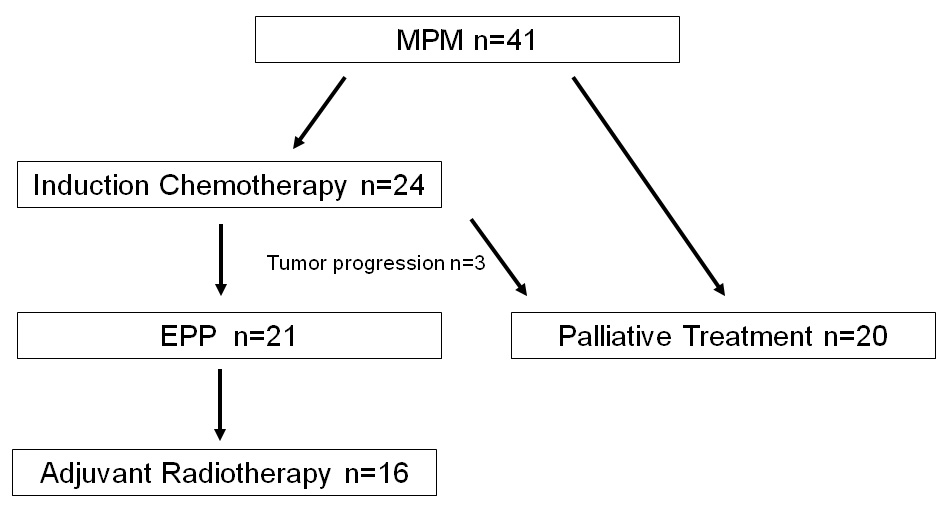
Figure 1
Flow chart showing the patient included in the trimodal or palliative treatment groups.
DOI: https://doi.org/10.4414/smw.2012.13686
Malignant pleural mesothelioma (MPM) arises in the pleural space and the clinical picture can be variable. Dyspnoea, chest pain, fever, and pleural effusion are the most frequent signs [1]. The most important aetiologic factor is asbestos exposure [1, 2]. The peak of MPM incidence in Western Europe is expected in this decade, unfortunately the asbestos exposure in developing countries is still high leading to a significant expected increase in the future [3, 4].
Since the introduction of trimodal treatment with neo-adjuvant chemotherapy, radical surgery and adjuvant radiotherapy better survival rates have been reported particularly in subpopulations with epithelial histology, R0-resection and absence of metastasis in extrapleural lymph nodes [5, 6]. However, prognosis remains dismal and most of the patients not undergoing trimodal treatment die within a year [7, 8]. Although controversial, extrapleural pneumonectomy (EPP) as a radical surgical technique offers an operative strategy in the setting of trimodal therapy with curative intent in treating MPM [9, 10, 11–13]. But, there are now some reports showing that trimodal therapy including EPP did not improve long term survival [14, 15]. On the basis of the latest reports, decortication/pleurectomy has become more important in the treatment of malignant pleural mesothelioma as survival rates seem to be better than after EPP [16, 17]. Complications after extrapleural pneumonectomy are frequent and frequently surgically related, for example bronchopleural fistula, diaphragmatic patch dehiscence, ARDS, cardiac arrhythmias, constrictive patch reconstruction of the pericardium, infection and bleeding [8, 18]. These can be successfully avoided and managed by proper selection of patients, meticulous technique and adequate pre- and postoperative management [13, 19, 20].
This retrospective cohort study presents the results of trimodal therapy of MPM in a single centre with the aim to analyse the benefit and long term outcome of a trimodal approach including EPP in the treatment of MPM.
According to the University of Bern Institutional Review Board guideline and in strict adherence to the ethical guidelines for human research of the Swiss Academy of Medical Sciences, data of 41 consecutive patients with histological proven MPM were obtained and analysed in a retrospective cohort analysis (table 1). No patient was excluded from this investigation during the studied time period. Data were obtained from health records of the Departments of Thoracic Surgery and Medical Oncology. Additionally, responsible general practitioners were contacted for long term follow up of each patient.
