Molecular pathogenesis of infections caused by Moraxella catarrhalis in children
DOI: https://doi.org/10.4414/smw.2012.13694
Sara
Bernhard, Violeta
Spaniol, Christoph
Aebi
Summary
Moraxella catarrhalis (M. catarrhalis) is a human-restricted commensal of the normal bacterial flora in the upper respiratory tract of children, and – during the previous two decades – has been recognised as a true human pathogen. M. catarrhalis is the third most common pathogen causing acute otitis media in children, which is the most common reason to visit a paediatrician during childhood. Acute otitis media thus causes a high clinical and economical burden. With the introduction of the conjugate pneumococcal vaccines the microbiomic pattern in the nasopharyngeal flora of children has changed, and the frequency of isolation of M. catarrhalis has increased. Compared to adults, children are more often colonised with M. catarrhalis.
Over the last three decades there has been a dramatic increase in the acquisition of β-lactam resistance in M. catarrhalis. Today 95–100% of clinically isolated M. catarrhalis produce β-lactamase. It is thus desirable to reduce the burden of M. catarrhalis disease by developing a vaccine. There are several potential vaccine antigen candidates in different stages of development, but none of them has entered clinical trials at the present time.
Abbreviations
AOM Acute otitis media
CEACAM Carcinoembryotic antigen -related cell adhesion molecule
COPD Chronic obstructive pulmonary disease
hag/MID Human erythrocyte agglutinin/Moraxella immunoglobulin D-binding protein
LOS Lipooligosaccharide
PCR Polymerase chain reaction
PRR Pathogen recognition receptors
RSV Respiratory syncytial virus
UspA Ubiquitous surface protein A
Introduction
Moraxella catarrhalis (M. catarrhalis) is an important, exclusively human respiratory tract pathogen. The Gram-negative diplococcus was first described in 1896 and was named Micrococcus catarrhalis[1]. In 1963 the pathogen was renamed Neisseria catarrhalis[2]. Microscopically M. catarrhalis resembles Neisseria meningitidis and Neisseria gonorrhoeae,but there is only limited chromosomal DNA-homology with these species. Therefore, it was moved to the new genus Branhamella as Branhamella catarrhalis in 1970 [3]. Its classification as a member of the Genus Moraxella as Moraxella catarrhalis was established in 1984 and it remained so since that time [4].
Two major phylogenetic subpopulations (type 1 and type 2 strains) of the species have been identified [5, 6]. Wirthet al. suggested that the older type 2 subpopulation has existed since ~50 million years, whereas the younger type 1 lineage appeared ~4 millions ago together with Homo sapiens[6]. The phylogenetically younger type 1 subpopulation has adapted to the human host, and possesses various virulence factors, such as human complement resistance (“seroresistance”) and adherence to human epithelial cells [6]. In a collection of 268 M. catarrhalis isolates from diverse geographic regions, 83% of the isolates were found to belong to the seroresistant subpopulation [6]. The seroresistant subpopulation has also been found to be naturally transformation competent [7], which in turn leads to frequent homologous recombination.
In children, M. catarrhalis causes mainly upper respiratory tract infections (otitis media), whereas in adults the pathogen causes lower respiratory tract infections in previously compromised airways (acute exacerbation of chronic obstructive pulmonary disease [COPD]). Invasive infections such as bacteraemia, meningitis, septic arthritis, ventriculitis and endocarditis, are very rare and during the past three decades less than 80 cases have been reported (summarised by [8]).
Microbiological diagnosis
Grown on blood agar, colonies of M. catarrhalis appear round, gray, opaque and convex and they can easily be pushed intact over the surface. This phenomenon is the so-called “hockey puck sign”.
The gram negative diplococcus is difficult to distinguish from Neisseria spp. in a typical Gram staining. Various biochemical test methods exist to distinguish the species. M. catarrhalis is DNAse, catalase and oxidase positive, and furthermore the pathogen hydrolyses tributyrin and reduces N2 to NH3 and is unable to produce acid from glucose, lactose, maltose fructose and sucrose. None of these tests are 100% sensitive or specific. The inforamative value of more sensitive DNA methods such as polymerase chain reaction (PCR) has been demonstrated [9], but as of today commercially available PCR assays are not available.
Epidemiology, colonisation and immune response
M. catarrhalis colonises the nasopharynx in early childhood [10–12]. Many factors affect nasopharyngeal carriage of M. catarrhalis, such as the presence of siblings, respiratory illnesses and visiting nursery schools [11–14]. By the age of 6 months the cumulative colonisation rate varies between 22% and 55% [11, 15].
Furthermore, several nosocomial outbreaks of M. catarrhalis infections in adults and in children have been reported [16, 17]. Winter and spring season as well as multi-bed wards were found to be significant risk factors for nosocomial transmission [17].
In healthy children, a seasonal cyclic variation of colonisation, with a peak in autumn/winter, has been demonstrated [12]. Other studies reported seasonal peaks of M. catarrhalis infections in winter and spring [18, 19]. This seasonality is also observed in viral respiratory tract infection such as respiratory syncytial virus (RSV) [20]. It has been demonstrated that children with a high nasopharyngeal RSV load have an increased risk for the development of acute otitis media (AOM), which suggests that viral infection often paves the way for subsequent bacterial AOM [21]. Another potential factor is the physiologic cold shock response of M. catarrhalis[22]. Cold shock describes the physiologic rapid reduction of temperature in the upper respiratory tract to approximately 26 °C when humans breathe cold air for a prolonged period of time, a phenomenon which occurs mainly during the winter season in temperate and cold climates. This physiologic cold shock has been shown to up-regulate the expression of important virulence factors, such as adherence to epithelial cells, iron acquisition, complement resistance and immune evasion [23]. An increased expression of the UspA1 adhesin on the surface of M. catarrhalis at 26 °C leads to an increased adherence to upper respiratory tract epithelial cells in vitro. Furthermore, cold shock increases the release of interleukin-8, a pro-inflammatory cytokine in pharyngeal epithelial cells [24]. These mechanisms in turn may lead to an increased bacterial density during the cold season, which has been shown to increase the risk of the development of AOM [15]. The seasonality of viral respiratory tract infections and the physiologic cold shock response appear to be important contributors to the seasonal peak in M. catarrhalis infections.
