Carotid intima-media thickness as a biomarker of subclinical atherosclerosis
DOI: https://doi.org/10.4414/smw.2012.13705
Marcus
Bauer, Seraina
Caviezel, Alexandra
Teynor, Raimund
Erbel, Amir A
Mahabadi, Arno
Schmidt-Trucksäss
Summary
Intima-media thickness of the carotid artery (CIMT) and its increase is associated with several cardiovascular risk factors and manifest cardiovascular diseases. CIMT is suggested to be an important biomarker of subclinical atherosclerosis. CIMT is measured in B-mode ultrasound images of the carotid tree as a typical double line of the arterial wall. CIMT is best visible in the measurement segment of the distal common carotid artery with lowest measurement variability. The measurement is most reliable over a one centimeter-segment with automatic or semi-automatic reading methods, which minimises reading errors. Further structured training of sonographer and reader is important for valid and reproducible results.
CIMT is an accepted predictor for future cardiovascular events independent of age, gender and cardiovascular risk factors. Measurement seems to be best applicable in patients with intermediate risk in order to readjust cardiovascular risk. Plaques in the carotid tree and thickening of the CIMT are different atherosclerotic processes. From childhood to early adulthood CIMT is the only atherosclerotic marker of the carotid tree; plaques occur later in life. Both parameters contribute independently to risk assessment for future cardio-vascular events.
Aims of this review are to outline measurement procedures, reproducibility, prognostic value and ability to discriminate healthy subject and patients with manifest disease in a practical and scientifically contemporary manner.
Abbreviations
CB Carotid bifurcation
CCA Common carotid artery
CIMT Carotid intima media thickness
ECA External carotid artery
ICA Internal carotid artery
Introduction
Carotid intima-media thickness (CIMT), recorded with B-mode sonography, is an important marker to quantify atherosclerotic burden in the common carotid artery (CCA). The last twenty years, the value of CIMT-measurement for risk estimation of atherosclerotic events (for e.g., myocardial infarction, stroke, sudden cardiac death) increased more than ever.
The aim of this review article is to (1.) present anatomical and histological background of the vessel wall structure; (2.) introduce the history of CIMT-imaging and (3.) demonstrate aspects of reproducibility. In addition to show (4.) prospective value in regard to cardiovascular endpoints and medical treatment; (5.) the power of discrimination between healthy and pathological values; (6.) the additional benefit of CIMT-measurement compared to established cardiovascular risk stratification algorithms and (7.) its implementation in daily clinical practice.
Anatomy and histology
The right CCA has its source in the Truncus brachiocephalicus, while the left CCA originates from the aortic arch. In its cervical course the CCA is medial located, slightly behind the internal jugular vein [1]. The carotid bifurcation (CB) into the internal (ICA) and external carotid artery (ECA) is located at the level of the fourth cervical vertebra [1]. The carotid artery has a superficial course, so that ultrasound examinations usually can be performed without bigger problems. In some cases the variability of the location of the carotid bulb makes the identification of the ICA and ECA somewhat difficult.
The histological mural structure of the CCA is composed of three layers:
1. Intima (Tunica intima): inner layer; monolayer of endothelial cells; between Tunica intima and Tunica media: internal elastic lamina.
2. Media (Tunica media): mainly composed of longitudinal smooth muscle cells, surrounded by connective tissue; containing elastic lamella that provides elastic property of vessels.
3. Adventitia (Tunica externa / adventitia): outer layer; generally embedded in the circumjacent tissue.
Histopathology
Atherosclerosis is a systemic and chronic inflammatory disease, which may cause cardiovascular disease, the most frequent cause of death in the world [2]. A long term thesis in the development of atherosclerosis was the “response-to-injury” theory [3], in which a physical injury of endothelium was considered to be responsible for atherosclerotic changes of vessel walls. This view was completed in the last three decades, since endothelial dysfunction was considered to be a functional trigger [4, 5]. Briefly, the infiltration of LDL-cholesterol through the endothelium, LDL-deposition in the intima and the following oxidative and enzymatic processes have been described [6]. Hence, the intima-media complex of arterial walls plays an essential role in the pathogenesis of atherosclerosis and may reflect different stages in the development of the disease: a hypertensive hypertrophic response of medial cells can be observed in early phases of atherosclerosis (quantified by CIMT-measurement), while carotid plaque formation are often seen in later stages of atherosclerosis, which may be caused by inflammation, oxidation, endothelial dysfunction, and/or smooth muscle cell proliferation [7]. An increased CIMT is typically seen at the CCA, while carotid plaque formation are more frequent at the CB or ICA. Plaque formation at the CB or ICA are more associated with hyperlipidaemia and MI, while an increased CIMT at the CCA shows a stronger relationship to hypertension and stroke [8].