For each patient, general data were preoperatively assessed for example: age, gender, asbestos exposure, clinical symptoms at time point of first diagnosis, and type of histology. Outcome parameters such as preoperative administration of chemotherapy, type of surgical procedure, postoperative radiotherapy, postoperative tumour stage, tumour response to chemotherapy, length of hospital stay were analysed. Postoperative complications, 30- and 90-day mortality as well as 1-, 2- and 5-year survival rates beginning from point of diagnosis were calculated and analysed retrospectively. Tumour recurrence after completion of the therapy was subdivided into local and distant tumour recurrence. Local recurrence was defined by manifestation in the ipsilateral chest or chest wall and tumour recurrence-free survival of patients with trimodal therapy was calculated. Postoperative complications during the first 30-days after EPP were categorised into minor (urinary tract infection, wound infection) and major complications (arrhythmia, chest wall infection, pneumonia, septicaemia, bleeding or requiring revisional surgery). Redo surgery because of intrathoracic blood loss or transfusion of more than two units of blood were classified as bleeding complication.
Trimodal treatment regimens consisted of an induction chemotherapy of three cycles of a platinum-based therapy with a combination of either pemetrexed or gemcitabine every three weeks. After 4–8 weeks after the last dose of chemotherapy and radiological control of tumour response surgery was performed. Adjuvant radiotherapy was initiated at least 30 days after surgery with a maximum dose of 60–65 Gy.
The EPP was performed using a modified technique of the previously described, by an experienced thoracic surgeon [10]. The chest was entered through an anterolateral thoracotomy in the 5th intercostal space. Additionally to this a second small thoracotomy in the 8th or 9th intercostal space allowed diaphragmatic resection and reconstruction. The tumour was excised by extrapleural dissection and en bloc resection of the pleura, lung, pericardium and hemidiaphragm on the involved side. The pericardium and the diaphragm were replaced by a Polypropylene mesh and sutured with interrupted 1–0 Polypropylene stitches. Formal mediastinal lymph node dissection was carried out to complete the staging. The bronchial stump was covered in every case with a pediculated latissimus dorsi or serratus anterior muscle flap. A single chest tube was left for drainage purpose.
Survival and tumour recurrence-free survival on the basis of tumour recurrence were analysed by Kaplan-Meier survival curves with Wilcoxon test. All results were stated as median and range with p <0.05 defined as statistically significant. For statistical analysis JMP 5.0 (SAS Institute Inc.) was used.
| Table 1: Patients demographics and clinical symptoms. | |||
| Trimodal therapy | Palliative therapy | ||
| Patients | n = 21 | n = 20 | |
| Male sex | n = 18 | n = 18 | |
| Median age in years (range) | 64 (40–75) | 67 (52–83) | |
| Asbestos exposure | n = 15 | n = 13 | |
| Histology | Epithelial | n = 17 | n = 11 |
| Mixed | n = 4 | n = 1 | |
| Sarcomatous | n = 0 | n = 6 | |
| No classification | n = 0 | n = 2 | |
| Clinical symptoms | Pleural effusion | n = 18 | n = 13 |
| Dyspnoea | n = 11 | n = 11 | |
| Chest pain | n = 9 | n = 12 | |
| Coughing | n = 9 | n = 4 | |
| Loss of weight | n = 1 | n = 9 | |
Between 1 October 2000 and 31 December 2005 41 patients with histological proven MPM were referred to our institution for evaluation of a trimodal therapy approach (table 1). After confirmation of the diagnosis by surgical biopsy or percutaneous CT guided biopsy and negative lymph node status with mediastinoscopy, 24 patients (59%) were enrolled, compared with the inclusion criteria of the Swiss Group for Clinical Cancer Research (SAKK) Trial [21] to a trimodal therapy. Three of these patients were excluded because of rapid tumour progression under chemotherapy. The remaining 21 patients in the trimodal therapy group had a median follow-up of 655 days (range 63–2,567 days). The trimodal treatment consisted of neoadjuvant platinum-based chemotherapy, extrapleural pneumonectomy (EPP) and adjuvant postoperative radiotherapy with 60–65 Gy of the involved hemithorax. The remaining 20 patients received palliative treatment due to either sarcomatous type of histology, advanced stage disease or limited functional reserves to undergo radical treatment (fig. 1). In the trimodal treatment group histology was epithelial (n = 17) and mixed type (n = 4). The postoperative pathological tumour staging according to the UICC (Union of International Cancer Control) classification is listed in table 2.