In adults the pharyngeal carriage rate is noticeably lower and varies between 1% and 5% [13, 14]. It increases again in adults older than 60 years of age [13]. Specific mucosal IgA antibody responses against outer membrane proteins have been detected in early childhood, but they do not prevent colonisation [25]. The presence of bactericidal serum anti-M. catarrhalis antibodies have been detected in both children and adults [26, 27]. The IgG3 antibody subclass response to M. catarrhalis is assumed to play an important role. The development of mature specific IgG antibodies is age-dependent. The subclass IgG1 develops during the first year of life and the subclass IgG3 after the second year of life, respectively. It has been demonstrated that children younger than 4 years of age have very low titers of IgG antibodies against M. catarrhalis [28]. This fact could explain the high colonisation rate of >80% and the high rate of AOM in children younger than 2 years of age.
After the introduction of the conjugate pneumococcal vaccines, the colonisation pattern in children has changed towards an increased prevalence of M. catarrhalis, H. influenzae and the non-vaccine serotypes of S. pneumonia. M. catarrhalis was found significantly more often in immunised children with AOM [29].
Paediatric infections
Acute otitis media
Acute otitis mediais the most common bacterial infection treated with antibiotics in children. M. catarrhalis is the second or third most common pathogen to cause acute otitis media together with Streptococcus pneumoniae and nontypable H. influenzae [30]. It should be noted that tympanocentesis and culture of middle ear fluid is required for the correct microbiologic diagnosis of bacterial AOM. Tympanocentesis is not routinely performed and the rate of M. catarrhalis AOM may thus be underestimated. Compared to S. pneumoniae and H. influenzae, M. catarrhalis causes a relatively mild course of AOM. S. pneumoniae AOM are clinically more severe than those caused by H. influenzae and M. catarrhalis and are more often associated with high fever, tympanic membrane bulging and redness and severe otalgia [31]. In one study, a lower spontaneous tympanic membrane perforation rate and no case of mastoiditis in children younger than 5 years of age with M. catarrhalis AOM was observed [30].
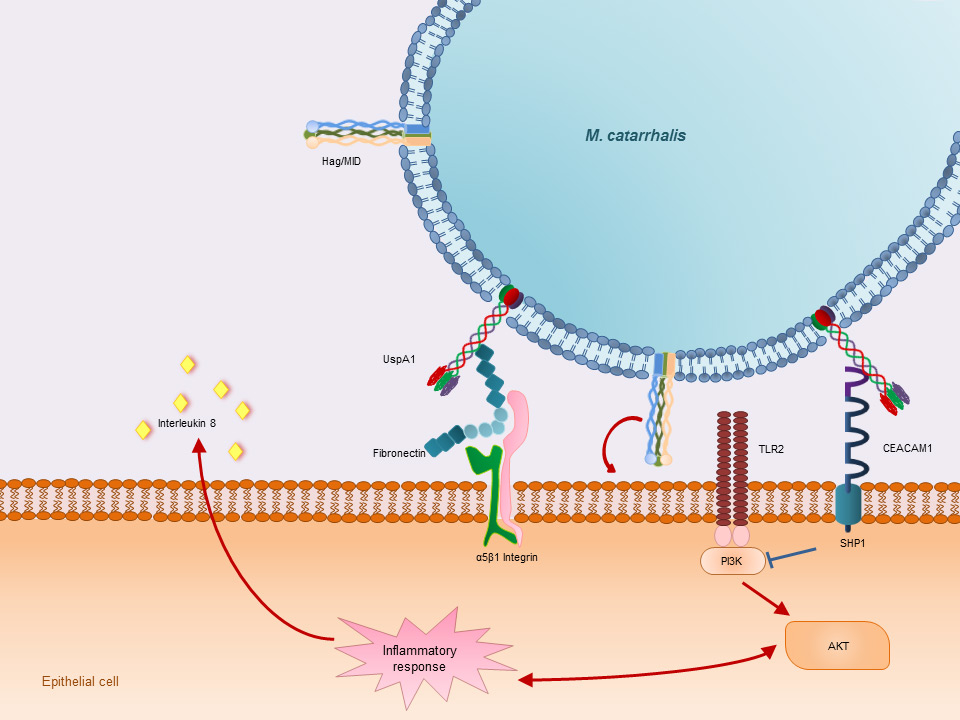
Figure 1
Adherence to host epithelial cells. UspA1 activates the carcino-embryonic antigen-related cell adhesion molecule 1 (CEACAM 1). Binding and activating of CEACAM 1 leads to recruitment of the SH2 containing protein tyrosin phosphatase 1 (SHP1). SHP1 inhibits the phosphoinositide 3-kinase (PI3K) phosphorylation which leads to a suppression of the AKT-mediated pro-inflammatory response. Furthermore UspA1 binds to the extracellular matrix glycoprotein fibronectin which in turn binds to α5β1 Integrin on the surface of the host epithelial cells. Hag/MID is known to be involved in the adherence of M. catarrhalis to host epithelial cells. The exact mechanism and the receptors involved are not yet known.