History of CIMT-measurements
Already in the 1980’s Pignoli et al. could demonstrate a highly significant association between histological findings of the CCA and respective ultrasound examinations [9]. Since then, associations between CIMT and a) traditional and non-traditional cardiovascular risk factors, b) the extent and severity of atherosclerosis c) as well as cardio- and cerebrovascular events (outcome) had been examined and described in different studies (4–6; table 1). Regarding cardiovascular risk factors, an increased age has the most impact on an increased CIMT: depending on different studies, age may explain 50%–80% of the variability for an increased CIMT. It has been described an annual increase of CIMT in the CCA about 0,007 mm [10], with nearly a 5 year delay in women compared to men of the same age.
|
Table 1: Prospective studies about the prediction of cardiovascular events via CIMT-measurement in subjects without manifest cardiovascular atherosclerotic disease. HR = hazard ratio; RR = relative risk; CCA = common carotid artery; CB = carotid bifurcation; ICA = internal carotid artery); * = follow-up [11]. |
|
Author
|
Study
|
Participants
|
Age
|
Follow-up
|
Endpoints
|
Region of interest
|
Risk estimation
|
Comment
|
| Chambless et al. 1997 [11] |
ARIC |
12,841 |
45–64 years |
4–7 years |
Fatal and non-fatal cardiovascular events |
CCA, CB, ICA |
For CIMT >1 vs. <1 mm, HR adjusted for Diabetes, HDL-, LDL-cholesterol, hypertension, smoking status, study center, age and race 1.18 (1.06–1.32) in men and 1.42 (1.24–1.64) in women. |
CIMT = mean CIMT from 6 different segments |
| O’Leary et al. 1999 [12] |
CHS |
4,476 |
72.5 years (mean age at beginning of the study) |
6.2 years (median) |
Myocardial infarction, stroke, combination |
CCA, ICA |
RR-combined endpoint (adjusted for age, gender, blood pressure (sys, dia), smoking (pack years), Diabetes, atrial fibrillation) for max. CIMT CCA: 2.22 (1.58-3.13) for >1.18 mm (highest) vs. <0.87 mm (lowest CIMT-quintile); 2.47 (1.59–3.85) for >1.81 mm (highest) vs. <0.90 mm (lowest CIMT-quintile). |
Primary measurement value: max. CIMT |
| Iglesias del Sol et al. 2002 [13] |
Rotterdam |
1,721 |
>55 years |
4.6 years (mean age) |
Cardio- or cerebro-vascular disease |
CCA, CB, ICA |
RR 1.41 (95% CI, 1.25–1.82) for stroke and 1.43 (95% CI, 1.16–1.78) for myocardial infarction. |
Primary measurement value: max. CIMT; separated for different segments |
| Lorenz et al. 2006 [14] |
CAPS |
5,056 |
19–90 years; mean age 50.1 years |
4.2 years (mean age) |
Myocardial infarction, stroke, combined endpoint |
CCA, CB, ICA |
HR (adjusted for risk factors) for CIMT-CCA >0.79mm vs. <0.63 mm 1.85 (1.09–3.15), for CIMT-CB >0.79mm vs. <0.63 mm 1.27 (0.80–1.99), for CIMT-ICA >0.79 mm vs. <0.63 mm 1.25 (0.84–1.86) for combined endpoint. |
Mean CIMT; far wall |
| *Nambi et al. 2010 [15] |
ARIC |
13,145 |
45–64 years |
15.1 years (mean age) |
Fatal and non-fatal cardiovascular events, revascularisation |
CCA, CB, ICA |
Men: CIMT increased AUC from 0.674 (only risk factors) up to 0.690 (95% CI for difference of the adjusted AUC: 0.009–0.022).
women: CIMT increased AUC from 0.759 (only risk factors) up to 0.762 (95% CI for difference of the adjusted AUC: –0.002–0.006). |
CIMT = mean CIMT from 6 different segments |
| Plichart et al. 2011 [16] |
Three-City |
5,895 |
65-85 years; mean age 73.3 years |
5.4 years (mean age) |
New diagnosed coronary heart disease, fatal cardiovascular event |
CCA |
HR 5. vs. 1. quintile = 0.8; 95% CI = 0.5–1.2; p for trend <0.48) (adjusted for risk factors). |
Mean CIMT; far wall; plaque-free segment |
Methods exposure
B-mode sonography of the carotid artery is a safe, cheap, quick and painless examination, free of radiation exposure to the patient and enables a detailed evaluation of different regions of the carotid artery. Currently, B-mode sonography of the carotid artery mainly allows a noninvasive visualisation and assessment of arterial wall changes via measurement and quantification of a) CIMT and b) carotid plaque formation:
a) CIMT is defined as the viewable distance between the lumen-intima- and the media- adventitia interface (see fig. 1).
b) Carotid plaque formation differ from CIMT (as defined) by an increase of CIMT about at least 0.5 mm or an increase about 50% compared the adjacent CIMT as well as an increase of thickness about more than 1.5 mm [17].