Figure 1
Flow chart showing the patient included in the trimodal or palliative treatment groups.
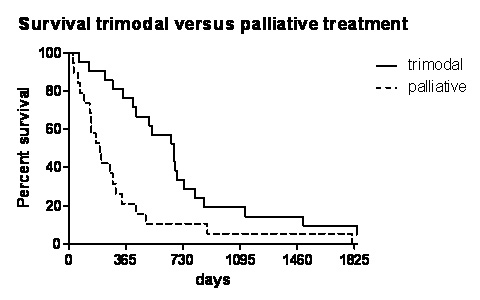
Figure 2
Long-term survival analysis by Kaplan-Meier survival curve of trimodal versus palliative treatment.
The median hospital stay of all 20 patients of the palliative therapy group after surgical biopsy was 7.5 days (range 3–30 days) with a 30- and 90-day mortality of 10% and 20% respectively. During the hospital stay there was a morbidity of 75%. The complications were infections (n = 4, 20%), arrhythmia (n = 4, 20%), respiratory insufficiency (n = 2, 10%), necrotising enterocolitis, cerebral stroke, pericardial effusion, pulmonary embolism and renal failure (each complication n = 1, 5%). Patients with palliative treatment obtained either chemotherapy (n = 13, 65%) with or without radiotherapy (n = 5, 25%).
Prior to the chemotherapy, port-site radiation with 3× 6–7 Gy was applied in the majority of the patients (n = 18, 86%). Neoadjuvant chemotherapy consisted of three cycles with a combination of platinum based agents (n = 19, 90%) and gemcitabine (n = 15, 71%). After the introduction of pemetrexed in 2005 it replaced gemcitabine (n = 4, 19%). The response rate, defined as tumour-regression or radiologically stable disease, was 87.5%. Three patients had tumour progression during chemotherapy and were included in the palliative treatment group. The remaining 21 patients underwent EPP. The median hospital stay after EPP was 16.5 days (range 2–30 days). Postoperative adjuvant radiotherapy with 60–65 Gy was administered in 76% after radical surgery.
After EPP there was a 30-day postoperative morbidity of 62% (table 3). Surgery-related complications occurred in three patients consisting of pericardial patch-avulsion (n = 1, 5%) and postoperative bleeding (n = 2, 10%). Neither bronchopleural fistula nor postoperative pleural empyema were seen. Reoperations were necessary in six patients (29%) because of programmed revision (n = 3, 14%), chest wall infection (n = 1, 5%), bleeding (n = 1, 5%) and patch avulsion (n = 1, 5%). Infectious complications were seen in six patients (29%) including major infections (chest wall infection n = 1, pneumonia n = 1, septicaemia n = 1) and minor infections (urinary tract infection n = 1, wound infection n = 1, no obvious source of infection n = 1). 30-day mortality was 4.8% and 90-day mortality was 9.5% (table 4).
During the first five years of follow up all patients that underwent palliative treatment died of tumour progression and associated co-morbidities. In the trimodal treatment group 20 patients died (95%) during the long term follow up and the majority due to tumour progression (n = 12). The other succumbed of heart failure (n = 3), septical complications (n = 1), haemorrhagic shock (n = 1) and unknown causes of death (n = 3).