Multiple factors influence the pathogenesis of acute otitis media in children. One of the most important factors is upper respiratory tract viral infection. During infection, pathogens migrate into the middle ear along the eustachian tube and cause inflammation, leading to congestion of the eustachian tube, which in turn causes a negative pressure in the middle ear [32]. Bacteria migrating or aspirated into the middle ear cavity can proliferate and cause AOM. M. catarrhalis possesses different virulence factors, which play an important role in this sequence of events.
Otitis prone children
Children with recurrent episodes of AOM are defined as otitis prone children if they have four or more episodes during one year [33]. Otitis prone children are at risk of developing delayed speech and language development due to conductive hearing loss caused by recurrent AOM with effusion [34]. Interestingly, M. catarrhalisDNA is more often detected in patients with middle ear effusion than in those with AOM [35]. This observation underlines the necessity of the development of a vaccine against M. catarrhalis, because as mentioned above, chronic or recurrent middle ear effusion can impair the child’s cognitive development.
Acute bacterial sinusitis
Acute bacterial sinusitis is not very common in children and accounts for approximately 5–10% of the complications of upper respiratory tract infections [36]. Acute bacterial sinusitis is defined as nasal discharge and cough during day and night-time for more than 10 days and less than 30 days [36]. The development of the paranasal sinuses is age dependent. The maxillary and ethmoidal sinuses are present at birth, whereas the sphenoidal sinus develops around 5–6 years of age. The frontal sinus develops last and completes the pneumatisation of the skull in young adulthood. As in AOM, bacteria migrate from the nasopharynx into the adjacent sinuses and proliferate if the secretions persist in the sinus cavity. Fluid retention occurs if the mucociliar clearance mechanism is disturbed, the ostia are obstructed, or if the viscosity of the discharge is increased. M. catarrhalis accounts for approximately 10–20% of the bacterial pathogens isolated in acute bacterial sinusitis [37].
Treatment
Today 90–100% of the M. catarrhalis isolates are resistant to ampicillin by producing β-lactamase. In the 1970s only a small proportion of M. catarrhalis isolates were β-lactamase producing and since then the number of resistant isolates has increased dramatically. Three different enzymes, BRO-1, BRO-2 and BRO-3, have been identified [38, 39]. BRO-1 is found in >90% of the resistant strains and is assumed to induce higher minimal inhibitory concentrations than BRO-2 producing strains [40]. Recent studies have reported an increase in resistance to trimethoprim/sulfamethoxazole (cotrimoxazole) of 18.5% in Taiwan to 82.5% in India [41, 42]. On the other hand, in two studies from Europe, a decrease in cotrimoxazole resistance was observed over the last decade [43, 44].
As tympanocentesis with subsequent bacterial culture of the middle ear effusion is not routinely performed, the treatment for AOM caused by M. catarrhalis is almost always empirical and is directed against the three most important bacterial pathogens (S. pneumoniae, H. influenzaeandM. catarrhalis). To date the first line treatment of AOM remains standard high dose amoxicillin in Europe and in the USA [45]. In case of treatment failure, the addition of clavulanat is recommended. Macrolides as an alternative treatment option should be reserved for patients with amoxicillin allergy, because treatment failure occurs more often [46]. Furthermore, the indication for the use of extended spectrum cephalosporins, tetracyclines and fluoroquinolons should be made very restrictively.
Pathogenesis – host-bacterium interaction – virulence factors
Long considered as a non-pathogenic commensal, recent research has established its clinical relevance, identifying a number of strategies, with which M. catarrhalis maintains its niche in the nasopharynx, and causes clinical disease [47–49]. M. catarrhalis exhibits different virulence mechanism to interact with the human host.
Adherence
Adherence to human epithelial cells and in particular to respiratory mucosal cells is considered a pivotal initial step in bacterial colonisation, which allows the bacteria to remain firmly attached to host epithelial cells. M. catarrhalis expresses various adhesins, which include the ubiquitous surface protein A family (UspA), the human erythrocyte agglutinin/Moraxella immunoglobulin D-binding protein (hag/MID) [50], the outer membrane protein CD (OMP CD) [51], M. catarrhalis adherence protein (McaP) [52], and lipoligosaccharide (LOS) [53] (fig. 1).
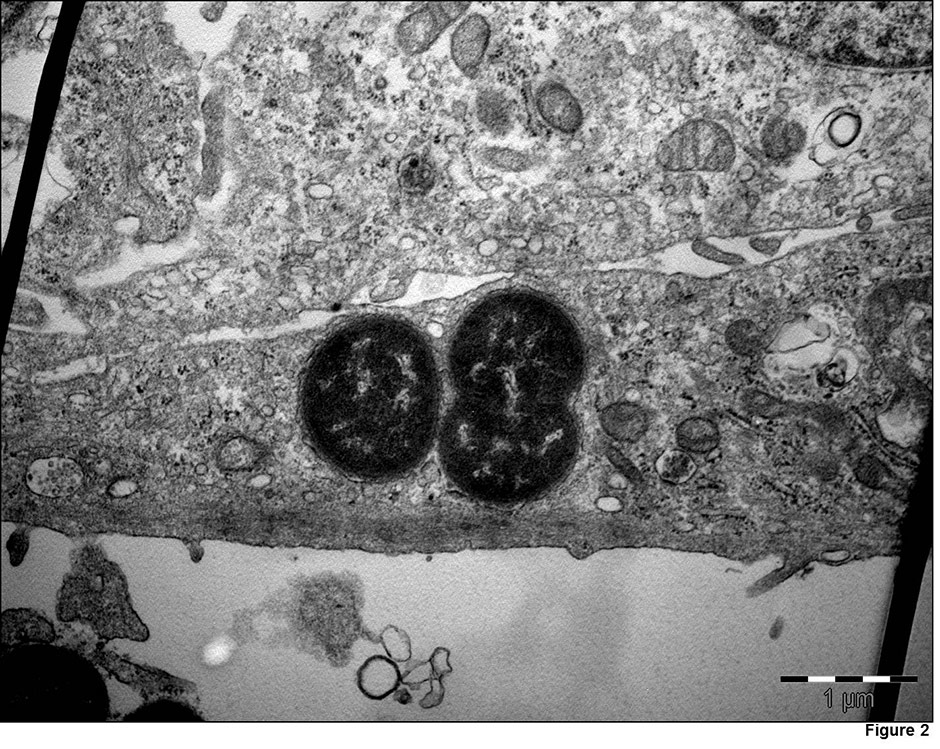
Figure 2
Transmission electron micrographs, demonstrating M. catarrhalis(strain 287) invasion in Detroit 526 pharyngeal cells.