Another possibility is to regard CIMT as a continuous distance that also integrates those vessel regions in CIMT-measurements that are defined as “plaque formation”. In those cases CIMT is defined as mean CIMT in CCA or the mean maximum CIMT in the CCA, the CB and / or the ICA [18].
Proposals for a standardised preparation and performance of carotid ultrasound examinations
Preparation
1. Dimly lit room;
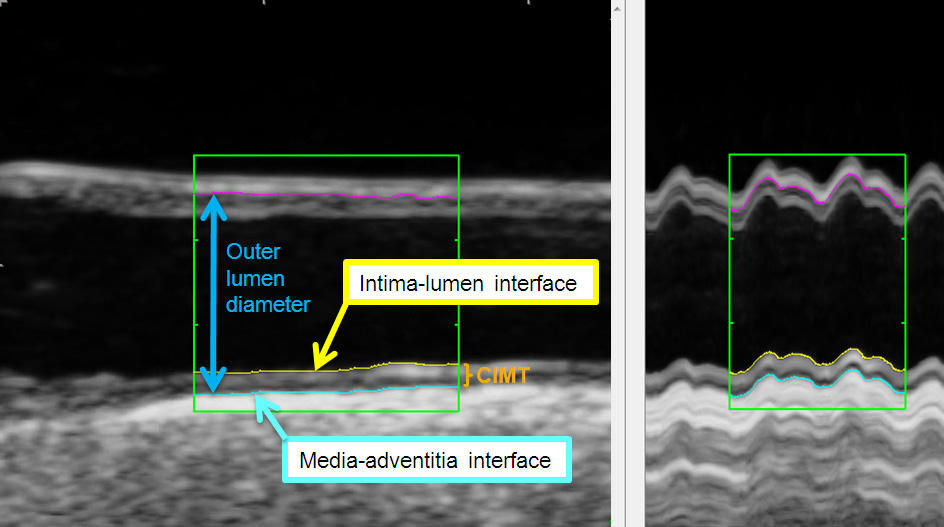
Figure 1
Ultrasound image of the common carotid artery (longitudinal axis) with tracing lines of automatic contour-detection at the lumen-intima- (yellow line) and the media- adventitia interface (blue line). Outer lumen diameter (between blue- and pink-coloured line) in the common carotid artery in B-mode (left) over two heart cycles and with M-mode (right) generated by 180 single images of a this clip. The mean CIMT at the far wall was 0.87 mm.
2. Room temperature 22–25° C,
3. Lying position;
4. Examination position: end-of-heading; with neck extended;
5. ECG-electrodes (control of heart cycles) [20];
6. Standardised head-position: (45°–50°-position to the right / left) ;
7. Minimum requirements transducer: ultrasound frequencies 5–15 MHz (better: 10 MHz linear ultrasound transducer), appropriate depth of focus;
8. Refresh rate: ≥25 Hz (minimal compression);
9. Gain: ~60 dB.
Performance
1. Sequential-based records over 3–4 heart cycles (longitudinal axis);
2. Optimal image-adjustment: visualising the double line pattern of the carotid artery (near and far wall of the CCA);
3. Record of at least three fixed images (longitudinal axis) → optimal: vertical dipping ultrasound rays (artery in horizontal focus);
4. End-diastolic records of images /sequences (see “Preparation”);
5. Image-acquisition: ICA and ECA
Using Pulse waved Doppler to distinguish between ICA and ECA;
ICA: greater caliber than ECA;
ICA: no extracranial vessel branches (ECA: supine thyroid artery).
6. Sequential-based record of the vessel diameter (detection plaque formation). Record of carotid plaque formation (longitudinal and transversal).
An ultrasound arc (see “Meijer-Arc”) can be used for longitudinal-records in anterior, middle and posterior position.
Different methods of CIMT-measurement
Originally, CIMT-measurements were performed via a manual method that could be integrated in ultrasound systems or per additional acquired software. However, the last few years it could be demonstrated that this method is associated with a higher reader-subjectivity compared to automatic or semiautomatic (automatic + manual correction) measurement software [21]. These can be implemented in the ultrasound system or can base a) on an image-analysis (contour-detection) (fig. 1, [22]) or b) on the analysis of radiofrequency signals. The radiofrequency – analysis is performed with single images or continuously over more heart cycles.