Overall 1-year-survival of all patients included in this investigation was 45%. Divided into the two therapy groups patients with trimodal therapy showed significantly better survival rates after 1-, 2- and 5-years respectively (71% vs 21%, 28% vs 5%, 10% vs 0%, p = 0.001) with a median survival of 707 (range 63–2,567) days versus 319 (range 25–1811) days (p = 0.001, fig. 2).
The median survival rates of the trimodal treatment group on the basis of age, histological type, UICC stage and lymph node status showed no differences between the groups (table 5).
During the follow-up, tumour recurrence was detected in 17 patients (81%). Clinical symptoms varied from pleural effusion and dyspnoea (n = 9), ascites (n = 4), pericardial effusion (n = 1), coughing (n = 1), pneumonia (n = 1) and reduced general condition (n = 1). Histological verification of the tumour recurrence was performed in six cases by surgical biopsy, in eleven patients by radiology (computed tomography, positron emission tomography, ultrasound). Local tumour recurrence was observed in 15 patients (71%) and distant tumour recurrence in 11 patients (53%) with affection of the contra lateral lung, liver, peritoneal space, and spine. Tumour-free survival in the trimodal treatment group was 47% after one year and 6% after five years (median tumour-free survival 572 (range 80–2,265) days).
| Table 2: Postoperative tumour staging according to the Union of International Cancer Control (UICC) classification [35]. | ||
| Trimodal therapy | Palliative therapy | |
| UICC I | n = 1 | n = 0 |
| UICC II | n = 0 | n = 1 |
| UICC III | n = 16 | n = 5 |
| UICC IV | n = 4 | n = 8 |
| Table 3: Postoperative minor and major complications of patients after EPP (n = 21). | ||
| Minor complications | Urinary tract infection | n = 1 (4.8%) |
| Wound infection | n = 1 (4.8%) | |
| Infection without source | n = 1 (4.8%) | |
| Major complications | Pneumonia, septicaemia, chest wall infection | n = 3 (14.3%) |
| Bleeding | n = 2 (9.5%) | |
| Reoperations | n = 6 (28.6%) | |
| Arrhythmia | n = 4 (19.1%) | |
| Table 4: Overall mortality after 30 and 90 days after EPP in the trimodal treatment group (n = 21) or surgical biopsy in the palliative treatment group (n = 20). | ||
| 30-day mortality | Palliative therapy | n = 2 (10%) |
| Trimodal therapy | n = 1 (4.8%) | |
| 90-day mortality | Palliative therapy | n = 4 (20%) |
| Trimodal therapy | n = 2 (9.5%) | |
| Table 5: Median survival rates of patients in the trimodal therapy group (n = 21) regarding age, type of histology, UICC stage and lymph node status. | |||
| Median survival in days (range) | [p] | ||
| Age younger vs older 65 years | 660 (126–1,498) | 751 (63–2567) | 0.83 |
| Epithelial vs mixed histology type | 701 (63–2,567) | 690 (520–866) | 0.33 |
| UICC stage III vs IV | 735 (126–2,567) | 432 (63–673) | 0.25 |
| Positive vs negative lymph node status | 765 (63–1,945) | 633 (232–2,567) | 0.77 |
| p <0.05 defined as statistical significant. | |||
In this single centre retrospective investigation a high percentage of patients did not qualify for a trimodal treatment concept including EPP as the tumour at the initial stage was already in an advanced tumour stage. Long term survival after trimodal therapy was only achieved in a minority of patients even though EPP was performed with acceptable morbidity and mortality rates.
MPM is a lethal disease with which thoracic surgeons will have to deal for decades to come [4]. The future outlook is yet unclear and there are only a few objective parameters to foresee the outcome of an individual patient. Untreated MPM is usually associated with a median survival of less than one year and the interval between onset of symptoms and diagnosis has been reported on average at approximately five months [7]. Given the low incidence of the disease and only a few prospective studies regarding therapeutic possibilities there is considerable controversy about the best treatment options even in the early stages of the disease [8, 14, 15, 22].