UspA1, UspA2 and the closely related hybrid protein UspA2H show a lollipop like structure with an N-terminal head based on a β-sheet-structure, a coiled-coil stalk region and a C-terminal anchor region with 4 β-strands forming a β-barrel in the membrane, which shows homology to the YadA protein of Yersinia spp. [54, 55]. These proteins are typical bacterial auto-transporter proteins [56]. The N-terminus of the UspA2H protein is similar to the UspA1 domain, and its C-terminus resembles that of UspA2 [57]. Homologies between UspA1 and UspA2 were found in the stalk region, whereas the anchor region and the N-terminal head domain diverge widely [57]. UspA1 belongs to the major adhesins of M. catarrhalis[58]. Interestingly, the UspA1 gene is present in both phylogenetic lineages, but only the the seroresistant type 1 expresses the corresponding protein on its surface [59]. UspA1 and UspA2 bind to host cells through multi-functional binding sites. UspA1 binds carcino-embryotic antigen -related cell adhesion molecules (CEACAMs) through a binding site on the stalk region. CEACAMs are expressed on the surface of human respiratory tract epithelial cells [60]. UspA1 and UspA2 were also found to bind to components of the extracellular matrix proteins such as fibronectin and laminin [61, 62]. UspA1 expression varies in accordance to phase variation, which in turns mediates the binding to host cells [63]. Furthermore, cold shock up-regulates the expression of UspA1, at least in part by prolonging uspA1 mRNA half-life [22].
Hag/MID is another important multifunctional outer membrane protein of M. catarrhalis.The adherence to different respiratory tract cell lines (e.g. human middle ear cells, Chang conjunctival cells, A549 lung cells) is mediated by hag/MID [50, 64]. Similar to UspA1, the expression of Hag/MID is regulated by phase variation [50, 65].
Lipooligosaccharide (LOS) is another essential major component of the M. catarrhalis outer membrane, and is involved in the adherence to human respiratory cells [66]. Three serotypes have been identified (LOS A, B and C) [13]. Interestingly, serotype B has only been found in the seroresistant type 1 lineage and is more prevalent in adults than in children [67]. Thus, M. catarrhalis is able to exhibit various binding properties, which could be beneficial for its survival in different conditions and on different sites of the human respiratory tract.
Invasion
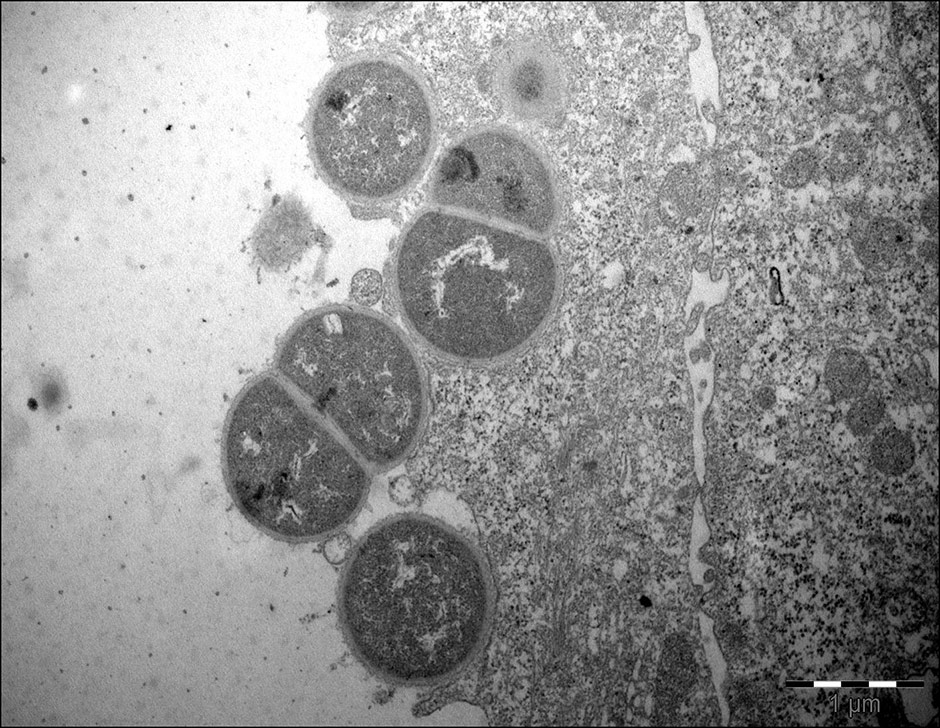
Invasion of human respiratory epithelial cells has been observed mainly in vitro [68, 69]. Invasion is regulated by the expression of LOS, UspA1 [68] and probably other outer membrane components. Slevogt et al. first described the capacity of M. catarrhalis to invade respiratory tract epithelial cells in vitro [69], and Heiniger et al. demonstrated its capacity to invade sub-epithelial pharyngeal lymphoid tissue in vivo [70]. It seems that invasion allows M. catarrhalis to escape killing by the host immune-system and extracellular antibiotics. The relevance and mechanisms of M. catarrhalisinvasion are incompletely understood, and further studies are warranted (fig. 2).