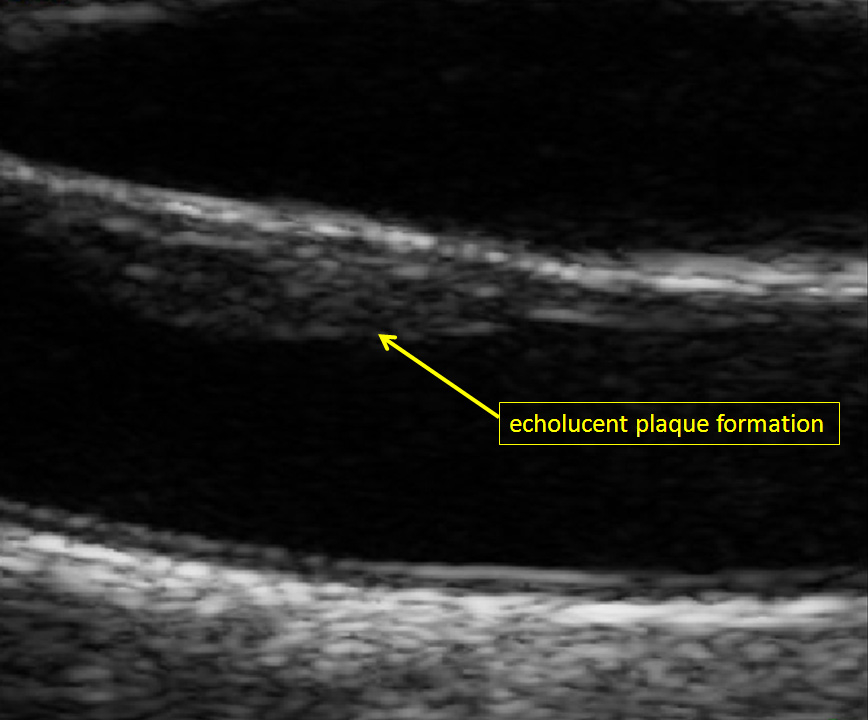
Figure 2
Echolucent carotid plaque formation at the near wall of the right common carotid artery (male study participant of the population-based Heinz Nixdorf Recall study) [19].
It has been shown that sequentially-based CIMT-measurement contributes to an improved differentiation between subjects with and without coronary heart disease, compared to the analysis of a single image (AUC for mean CIMT 0.82 [95% CI 0.68–0.94] versus 0.64 [95% CI 0.55–0.80]) [23].
CIMT-measurement is possible in different segments of the carotid artery: in the CCA, CIMT is measured automatically over a distance about one cm [24]. Further vessel segments, like the ICA and ECA or CB are commonly only depictable over a limited distance. Therefore, maximum CIMT is measured in these segments. Up to now, the different measurement-localisations and -methods resulted in inconsistent CIMT-protocols. The limited standardisation is the vulnerable weakness of this non-imaging method.
Per electrocardiogram it is possible to standardise the CIMT-measurement during a complete heart cycle (R-wave) which is especially important in single images, because CIMT may vary between 5%–10% during a heartbeat [22].
Regarding the R-wave in the electrocardiogram, the wave represents the end-diastolic moment – the moment of the thickest CIMT [20, 25].
Utilisation of Meijer’s carotid arc is a further instrument to optimise reproducibility of CIMT-measurements over specific carotid segments and time. This semicircle is divided into 30° angles one after another (120°, 150°, 180°, …) and was used in different studies (e.g., METEOR study) to optimise data acquisition [26]. Best visibility has been observed at semi-lateral angles, thus left side at 120°–150° and right side at 210°–240° angle of insonation.
Reproducibility
Measurement-variability of CIMT depends on the carotid segment that is examined. The inter-observer variability of maximum CIMT in the CCA was found to be 0.14 ± 0.16 mm and 0.13 ± 011 mm for mean CIMT. The inter-observer variability is reported to be slightly higher (0.20 ± 0.26 mm and 0.18 ± 0.24 mm, respectively) [27]. In repetitive CIMT-measurements of the CCA the absolute mean difference in the CCA was only 20%–25% of ICA or the CB (0.11 ± 0.08 mm vs 0.60 up to 0.66 mm) [28]. Due to the speckle pattern caused by the differences of acoustic impedance between wall components the near wall of the CCA and CB have a slightly higher CIMT-variability compared to the far wall [29].
Compared to CB (76% up to 96%) and ICA (54% up to 81%) the CCA can be depicted in nearly all patients (94% up to 99%). This applies also for the far wall compared to near wall (CCA 97% vs 88%, CB 87% vs 80% and ICA 76% vs 49%) [30, 31]. Additionally, anatomic conditions and especially the experience of the reader influence the visibility considerably.
Normal CIMT-values
Classification of CIMT-values in healthy and pathological values varies considerably, depending on the CIMT measurement protocol. Therefore, conclusions from measured CIMT must be drawn, by consideration of the underlying protocol. Every reader should pay attention that the same measurement value in two different studies may be considered as normal on one and as pathologic on the other hand.
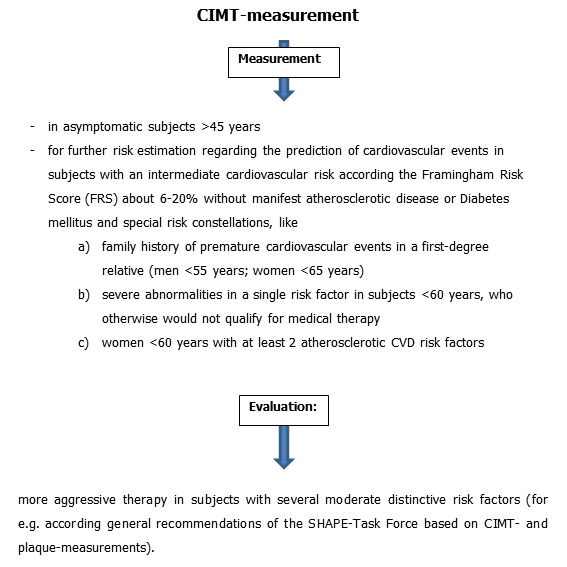
Figure 3
Potential implementation of CIMT-measurements in the daily clinical practice. General recommendation of SHAPE-Task Force [8].