Since the introduction of EPP for radical surgery with curative intent in the seventies, the mortality and complication rate of this major surgical intervention has fallen noticeably [23]. Mortality after EPP in most reported series is below 10%. Because it represents the maximal possible cytoreduction several authors recommend it as the standard operative approach for therapy aimed to obtain long term disease free survival. Most series; although, report a favourable median survival of around two years with a five year survival of less than 20% in cohorts which have undergone trimodal treatment and therefore reflect the survival rates of trimodal therapy including EPP and not EPP alone. Our longterm survival is in line with the published data and equally low. The fact that we only had a 30-day-mortality underscores that median disease free survival reflects more the disease progression than treatment related mortality. With a median disease free survival of 572 days and 6% survival at five years it is obvious that most patients finally succumb because of their disease despite aggressive treatment. However, a significant number of patients are disease free for a meaningful time and the results are comparable with previously published data and support the hypothesis that EPP in the setting of trimodal therapy possibly achieves a better disease control [6, 24]. Patients with advanced tumour stage or severe comorbidities which did not allow performing radical trimodal therapy were enrolled in the palliative treatment group. Even after thoracoscopic pleurodesis, reflecting the weakened general condition, this group showed a high postoperative morbidity and even mortality.
Trimodal therapy is a resource consuming endeavour and physically and emotionally stressful for the patient. A quite high response rate or stable disease was reported after the preoperative chemotherapy. One reason might be that the radiologists evaluating the CT scans were not specialised thoracic radiologists. Our series demonstrates, as in previous studies, that in selected patients the therapy can be safely done and that induction chemotherapy does not increase the risk of major postoperative complications and is reasonably well tolerated [21, 24, 25]. We strongly support the coverage of bronchial stump with a viable muscle flap to help avoid bronchopleural fistula. We prefer the anterolateral approach which allows preserving the latissimus dorsi muscle. Our complication rate reflects the already published data and is high [10, 11, 18, 19]. However, not all patients were able to undergo adjuvant radiation which is comparable to the most recent prospective study [16].
The longterm outcome of patients undergoing trimodal treatment has so far been reported mostly in retrospective studies [5, 14, 26–28]. Latest prospective reports have now shown that trimodal therapy including EPP was not associated with an improved survival in comparison to patients without radical surgery [14, 15]. A certain bias of the results in our study is inevitable. Prognostically important factors are reported controversially in the literature. Histology, nodal involvement and pathologic negative margins seem to be the most important prognostic factors [5, 27, 29]. Our data is not significant in this respect. A recent prospective trial showed however that response rate to induction therapy is a relevant factor for outcome and long term results after trimodal therapy but there is no evident criteria to assess which patients will profit from this therapeutic option [24].
Recurrence of MPM is almost inevitable and well reported in the literature. Patterns of failure are generally local. However, after trimodal therapy including EPP there seems to be a better local control with a shift towards distant metastasis, but no controlled trial compared different therapy strategies in relation to failure pattern [30]. Even if EPP is considered as a radical resection it is difficult to imagine a complete removal of the tumour and adjuvant local therapy and systemic therapy may contribute to better local control. Recent data suggest that adjuvant Intensity Modulated Radiotherapy (IMRT) may provide better local control [18, 31]. Pleurectomy/Decortication (PD) as less radical surgical procedure with the intent of gross tumour clearance has been shown in selected cases to have advantages due to the generally lower morbidity [32, 33]. A recent randomised study has shown that PD is even superior to EPP in respect to 30-day mortality and 5-year survival [16]. In addition to that another study has shown that the tumour clearance with PD was achievable in 97% of patients in stage III/IV disease, which seems to be quite high [17]. The question of whether radical surgery after induction chemotherapy is better than chemotherapy alone was object of the prospective conducted MARS Trial, which showed that survival after chemotherapy alone was better and less associated with adverse events than with radical surgery [15, 34].