Biofilm formation
Microbial communities, enclosed by a self-produced extracellular polymeric matrix substance adhering to surfaces are defined as biofilms [71]. Biofilm formation is a well-known virulence factor in many respiratory tract pathogens [71, 72]. M. catarrhalis has been found to be able to form biofilms in vitro and in vivo [71, 73]. The UspA family and Hag/MID are involved in the regulation of biofilm formation [73]. Verhaegh et al. demonstrated age-dependence in the capacity to from biofilms, with clinical isolates from children being able to form more extensive biofilms than those isolated from adults [67]. Further, biofilms of M. catarrhalis have been detected in middle ear effusion of children with otitis media [71]. These two observations may be clinically relevant and could contribute to the understanding of the pathogenesis of recurrent/chronic AOM with effusion.
Immune evasion strategies – complement resistance
To escape immune mediated killing is another important challenge for M. catarrhalis. The innate immune system consists of several components, such as the complement system and pathogen recognition receptors (PRR). PRR recognise pathogen-associated molecular patterns and include toll-like receptors. Complement resistance is likely to be an important virulence factor of M. catarrhalis. This statement is emphasised by the observation that clinical isolates able to survive in normal human serum are more prevalent in strains recovered from infected patients, than in those isolated from colonised but asymptomatic patients [74]. M. catarrhalis exhibits different mechanisms to inhibit complement mediated killing. It is able to activate all three pathways of the human complement system and both major outer membrane proteins (UspAs, OMP E, OMP CD, CopB) and surface exposed structures (LOS) are involved in complement defence (summarised by [47, 48]). M.catarrhalis is able to bind to the regulator protein C4b binding protein through UspA1 and UspA2 [75]. In addition, UspA2 is able to bind C3 and vitronectin and thereby inhibits the activation of the alternative complement pathway [75–77]. Another interesting observation is that outer membrane vesicles of M. catarrhalis carrying UspA1 and UspA2 are able to contribute to an improved survival of serum sensitive H. influenzaethrough inactivation of C3 [78].
Vaccines
Both, AOM and exacerbation of COPD cause a significant human and economical burden. The development of a M. catarrhalis vaccine is desirable to reduce this burden. One challenge in the development of a vaccine to prevent M. catarrhalis infection is the lack of a suitable animal model. Several antigens are currently under investigation as potential vaccine antigens (summarised by [49, 79, 80]). A requirement for such an antigen is its conserved expression on the bacterial surface during infection. The gene expression of several potential antigen candidate proteins (e.g. UspA1 and hag/MID) of M. catarrhalis undergoes phase variation [63, 65], which in turn represents another obstacle in the development of a successful vaccine.
Concluding remarks
M. catarrhalishas been recognised as an important, exclusively human pathogen and commensal, causing upper respiratory tract infections in children and lower respiratory tract infections in adults. In children, M. catarrhalis is responsible for up 20% of all AOM episodes. With the widespread introduction of the conjugate vaccines against S. pneumoniae a change in the colonisation and infection pattern may appear with a consequent increase in the number of M. catarrhalis infections. Further research is needed to understand the pathogenetic mechanisms, especially with a focus on the direction of a vaccine development against M. catarrhalis.
Acknowledgments:We thank Marion Jetter, PhD, for providing the transmission electron micrographs.
References
1 Frosch P, Kolle W. Die Mikrokokken. Die Mikroorganismen. Leipzig: Verlag von Vogel; 1896. p. 154–5.
2 Berger U. Die anspruchslosen Neisserien. Exp Ther. 1963;(35):97–167.
3 Catlin B. Transfer ot the organsim named Neisseria catarrhalis to Branhamella gen. nov. Int J Syst Bacteriol. 1970;20(2):155–9.
4 Bovre K. The genus Moraxella Bergy’s Manual of Systematic Bacteriology. Baltimore: The Williams & Wilkens Co; 1984.
5 Bootsma HJ, van der Heide HG, van de Pas S, Schouls LM, Mooi FR. Analysis of Moraxella catarrhalis by DNA typing: evidence for a distinct subpopulation associated with virulence traits. J Infect Dis. 2000;181(4):1376–87.
6 Wirth T, Morelli G, Kusecek B, van Belkum A, van der Schee C, Meyer A, et al. The rise and spread of a new pathogen: seroresistant Moraxella catarrhalis. Genome Res. 2007;17(11):1647–56.
7 Meier PS, Troller R, Heiniger N, Hays JP, van Belkum A, Aebi C. Unveiling electrotransformation of Moraxella catarrhalis as a process of natural transformation. FEMS Microbiol Lett. 2006;262(1):72–6.
8 Sano N, Matsunaga S, Akiyama T, Nakashima Y, Kusaba K, Nagasawa Z, et al. Moraxella catarrhalis bacteraemia associated with prosthetic vascular graft infection. J Med Microbiol. 2010;59(Pt 2):245–50.