Nowadays, there are two main possibilities for the evaluation of CIMT-values: the utilisation of a) fixed cut-off values or b) percentile-distribution. For fixed cut-off values, a prognostic value for prediction of future cardiovascular events has been established [11, 16, 32].
Fixed CIMT-values ≥1 mm were described in the literature, however, such high values are not achieved in different studies which can be explained by in- or exclusion of plaque formation (see also page 3) [10, 33, 34]. The differences in observations have resulted in the utilisation of percentile-distributions [35]. Both, fixed cut-off- and percentile values have been previously used based on different measurement protocols [36]. Like other markers of subclinical atherosclerosis (e.g., coronary artery calcium, ankle brachial index), CIMT increases with an increase in age [10]. In contrast to other well-known cardiovascular risk factors, normal and pathological values differ over the years. Hence, it is reasonable to provide gender and age-dependent CIMT-values in consideration of the CIMT-protocol. Generally, Stein et al. provided very detailed information about gender- and aged-stratified CIMT-values in different international studies and the respective measured carotid segments [36]. In order to simplify these values, Jäger et al. developed an equation for calculating an individual IMT threshold value (mm) from calculations of average CIMT as a function of age: decade of life/10 + 0.2 mm [37]. CIMT-based percentile-values are supplied on the homepage of the Heinz Nixdorf Recall study for a general population between 45 and 75 years [19].
CIMT-Progression
CIMT-progression has been examined in different studies and depends on included regions of CIMT-measurement [10, 38]. Recently, Lorenz et al. included n = 36.984 participants from 16 different studies in a meta-analysis to investigate CIMT-progression as a predictor of cardiovascular events; the association remained unproven [39]. However, due to well-known histopathological mechanisms in the intima-media complex (see above) and calculated cardiovascular risks being associated with an increased CIMT, the question of CIMT-interference via medical treatment arose in the last decade. Therefore, in many studies CIMT-regression and –progression, induced by medical treatment of lipid metabolism, were conducted. It could be demonstrated that a 40-mg dose of rosuvastatin significantly decreased maximum CIMT-progression over 12 carotid segments (common carotid, carotid bulb, internal carotid) in middle-aged individuals [38]. Based on these METEOR-results, the predictive value of CIMT-measurement as a detection and monitoring tool in subjects with low Framingham risk score was shown [38]. The effect of other different LDL-lowering therapies on the regression of CIMT could be proven in different studies [40–42]. Furthermore, in some studies the association between different statins and CIMT-regression were examined [43, 44]. CIMT-measurement, used as a monitoring tool in lipid-lowering studies, was subjected to a practical test and passed it, when Kastelein et al. examined the “null-effect” of LDL-cholesterol: A combined therapy with ezetimibe and simvastatin did not result in a significant difference in changes in intima-media thickness, as compared with simvastatin alone [45]. Despite many comments in public, Brown et al. pointed out, that different preconditions led to the observed results [46].
Benefit of CIMT-measurements in addition to traditional and established cardiovascular risk stratification algorithms
Actual cardiovascular risk stratification relies on risk calculations that are based on the inclusion of different traditional and established risk factors (e.g., Framingham Risk Score, European HEART Score). However, the solely utilisation of these risk scores is afflicted with different problems. There is a variation in risk estimation in different populations [47], different endpoints in risk algorithms are evaluated (e.g., coronary morbidity, CV-mortality), many important lifestyle and other risk factors are not included in stratification algorithms [48], and cross-sectional risk assessment does not account for variation in risk factor exposure.
Up to today, risk factors are used to classify cardiovascular risk as low, intermediate and high risk. Subjects with a low risk are recommended to modify risk factors, while high-risk subjects receive medical therapy. The management for subjects with an intermediate risk is unsettled. These persons are recommended for further risk assessment including new markers of a subclinical atherosclerosis (for example CIMT-measurement). However, the value of CIMT-measurement in the cardiovascular risk stratification is still under debate.
For reclassification of cardiovascular risk via CIMT-measurement (according the Framingham Risk Score), the investigators of the Atherosclerosis Risk in Communities Study (ARIC-Study) provided data on 13,145 subjects (mean observation: 15.1 years) that were observed as regard to the onset of acute myocardial infarction, coronary death and cardiovascular revascularisation [15]. A total of 16.7% subjects with intermediate risk (5%–20% 10-year risk) could be reclassified.