Limitations of our retrospective cohort study should be noted. By nature, this study was performed in a retrospective manner, without the possibility of randomisation towards the trimodal treatment group or palliative group. Despite this, the study investigated the course of all patients referred with MPM without exclusion in one centre, the number of patients was small and the conclusions must be drawn cautiously. Nevertheless, we are convinced that our results reflect the situation in the treatment of patients with MPM in a Swiss tertiary referral centre.
In conclusion, on the basis of this single centre investigation trimodal therapy for selected patients with MPM is feasible and safe with acceptable perioperative morbidity and mortality. The long term outcome however is not satisfactory, despite an increased disease free survival. Local control of the disease is achieved only in a minority of patients by trimodal treatment including induction chemotherapy, radical surgery and radiotherapy and warrants further efforts in better adjuvant radiation techniques and better selection of patients qualifying for this treatment regime. Trimodal therapy including EPP should be performed in the setting of further controlled trials to obtain more solid evidence for the utility of this treatment and to prove any superiority in comparison to other treatment strategies.
1 Pistolesi M, Rusthoven J. Malignant pleural mesothelioma: update, current management, and newer therapeutic strategies. Chest. 2004;126(4):1318–29.
2 Godleski JJ. Role of asbestos in etiology of malignant pleural mesothelioma. Thorac Surg Clin. 2004;14(4):479–87.
3 Mahe MA, Cellerin L, Michaud JL, Sagan C, Supiot S, Le Pechoux C. Recent progress in treatment of malignant pleural mesothelioma. Cancer Radiother. 2005;9(6–7):362–5.
4 Hodgson JT, McElvenny DM, Darnton AJ, Price MJ, Peto J. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br J Cancer. 2005;92(3):587–93.
5 Sugarbaker DJ, Flores RM, Jaklitsch MT, Richards WG, Strauss GM, Corson JM, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg. 1999;117(1):54–63; discussion 63–5.
6 Stewart DJ, Martin-Ucar A, Pilling JE, Edwards JG, O’Byrne KJ, Waller DA. The effect of extent of local resection on patterns of disease progression in malignant pleural mesothelioma. Ann Thorac Surg. 2004;78(1):245–52.
7 Merritt N, Blewett CJ, Miller JD, Bennett WF, Young JE, Urschel JD. Survival after conservative (palliative) management of pleural malignant mesothelioma. J Surg Oncol. 2001;78(3):171–4.
8 Trousse DS, Avaro JP, D’Journo XB, Doddoli C, Astoul P, Giudicelli R, et al. Is malignant pleural mesothelioma a surgical disease? A review of 83 consecutive extra-pleural pneumonectomies. Eur J Cardiothorac Surg. 2009;36(4):759–63.
9 Treasure T, Sedrakyan A. Pleural mesothelioma: little evidence, still time to do trials. Lancet. 2004;364(9440):1183–5.
10 Sugarbaker DJ, Mentzer SJ, Strauss G. Extrapleural pneumonectomy in the treatment of malignant pleural mesothelioma. Ann Thorac Surg. 1992;54(5):941–6.
11 Neragi-Miandoab S. Multimodality approach in management of malignant pleural mesothelioma. Eur J Cardiothorac Surg. 2006;29(1):14–9.
12 Yoshino I, Yamaguchi M, Okamoto T, Ushijima C, Fukuyama Y, Ichinose Y, et al. Multimodal treatment for resectable epithelial type malignant pleural mesothelioma. World J Surg Oncol. 2004;2:11.
13 Yan TD, Boyer M, Tin MM, Wong D, Kennedy C, McLean J, et al. Extrapleural pneumonectomy for malignant pleural mesothelioma: outcomes of treatment and prognostic factors. J Thorac Cardiovasc Surg. 2009;138(3):619–24.
14 van Schil PE, Baas P, Gaafar R, Maat AP, van de Pol M, Hasan B, et al. Trimodality therapy for malignant pleural mesothelioma: results from an EORTC phase II multicentre trial. Eur Resp J. 2010;36:1362–9.
15 Treasure T, Lang-Lazdunski L, Waller D, Bliss JM, Tan C, Entwisle J, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12:763–72.