9 Post JC, White GJ, Aul JJ, Zavoral T, Wadowsky RM, Zhang Y, et al. Development and validation of a multiplex PCR-based assay for the upper respiratory tract bacterial pathogens haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. Mol Diagn. 1996;1(1):29–39.
10 Faden H, Harabuchi Y, Hong JJ, Epidemiology of Moraxella catarrhalis in children during the first 2 years of life: relationship to otitis media. J Infect Dis. 1994;169(6):1312–7.
11 Verhaegh SJ, Lebon A, Saarloos JA, Verbrugh HA, Jaddoe VW, Hofman A, et al. Determinants of Moraxella catarrhalis colonization in healthy Dutch children during the first 14 months of life. Clin Microbiol Infect. 2010;16(7):992–7.
12 Verhaegh SJ, Snippe ML, Levy F, Verbrugh HA, Jaddoe VW, Hofman A, et al. Colonization of healthy children by Moraxella catarrhalis is characterized by genotype heterogeneity, virulence gene diversity and co-colonization with Haemophilus influenzae. Microbiology. 2011;157(Pt 1):169–78.
13 Vaneechoutte M, Verschraegen G, Claeys G, Weise B, Van den Abeele AM. Respiratory tract carrier rates of Moraxella (Branhamella) catarrhalis in adults and children and interpretation of the isolation of M. catarrhalis from sputum. J Clin Microbiol. 1990;28(12):2674–80.
14 Ejlertsen T, Thisted E, Ebbesen F, Olesen B, Renneberg J. Branhamella catarrhalis in children and adults. A study of prevalence, time of colonisation, and association with upper and lower respiratory tract infections. J Infect. 1994;29(1):23–31.
15 Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y, Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics. J Infect Dis. 1997;175(6):1440–5.
16 Cook PP, Hecht DW, Snydman DR. Nosocomial Branhamella catarrhalis in a paediatric intensive care unit: risk factors for disease. J Hosp Infect. 1989;13(3):299–307.
17 Levy F, Leman SC, Sarubbi FA, Walker ES. Nosocomial transmission clusters and risk factors in Moraxella catarrhalis. Epidemiol Infect. 2009;137(4):581–90.
18 Wood GM, Johnson BC, McCormack JG. Moraxella catarrhalis: pathogenic significance in respiratory tract infections treated by community practitioners. Clin Infect Dis. 1996;22(4):632–6.
19 Sarubbi FA, Myers JW, Williams JJ, Shell CG. Respiratory infections caused by Branhamella catarrhalis. Selected epidemiologic features. Am J Med. 1990;88(5A):9S–14S.
20 Duppenthaler A, Gorgievski-Hrisoho M, Frey U, Aebi C. Two-year periodicity of respiratory syncytial virus epidemics in Switzerland. Infection. 2003;31(2):75–80.
21 Pettigrew MM, Gent JF, Pyles RB, Miller AL, Nokso-Koivisto J, Chonmaitree T. Viral-bacterial interactions and risk of acute otitis media complicating upper respiratory tract infection. J Clin Microbiol. 2011;49(11):3750–5.
22 Heiniger N, Troller R, Meier PS, Aebi C. Cold shock response of the UspA1 outer membrane adhesin of Moraxella catarrhalis. Infect Immun. 2005;73(12):p. 8247–55.
23 Spaniol V, Troller R, Schaller A, Aebi C. Physiologic cold shock of Moraxella catarrhalis affects the expression of genes involved in the iron acquisition, serum resistance and immune evasion. BMC Microbiol. 2011;11:182.
24 Spaniol V, Troller R, Aebi C. Physiologic cold shock increases adherence of Moraxella catarrhalis to and secretion of interleukin 8 in human upper respiratory tract epithelial cells. J Infect Dis. 2009;200(10):1593–601.
25 Stutzmann Meier P, Heiniger N, Troller R, Aebi C, Salivary antibodies directed against outer membrane proteins of Moraxella catarrhalis in healthy adults. Infect Immun. 2003;71(12):6793–8.
26 Faden H, Hong J, Murphy T. Immune response to outer membrane antigens of Moraxella catarrhalis in children with otitis media. Infect Immun. 1992;60(9):3824–9.
27 Bakri F, Brauer AL, Sethi S, Murphy TF. Systemic and mucosal antibody response to Moraxella catarrhalis after exacerbations of chronic obstructive pulmonary disease. J Infect Dis. 2002;185(5):632–40.
28 Goldblatt D, Turner MW, Levinsky RJ. Branhamella catarrhalis: antigenic determinants and the development of the IgG subclass response in childhood. J Infect Dis. 1990;162(5):1128–35.
29 Revai K, McCormick DP, Patel J, Grady JJ, Saeed K, Chonmaitree T. Effect of pneumococcal conjugate vaccine on nasopharyngeal bacterial colonization during acute otitis media. Pediatrics. 2006;117(5):1823–9.
30 Broides A, Dagan R, Greenberg D, Givon-Lavi N, Leibovitz E. Acute otitis media caused by Moraxella catarrhalis: epidemiologic and clinical characteristics. Clin Infect Dis. 2009;49(11):1641–7.
31 Rodriguez WJ, Schwartz RH. Streptococcus pneumoniae causes otitis media with higher fever and more redness of tympanic membranes than Haemophilus influenzae or Moraxella catarrhalis. Pediatr Infect Dis J. 1999;18(10):942–4.
