that may on the one hand be a consequence of incomplete revascularisation, and on other hand a progression of native coronary sclerosis.
These results have to be interpreted with caution, as a 5% difference in the transthoracic echo investigation is a relative number and may have a strong investigator correlation. When looking at the numerical difference in EF, one has to note that the evolution of EF per se plays an important role. This would suggest the positive influence of viable myocardium and recuperation of hibernating musculature after revascularisation was performed.
According to our results, we believe that CABG may be successfully performed in octogenarians with impaired EF preoperatively with acceptable perioperative as well as mid-term results. This is also true for low EF patient groups. Perioperative mortality was inferior by 15% and is comparable to the in-hospital mortality rates of younger patients in the literature. Furthermore, we believe that in-hospital mortality may be reduced with advanced therapy modalities in the ICU to reduce intubation time and ICU stay. Also worth noting is that EF changes during follow up plays an important role in mortality. Thus may suggest that the assessment of viable myocardium is an essential component to predict mortality in this patient group.
Study limitations
One limitation is that preoperative as well as follow up transthoracic echocardiography investigations were not performed by the same investigators in all cases. In an ideal scenario there would be only a few investigators involved in performing preoperative procedures, as well as in follow-up echocardiography. A further negative influence of free IMA graft on survival cannot be clearly explained. This is due to a very low case load (n = 4). As such, a larger cohort of elective CABG cases should be investigated to compare free IMA groups versus in-situ IMA.
References
1 Passamani E, Davis KB, Gillespie MJ, Killip T and the CASS principal investigators and their associates. A randomized trial of coronary artery bypass surgery. Survival of patients with low ejection fraction. N Engl J Med. 1985;312:1665–71.
2 The Veterans Administration coronary Artery Bypass Surgery Cooperative Study Group. Eleven year survival iin the veteransadministartion randomised trial of coronary bypass surgery for stable angina. N Engl J Med. 1984;311:1333–9.
3 John R, Rajasinghe HA, Chen JM, Weinberg AD, Sinha P, Mancini DM, et al. Long-term outcomes after cardiac transplantation: and experiance based on diferent erase of immunosupressive therapy. Ann Thorac Surg. 2001;72:440–9.
4 Alderman EL, Fisher LD, Litwin P, Kaiser GC, Myers WO, Maynard C, et al. Results of coronary artery surgery in patients with poor left ventricular function (CASS). Circulation. 1983;68:785–95.
5 Eur Heart J. 2012;33:1787–847.
6 Kunadian V, Zaman A, Qiu W. Revascularization among patients with severe left ventricular dysfunction: a meta-analysis of observatinal studies. Eur J Heart Fail. 2011;13:773–84.
7 Islamoglu F, Apaydin AZ, Posacioglu H, Ozbaran M, Hamulu A, Buket S, et al. Coronary artery bypass grafting in patients with poor left ventricular function. Jpn Heart J. 2002;43:343–56.
8 Carr JA, Haithcock BE, Paone G, Bernabei A, Silverman NA. Long term outcome after coronary bypass grafting in patients with severe left ventricular dysfunction. Ann Thorac Surg. 2002;74:1531–6.
9 Topkara VK, Cheema FH, Kesavaramanujam S, Mercando ML, Cheema AF, Namerow PB, et al. Coronary artery bypass grafting in patients with low ejection fraction. Circulation. 2005;30:112(9 Suppl):I344–50.
10 Jones RH, Hannan EL, Hammermeister KE, Delong ER, O’Connor GT, Luepker RV, et al. Identification of preoperative variables needed for risk adjustment of short-term mortality after coronary artery bypass graft surgery. The Working Group Panel on the Cooperative CABG Database Project. J Am Coll Cardiol. 1996;28:1478.
11 Lee S, Chang BC, Yoo KJ, Hong YS, Kang MS. Clinical results of coronary revascularization in left ventricular dysfunction. Circ J. 2007;71:1862–6.
12 Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, et al; STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;28;364(17):1607–16.
13 Siregar S, Groenwold RH, de Heer F, Bots ML, van der Graaf Y, van Herwerden LA. Performance of the original EuroSCORE Eur J Cardiothorac Surg. 2012;26(ahead of publication).
14 Schoenenberger AW, Kobza R, Jamshidi P, Zuber M, Abbate A, Stuck AE, et al. Am J Cardiol. 2009;104(2):158–63.