16 Lang-Lazdunski L, Bille A, Lal R, Cane P, McLean E, Landau D, et al. Pleurectomy/decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J Thorac Oncol. 2012;7:737–43.
17 Friedberg JS, Culligan MJ, Mick R, Stevenson J, Hahn SM, Sterman D, et al. Radical pleurectomy and intraoperative photodynamic therapy for malignant pleural mesothelioma. Ann Thorac Surg. 2012;93:1658–67.
18 Buduhan G, Menon S, Aye R, Louie B, Mehta V, Vallieres E. Trimodality therapy for malignant pleural mesothelioma. Ann Thorac Surg. 2009;88(3):870–5; discussion 876.
19 Opitz I, Kestenholz P, Lardinois D, Muller M, Rousson V, Schneiter D, et al. Incidence and management of complications after neoadjuvant chemotherapy followed by extrapleural pneumonectomy for malignant pleural mesothelioma. Eur J Cardiothorac Surg. 2006;29(4):579–84.
20 Sugarbaker DJ, Jaklitsch MT, Bueno R, Richards W, Lukanich J, Mentzer SJ, et al. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg. 2004;128(1):138–46.
21 Weder W, Kestenholz P, Taverna C, Bodis S, Lardinois D, Jerman M, et al. Neoadjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. J Clin Oncol. 2004;22(17):3451–7.
22 Treasure T, Waller D, Swift S, Peto J. Radical surgery for mesothelioma. BMJ. 2004;328(7434):237–8.
23 Butchart EG, Ashcroft T, Barnsley WC, Holden MP. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax. 1976;31(1):15–24.
24 Krug LM, Pass HI, Rusch VW, Kindler HL, Sugarbaker DJ, Rosenzweig KE, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol. 2009;27(18):3007–13.
25 Flores RM. Induction chemotherapy, extrapleural pneumonectomy, and radiotherapy in the treatment of malignant pleural mesothelioma: the Memorial Sloan-Kettering experience. Lung Cancer. 2005;49(Suppl 1):S71–4.
26 Hasani A, Alvarez JM, Wyatt JM, Bydder S, Millward M, Byrne M, et al. Outcome for patients with malignant pleural mesothelioma referred for Trimodality therapy in Western Australia. J Thorac Oncol. 2009;4(8):1010–6.
27 Flores RM, Zakowski M, Venkatraman E, Krug L, Rosenzweig K, Dycoco J, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol. 2007;2(10):957–65.
28 de Perrot M, Uy K, Anraku M, Tsao MS, Darling G, Waddell TK, et al. Impact of lymph node metastasis on outcome after extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2007;133(1):111–6.
29 Edwards JG, Stewart DJ, Martin-Ucar A, Muller S, Richards C, Waller DA. The pattern of lymph node involvement influences outcome after extrapleural pneumonectomy for malignant mesothelioma. J Thorac Cardiovasc Surg. 2006;131(5):981–7.
30 Janne PA, Baldini EH. Patterns of failure following surgical resection for malignant pleural mesothelioma. Thorac Surg Clin. 2004;14(4):567–73.
31 Rice DC, Stevens CW, Correa AM, Vaporciyan AA, Tsao A, Forster KM, et al. Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg. 2007;84(5):1685–92; discussion 1692–3.
32 Flores RM. Surgical options in malignant pleural mesothelioma: extrapleural pneumonectomy or pleurectomy/decortication. Semin Thorac Cardiovasc Surg. 2009;21(2):149–53.
33 Flores RM, Pass HI, Seshan VE, Dycoco J, Zakowski M, Carbone M, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg. 2008;135(3):620–6, 6 e1–3.
34 Treasure T, Waller D, Tan C, Entwisle J, O’Brien M, O’Byrne K, et al. The mesothelioma and radical surgery randomized controlled trial: the Mars feasibility study. J Thorac Oncol. 2009;4(10):1254–8.
35 Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M (eds). AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.