32 Bluestone CD. Pathogenesis of otitis media: role of eustachian tube. Pediatr Infect Dis J. 1996;15(4):281–91.
33 Alho OP, Koivu M, Sorri M. What is an “otitis-prone” child? Int J Pediatr Otorhinolaryngol. 1991;21(3):201–9.
34 Teele DW. Long term sequelae of otitis media: fact or fantasy? Pediatr Infect Dis J. 1994;13(11):1069–73.
35 Post JC, Preston RA, Aul JJ, Larkins-Pettigrew M, Rydquist-White J, Anderson KW, et al. Molecular analysis of bacterial pathogens in otitis media with effusion. JAMA. 1995;273(20):1598–604.
36 Wald ER. Sinusitis in children. N Engl J Med. 1992;326(5):319–23.
37 Brook I, Foote PA, Hausfeld JN. Frequency of recovery of pathogens causing acute maxillary sinusitis in adults before and after introduction of vaccination of children with the 7-valent pneumococcal vaccine. J Med Microbiol. 2006;55(Pt 7):943–6.
38 Eliasson I, Kamme C, Vang M, Waley SG. Characterization of cell-bound papain-soluble beta-lactamases in BRO-1 and BRO-2 producing strains of Moraxella (Branhamella) catarrhalis and Moraxella nonliquefaciens. Eur J Clin Microbiol Infect Dis. 1992;11(4):313–21.
39 Bootsma HJ, van Dijk H, Vauterin P, Verhoef J, Mooi FR. Genesis of BRO beta-lactamase-producing Moraxella catarrhalis: evidence for transformation-mediated horizontal transfer. Mol Microbiol. 2000;36(1):93–104.
40 Schmitz FJ, Beeck A, Perdikouli M, Boos M, Mayer S, Scheuring S, et al. Production of BRO beta-lactamases and resistance to complement in European Moraxella catarrhalis isolates. J Clin Microbiol. 2002;40(4):1546–8.
41 Hsu SF, Lin YT, Chen TL, Siu LK, Hsueh PR, Huang ST, et al. Antimicrobial resistance of Moraxella catarrhalis isolates in Taiwan. J Microbiol Immunol Infect, 2011. 2011 Dec 8. [Epub ahead of print].
42 Gupta N, Arora S, Kundra S. Moraxella catarrhalis as a respiratory pathogen. Indian J Pathol Microbiol. 2011;54(4):769–71.
43 Melo-Cristino J, Santos L, Silva-Costa C, Friaes A, Pinho MD, Ramirez M. The Viriato study: update on antimicrobial resistance of microbial pathogens responsible for community-acquired respiratory tract infections in Portugal. Paediatr Drugs. 2010;12(Suppl 1):11–7.
44 Karpanoja P, Nyberg ST, Bergman M, Voipio T, Paakkari P, Huovinen P, et al. Connection between trimethoprim-sulfamethoxazole use and resistance in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob Agents Chemother. 2008;52(7):2480–5.
45 PIGS. Pediatric Infectious Disease Group of Switzerland, Empfehlungen zur Diagnose und Behandlung von Otitis media, Sinusitis, Pharyngitis und Pneumonie. Accessed April 4th 2012; Available from: http://www.pigs.ch/pigs/02-news/doc/reco2010-d.pdf.
46 Courter JD, Baker WL, Nowak KS, Smogowicz LA, Desjardins LL, Coleman CI, et al. Increased clinical failures when treating acute otitis media with macrolides: a meta-analysis. Ann Pharmacother. 2010;44(3):471–8.
47 Aebi C. Moraxella catarrhalis – pathogen or commensal? Adv Exp Med Biol. 697:107–16.
48 de Vries SP, Bootsma HJ, Hays JP, Hermans PW. Molecular aspects of Moraxella catarrhalis pathogenesis. Microbiol Mol Biol Rev. 2009;73(3):389–406, Table of Contents.
49 Murphy TF, Parameswaran GI. Moraxella catarrhalis, a human respiratory tract pathogen. Clin Infect Dis. 2009;49(1):124–31.
50 Bullard B, Lipski SL, Lafontaine ER. Hag directly mediates the adherence of Moraxella catarrhalis to human middle ear cells. Infect Immun. 2005;73(8):5127–36.
51 Meier PS, Freiburghaus S, Martin A, Heiniger N, Troller R, Aebi C. Mucosal immune response to specific outer membrane proteins of Moraxella catarrhalis in young children. Pediatr Infect Dis J. 2003;22(3):256–62.
52 Timpe JM, Holm MM, Vanlerberg SL, Basrur V, Lafontaine ER. Identification of a Moraxella catarrhalis outer membrane protein exhibiting both adhesin and lipolytic activities. Infect Immun. 2003;71(8):4341–50.
53 Peng D, Hong W, Choudhury BP, Carlson RW, Gu XX. Moraxella catarrhalis bacterium without endotoxin, a potential vaccine candidate. Infect Immun. 2005;73(11):7569–77.
54 Hoiczyk E, Roggenkamp A, Reichenbecher M, Lupas A, Heesemann J. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 2000;19(22):5989–99.
55 Koretke KK, Szczesny P, Gruber M, Lupas AN. Model structure of the prototypical non-fimbrial adhesin YadA of Yersinia enterocolitica. J Struct Biol. 2006;155(2):154–61.
56 Ackermann N, Tiller M, Anding G, Roggenkamp A, Heesemann J. Contribution of trimeric autotransporter C-terminal domains of oligomeric coiled-coil adhesin (Oca) family members YadA, UspA1, EibA, and Hia to translocation of the YadA passenger domain and virulence of Yersinia enterocolitica. J Bacteriol. 2008;190(14):5031–43.