Using CIMT- and carotid plaque-measurement in all carotid segments, a total of 9.9% subjects could be reclassified. In subjects with an intermediate risk (5%–20%) 12.4% persons were reclassified in the low risk group and 10.8% in the high risk group. The number of reclassification was considerably lower in the Carotid Atherosclerosis Progression Study (CAPS-Study) (5.3%) [49]. However, in this study cardiovascular risk has been evaluated according the European Heart Score that calculates fatal cardiovascular events as the outcome [49]. So the data are not comparable with each other.
For subjects with a 10-year risk for cardiovascular events of 6%–20%, the American Heart Association (AHA) finds CIMT-measurements reasonable. Hence, CIMT-measurement is one of the few biomarkers that is attributed to the class IIa / level of evidence B. However, suitable technical equipment and a profound course of instruction and experience of the reader are conditions to keep up a high quality of the measurement methodology [50].
Whether an increased CIMT is able to support correct clinical decision making and lead to specified anti-atherosclerotic therapy has not been investigated in meaningful endpoint-studies until today. Additionally, appropriate clinical guidelines, with inclusion of CIMT-measurements in the treatment of patients as well as proof of cost-effectiveness are still missing.
|
Table 2: Potential clinical implementation of CIMT-measurements with respective suggestions of therapy.Lifestyle modification = lifestyle modifications and a LDL cholesterol target of <130 mg/dl (<3.37 mmol/l); targeting to <100 mg/dl (<2.59 mmol/l) is optional. Aggressive lifestyle modification = lifestyle modifications and a LDL cholesterol target of <100 mg/dl (<2.59 mmol/l); targeting to <70 mg/dl (<1.82 mmol/l) is optional. |
| |
CIMT
|
Carotid plaque formation
|
Therapy
|
LDL
|
|
Moderate high risk
|
<1 mm and 50.–75. percentile |
No plaque |
Lifestyle modification |
<130 mg/dl (<3.37 mmol/l) |
|
High risk
|
≥1 mm or >75. percentile |
<50% stenosis |
Agressive lifestyle modification |
<100 mg/dl (<2.59 mmol/l) |
|
Very high risk
|
≥1 mm or >75. percentile |
≥50% stenosis |
Agressive lifestyle modification |
<70 mg/dl (<1.82 mmol/l) myocardial ischemic test |
Possible implementation of CIMT-measurements in the daily clinical practice, based on a combination of ASE-, AHA- and SHAPE-Task Force-recommendations (table 2)
Quantification of carotid plaque formation or CIMT-measurement
Embedding carotid plaque formation in CIMT-measurements is debatable, since they are reported to be different biological and genetic atherosclerotic phenotypes [51]. It is certain that plaque formation is always pathologic. In a meta-analysis about 11 population-based studies (n = 54,336 subjects) carotid plaque formation had a significantly higher diagnostic precision in the prediction of myocardial infarction compared to an increased CIMT (AUC 0.64 vs. 0.61) [52]. The additive inclusion of plaque formation in CIMT-measurements may improve risk prediction of coronary heart disease [15].
The last three decades, quantification and evaluation of carotid plaque formation has changed remarkably. In addition to cursory assessments like degree of stenosis and echogenicity, other distinctive features have been used to investigate carotid plaques. The most common used criteria for plaque investigation are echogenicity (echolucent, echogenic, mixed echogenicity), echogenic distribution pattern (homogeneous versus inhomogeneous) and evaluation of surface structure (regular versus irregular). Furthermore, measurement of two dimensions (2D) and three-dimensions (3D) are used to quantify total plaque area and total plaque volume [53, 54]. Lastly, plaque vascularisation on contrast-enhanced ultrasound are developed to optimise cardiovascular risk prediction [55, 56]. Because the prevalence of carotid plaques in a population at 60 years is 60%–90% [57] it seems to be of additional benefit for risk prediction to take this different ultrasound derived pattern into consideration at least in an elderly population. Finally both, quantification of CIMT and carotid plaque formation provide different information of the atherosclerotic status and burden in the carotid artery. Taken together these two parameters have been shown to result in a superior risk prediction for coronary heart disease than with one of the parameters alone [15].
Conclusion
CIMT-measurement and plaque detection are already established measurement methods for detection of subclinical atherosclerosis in many studies. However, solid and efficient trainings of sonographer and reader are required for sufficient CIMT-quantification in studies as well as daily clinical practice. Among different CIMT- measurement methods, automatically based methods show the highest reproducibility. To differ between normal and pathological CIMT values an exact consideration of the underlying measurement protocol is obligatory. CIMT-measurement is a suitable method for an improvement of risk stratification in subjects with an intermediate risk factor profile above traditional atherosclerotic risk factors. The combination of CIMT-measurement and quantification of carotid plaque formation further increases the predictive value for first cardiovascular events.
References
1 Maier H. Präparierkurs Präparieranweisungen und Theorie. 2., erw. Aufl. Köln: Deutscher Ärzte-Verlag; 1987.
2 Faxon DP, Creager MA, Smith SC Jr, Pasternak RC, Olin JW, Bettmann MA, et al. Atherosclerotic Vascular Disease Conference: executive summary: Atherosclerotic Vascular Disease Conference proceeding for healthcare professionals from a special writing group of the American Heart Association. Circulation. 2004;109(21):2595–604.
3 Ross R, Faggiotto A, Bowen-Pope D, Raines E. The role of endothelial injury and platelet and macrophage interactions in atherosclerosis. Circulation. 1984;70(5 Pt 2):III77–82.
4 Gossl M, Lerman LO, Lerman A. Frontiers in nephrology: early atherosclerosis – a view beyond the lumen. J Am Soc Nephrol. 2007;18(11):2836–242.
5 Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17(11):1410–22.
6 Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–95.
7 Hegele RA. The pathogenesis of atherosclerosis. Clin Chim Acta. 1996;246(1–2):21–38.
8 Johnsen SH, Mathiesen EB. Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Curr Cardiol Rep. 2009;11(1):21–7.
9 Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74(6):1399–406.
10 Bauer M, Möhlenkamp S, Lehmann N, Schmermund A, Roggenbuck U, Moebus S, et al. The effect of age and risk factors on coronary and carotid artery atherosclerotic burden in males – results of the Heinz Nixdorf Recall Study. Atherosclerosis. 2009;205(2):595–602.
11 Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146(6):483–94.
12 O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340(1):14–22.
13 Iglesias del Sol A, Bots ML, Grobbee DE, Hofman A, Witteman JCM. Carotid intima-media thickness at different sites: relation to incident myocardial infarction. The Rotterdam Study. Eur Heart J. 2002;23(12):934–40.
14 Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS). Stroke. 2006;37(1):87–92.
15 Nambi V, Chambless L, Folsom AR, He M, Hu Y, Mosley T, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55(15):1600–7.
16 Plichart M, Celermajer DS, Zureik M, Helmer C, Jouven X, Ritchie K, et al. Carotid intima-media thickness in plaque-free site, carotid plaques and coronary heart disease risk prediction in older adults. The Three-City Study. Atherosclerosis. 2011;219(2):917–24.
17 Touboul P-J, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness consensus (2004–2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23(1):75–80.
18 Dogan S, Kastelein JJP, Grobbee DE, Bots ML. Mean common or mean maximum carotid intima-media thickness as primary outcome in lipid-modifying intervention studies. J Atheroscler Thromb. 2011;18(11):946–57.
19 Heinz Nixdorf Recall Studie [Internet]. [cited 2012 Jan 10].
20 Oren A, Vos LE, Uiterwaal CSPM, Grobbee DE, Bots ML. Cardiovascular risk factors and increased carotid intima-media thickness in healthy young adults: the Atherosclerosis Risk in Young Adults (ARYA) Study. Arch Intern Med. 2003;163(15):1787–92.
21 Kanters SD, Algra A, van Leeuwen MS, Banga JD. Reproducibility of in vivo carotid intima-media thickness measurements: a review. Stroke. 1997;28(3):665–71.
22 Teynor A, Caviezel S, Dratva J, Künzli N, Schmidt-Trucksäss A. An automated, interactive analysis system for ultrasound sequences of the common carotid artery. Ultrasound Med. Biol. 2012;38(8):1440–50.
23 Haller C, Schulz J, Schmidt-Trucksäss A, Burkardt H, Schmitz D, Dickhuth H-H, et al. Sequential based analysis of Intima-Media Thickness (IMT) in common carotid artery studies. Atherosclerosis. 2007;195(2):e203–e209.
24 Cheng D, Schmidt-Trucksäss A, Cheng K, Burkhardt H. Using snakes to detect the intimal and adventitial layers of the common carotid artery wall in sonographic images. Comput Methods Programs Biomed. 2002;67(1):27–37.
25 Jourdan C, Wühl E, Litwin M, Fahr K, Trelewicz J, Jobs K, et al. Normative values for intima-media thickness and distensibility of large arteries in healthy adolescents. J Hypertens. 2005;23(9):1707–15.
26 Bots ML, Evans GW, Riley WA, Grobbee DE. Carotid intima-media thickness measurements in intervention studies: design options, progression rates, and sample size considerations: a point of view. Stroke. 2003;34(12):2985.
27 O’Leary DH, Polak JF, Wolfson SK, Bond MG, Bommer W, Sheth S, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22(9):1155–63.
28 Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness. Circulation. 2007;115(4):459–67.
29 Stensland-Bugge E, Bønaa KH, Joakimsen O. Reproducibility of ultrasonographically determined intima-media thickness is dependent on arterial wall thickness. The Tromsø Study. Stroke. 1997;28(10):1972–80.