57 Brooks MJ, Sedillo JL, Wagner N, Laurence CA, Wang W, Attia AS, et al. Modular arrangement of allelic variants explains the divergence in Moraxella catarrhalis UspA protein function. Infect Immun. 2008;76(11):5330–40.
58 Aebi C, Lafontaine ER, Cope LD, Latimer JL, Lumbley SL, McCracken GH, Jr, et al. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis 035E. Infect Immun. 1998;66(7):3113–9.
59 Meier PS, Troller R, Heiniger N, Grivea IN, Syrogiannopoulos GA, Aebi C. Moraxella catarrhalis strains with reduced expression of the UspA outer membrane proteins belong to a distinct subpopulation. Vaccine. 2005;23(16) 2000–8.
60 Hill DJ, Edwards AM, Rowe HA, Virji M. Carcinoembryonic antigen-related cell adhesion molecule (CEACAM)-binding recombinant polypeptide confers protection against infection by respiratory and urogenital pathogens. Mol Microbiol. 2005;55(5):1515–27.
61 Tan TT, Forsgren A, Riesbeck K. The respiratory pathogen moraxella catarrhalis binds to laminin via ubiquitous surface proteins A1 and A2. J Infect Dis. 2006;194(4):493–7.
62 Tan TT, Nordstrom T, Forsgren A, Riesbeck K. The respiratory pathogen Moraxella catarrhalis adheres to epithelial cells by interacting with fibronectin through ubiquitous surface proteins A1 and A2. J Infect Dis. 2005;192(6):1029–38.
63 Lafontaine ER, Wagner NJ, Hansen EJ. Expression of the Moraxella catarrhalis UspA1 protein undergoes phase variation and is regulated at the transcriptional level. J Bacteriol. 2001;183(5):1540–51.
64 Forsgren A, Brant M, Karamehmedovic M, Riesbeck K. The immunoglobulin D-binding protein MID from Moraxella catarrhalis is also an adhesin. Infect Immun. 2003;71(6):3302–9.
65 Mollenkvist A, Nordstrom T, Hallden C, Christensen JJ, Forsgren A, Riesbeck K. The Moraxella catarrhalis immunoglobulin D-binding protein MID has conserved sequences and is regulated by a mechanism corresponding to phase variation. J Bacteriol. 2003;185(7):2285–95.
66 Peng D, Hu WG, Choudhury BP, Muszynski A, Carlson RW, Gu XX. Role of different moieties from the lipooligosaccharide molecule in biological activities of the Moraxella catarrhalis outer membrane. FEBS J. 2007;274(20):5350–9.
67 Verhaegh SJ, Streefland A, Dewnarain JK, Farrell DJ, van Belkum A, Hays JP. Age-related genotypic and phenotypic differences in Moraxella catarrhalis isolates from children and adults presenting with respiratory disease in 2001–2002. Microbiology. 2008;154(Pt 4):1178–84.
68 Spaniol V, Heiniger N, Troller R, Aebi C. Outer membrane protein UspA1 and lipooligosaccharide are involved in invasion of human epithelial cells by Moraxella catarrhalis. Microbes Infect. 2008;10(1):3–11.
69 Slevogt H, Seybold J, Tiwari KN, Hocke AC, Jonatat C, Dietel S, et al. Moraxella catarrhalis is internalized in respiratory epithelial cells by a trigger-like mechanism and initiates a TLR2- and partly NOD1-dependent inflammatory immune response. Cell Microbiol. 2007;9(3):694–707.
70 Heiniger N, Spaniol V, Troller R, Vischer M, Aebi C. A reservoir of Moraxella catarrhalis in human pharyngeal lymphoid tissue. J Infect Dis. 2007;196(7):1080–7.
71 Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296(2):202–11.
72 Hall-Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cell Microbiol. 2009;11(7):1034–43.
73 Pearson MM, Laurence CA, Guinn SE, Hansen EJ. Biofilm formation by Moraxella catarrhalis in vitro: roles of the UspA1 adhesin and the Hag hemagglutinin. Infect Immun. 2006;74(3):1588–96.
74 Murphy S, Fitzgerald M, Mulcahy R, Keane C, Coakley D, Scott T. Studies on haemagglutination and serum resistance status of strains of Moraxella catarrhalis isolated from the elderly. Gerontology. 1997;43(5):277–82.
75 Nordstrom T, Blom AM, Forsgren A, Riesbeck K. The emerging pathogen Moraxella catarrhalis interacts with complement inhibitor C4b binding protein through ubiquitous surface proteins A1 and A2. J Immunol. 2004;173(7):4598–606.
76 Singh B, Blom AM, Unal C, Nilson B, Morgelin M, Riesbeck K. Vitronectin binds to the head region of Moraxella catarrhalis ubiquitous surface protein A2 and confers complement-inhibitory activity. Mol Microbiol. 2010;75(6):1426–44.
77 Attia AS, Ram S, Rice PA, Hansen EJ. Binding of vitronectin by the Moraxella catarrhalis UspA2 protein interferes with late stages of the complement cascade. Infect Immun. 2006;74(3):1597–611.
78 Tan TT, Morgelin M, Forsgren A, Riesbeck K. Haemophilus influenzae survival during complement-mediated attacks is promoted by Moraxella catarrhalis outer membrane vesicles. J Infect Dis. 2007;195(11):1661–70.
79 Tan TT, Riesbeck K. Current progress of adhesins as vaccine candidates for Moraxella catarrhalis. Expert Rev Vaccines. 2007;6(6):949–56.
80 Mawas F, Ho MM, Corbel MJ. Current progress with Moraxella catarrhalis antigens as vaccine candidates. Expert Rev Vaccines. 2009;8(1):77–90.