30 Montauban van Swijndregt AD, De Lange EE, De Groot E, Ackerstaff RG. An in vivo evaluation of the reproducibility of intima-media thickness measurements of the carotid artery segments using B-mode ultrasound. Ultrasound in Medicine & Biology. 1999;25(3):323–30.
31 Espeland MA, Craven TE, Riley WA, Corson J, Romont A, Furberg CD. Reliability of longitudinal ultrasonographic measurements of carotid intimal-medial thicknesses. Stroke. 1996;27(3):480–5.
32 Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: The Rotterdam Study. Circulation. 1997;96(5):1432–7.
33 Salonen J, Salonen R. Ultrasonographically assessed carotid morphology and the risk of coronary heart disease. Arterioscler Thromb Vasc Biol. 1991;11(5):1245–9.
34 Chambless LE, Folsom AR, Clegg LX, Sharrett AR, Shahar E, Nieto FJ, et al. Carotid wall thickness is predictive of incident clinical stroke: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 2000;151(5):478–87.
35 Naghavi M, Falk E, Hecht HS, Jamieson MJ, Kaul S, Berman D, et al. From vulnerable plaque to vulnerable patient—part III: Executive Summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force Report. Am J Cardiol. 2006;98(2, Supplement 1):2–15.
36 Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21(2):93–111.
37 Jäger KA, Staub D. Did you measure the intima-media thickness? Ultraschall Med. 2009;30(5):434–7.
38 Crouse JR 3rd, Raichlen JS, Riley WA, Evans GW, Palmer MK, O’Leary DH, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA. 2007;297(12):1344–53.
39 Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Völzke H, Tuomainen T-P, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012;379(9831):2053–62.
40 Salonen R, Nyyssönen K, Porkkala E, Rummukainen J, Belder R, Park JS, et al. Kuopio Atherosclerosis Prevention Study (KAPS). A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation. 1995;92(7):1758–64.
41 Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu C, Liu C, et al. Reduction in carotid arterial wall thickness using lovastatin and dietary therapy: a randomized controlled clinical trial. Ann Intern Med. 1996;124(6):548–56.
42 de Groot E, Jukema JW, van Boven AJ, Reiber JH, Zwinderman AH, Lie KI, et al. Effect of pravastatin on progression and regression of coronary atherosclerosis and vessel wall changes in carotid and femoral arteries: a report from the Regression Growth Evaluation Statin Study. Am J Cardiol. 1995;76(9):40C–46C.
43 Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN. ARBITER: Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation. 2002;106(16):2055–60.
44 Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet. 2001;357(9256):577–81.
45 Kastelein JJP, Akdim F, Stroes ESG, Zwinderman AH, Bots ML, Stalenhoef AFH, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358(14):1431–43.
46 Brown BG, Taylor AJ. Does ENHANCE diminish confidence in lowering LDL or in ezetimibe? N Engl J Med. 2008;358(14):1504–7.
47 Hense H-W, Schulte H, Löwel H, Assmann G, Keil U. Framingham risk function overestimates risk of coronary heart disease in men and women from Germany – results from the MONICA Augsburg and the PROCAM cohorts. Eur Heart J. 2003;24(10):937–45.
48 Mozaffarian D, Wilson PWF, Kannel WB. Beyond established and novel risk factors lifestyle risk factors for cardiovascular disease. Circulation. 2008;117(23):3031–8.
49 Lorenz MW, Schaefer C, Steinmetz H, Sitzer M. Is carotid intima media thickness useful for individual prediction of cardiovascular risk? Ten-year results from the Carotid Atherosclerosis Progression Study (CAPS). Eur Heart J. 2010;31(16):2041–8.
50 Greenland P, Lauer MS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA Guideline for Assessment of Cardiovascular Risk in Asymptomatic Adults. Circulation. 2010;122(25):e584–e636.
51 Spence J. The importance of distinguishing between diffuse carotid intima-media thickening and focal plaque. Can J Cardiol. 2008;24:61C–64C.
52 Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: A meta-analysis. Atherosclerosis. 2012;220(1):128–33.
53 Spence JD, Hegele RA. Non-invasive assessment of atherosclerosis risk. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4(2):125–8.
54 Landry A, Spence JD, Fenster A. Quantification of carotid plaque volume measurements using 3D ultrasound imaging. Ultrasound Med Biol. 2005;31(6):751–62.
55 Staub D, Partovi S, Schinkel AFL, Coll B, Uthoff H, Aschwanden M, et al. Correlation of carotid artery atherosclerotic lesion echogenicity and severity at standard US with intraplaque neovascularization detected at contrast-enhanced US. Radiology. 2011;258(2):618–26.
56 Staub D, Patel MB, Tibrewala A, Ludden D, Johnson M, Espinosa P, et al. Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke. 2010;41(1):41–7.
57 Joakimsen O, Bonaa KH, Stensland-Bugge E, Jacobsen BK. Age and sex differences in the distribution and ultrasound morphology of carotid atherosclerosis: the Tromsø Study. Arterioscler Thromb Vasc Biol. 1999;19(12):3007–13.


