DOI: https://doi.org/10.4414/smw.2012.13699
Abbreviations:
NFPA Non-functioning pituitary adenomas
iMRI Intraoperative magnetic resonance imaging
PA Pituitary adenoma
CT Computed tomography
GH Growth hormone
ACTH Adrenocorticotropin hormone
TSH Thyroid stimulating hormone
LH Luteinising hormone
FSH Follicle stimulating hormone
hCG Human chorionic gonadotropin
PACS Picture archiving and communication system
CSF Cerebrospinal fluid
DI Diabetes insipidus
Pituitary adenomas (PA) are rare neoplasm with an estimated incidence of 0.4–8.2 per 105 inhabitants [1–3]. Symptoms range from headache and cranial nerve palsy – being signs of elevated intracranial pressure or direct compression of neural structures [4, 5], to endocrinological deficiency or excessive output of hormones with associated disease [6, 7]. Diagnosis is usually established by computed tomography (CT) or magnetic resonance imaging (MRI) performed for investigation of neurological symptoms or endocrinological disturbances and endocrinological examination including hormone blood levels.
Acromegaly is a rare disease with an estimated incidence of about 4 per 106 inhabitants. In most cases, it is caused by a growth-hormone (GH)-producing pituitary adenoma (PA). If excessive output of GH is not normalised, severe cardiovascular and metabolic disturbances as well as cosmetic and orthopedic deformities will result. Previously published studies have shown a 2–3-fold increased mortality for treatment-resistant cases as compared to successfully treated patients and healthy individuals [8–10]. Correction of GH values to normal can restore life expectancy towards normal. First surgical treatment using a trans nasal approach in an acromegalic patient was performed by Dr. Schloffer in Vienna in 1907 [11].
Non-functioning pituitary adenomas (NFPAs) add up to 25–30% of all PAs with an estimated incidence of 0.1–3.0 per 105 inhabitants [7, 12, 13]. The symptoms of NFPAs are caused by the tumour’s mass effect, which results in headache due to stretching of the diaphragma sellae and the sellar dura and visual deficits due to chiasmal compression. Diplopia may occur as a result of lateral tumour growth and/or haemorrhage into the cavernous sinus. In addition, patients can develop symptoms of secondary pituitary insufficiency due to compression of the pituitary gland [6, 7]. Visual disturbances occur in 53.8–74% [4, 5] headaches in 9.7–56% [4, 14], and third nerve palsy in 12% [4].
Except for asymptomatic micro-PAs and for prolactinomas, surgery is generally recommended as treatment of choice [1, 6, 15–21]. As most pituitary adenomas are benign neoplasms, tumour control can be achieved by complete resection. Today, most patients receive surgery as first line treatment using a transnasal, transsphenoidal approach introduced by Cushing [22] and reintroduced using microsurgical techniques by Hardy in 1979 [23]. In cases successfully treated by surgery, the outcome is defined by persisting neurological symptoms and pituitary function. A requirement for hormone replacement therapy is associated with the inconvenience of daily medication and regular tests whether the treatment is appropriate; moreover, hormone substitution is also an important socioeconomic factor as replacement therapy can generate costs being a multiple of the initial surgical treatment.
In recent years there have been a lot of refinements in the surgical treatment of PA like improvement of endoscopy and introduction of intraoperative magnetic resonance imaging (iMRI) [21, 24–33]. Those treatment options have been analysed regarding the clinical outcome and tumour control and showed superior results in the treatment of certain PAs (i.e., Growth hormone producing PA) compared to the conventional surgical approach. Endocrinological outcome has only been a minor part in those studies as the research focus concentrated mainly on tumour control.
In this largest study to date we analysed the postoperative pituitary function and the general outcome of 148 consecutive surgical procedures for non-functioning and GH-producing PAs being treated using a transnasal, transsphenoidal microsurgical iMRI-assisted approach using the PoleStar N20 imager.
A total of 148 surgical procedures were performed in 145 patients (82 male, 63 female) suffering from non-functioning- or GH-producing PA between September 2005 and August 2009. The data of those patients was prospectively collected and retrospectively analysed. Inclusion criteria of this study were diagnosis of a GH-producing- or NFPA and iMRI-assisted tumour removal. There were no exclusion criteria.
A total of 39 patients presented with GH-producing PA and 109 patients with NFPA. Three patients (1 male, 2 female) underwent surgery twice for removal of recurrent tumour. All patients were operated on by the senior author (R.L.B.) using the same standardised transsphenoidal approach with the aid of ultra low-field iMR imaging. The mean patient age was 55 ± 15 years (range 19–80 years). The median preoperative tumour volume was 6797 mm3 (range 50–65, 450 mm3; mean diameters 23mm*20mm*19mm). Ten cases (6.9%) presented with microadenomas, 118 (82.5%) with macroadenomas, and 15 (10.5%) with giant adenomas. A total of 24 patients (17.1%) had previous pituitary surgery. In 51 cases (35.7%) the tumour invaded the cavernosus sinus. Overall, 89 patients (62.2%) had cranial nerve symptoms, among them 79 (55.2%) with visual field deficits. 56 patients (37.8%) complained about headache. In 37 out of the 39 cases (94.8%) with GH-producing PA, patients had symptoms of acromegaly. There were no patients with somatostatin receptors ligands therapy in the GH-producing PA group and no patients with previous radiotherapy.
| Table 1: Study and patients characteristics. | |
| Number of patients | 145 |
| Number of operations | 148 |
| Sex (male/female) | 82/63 (56.6% / 43.4%) |
| Mean age in years | 55 ± 15 (19-80) |
| Mean preoperative tumour size in mm3 | 6797 (50–65,450) |
| Infiltration of cavernous sinus | 51 (35.7%) |
| Patients with micro-adenoma | 10 (6.9%) |
| Patients with macro-adenoma | 118 (82.5%) |
| Patients with giant adenoma | 15 (10.5%) |
| Patients with previous surgery | 24 (16.8%) |
| Patients with cranial nerve symptoms | 89 (62.2%) |
| Patients with visual field deficits | 79 (55.2%) |
| Patients with pan-/hypopituarism | 62 (41.9%) |
| Patients with pre-op. hormone substitution | 29 (17.5%) |
Patients were seen as outpatients before surgery. After decision for surgical treatment, patients arrived in hospital the day before surgery. Preoperative diagnostics included computed tomography (CT) scan and magnetic resonance imaging (MRI) for determination of the pituitary adenoma, bony and neurovascular structures. Pre- and postoperative imaging studies were performed at the Department of Neuroradiology, University Hospital of Zurich. CT studies were performed on a 16 slice CT scanner (Siemens SOMATON® Sensation, München, Germany) acquiring non contrast-enhanced and contrast-enhanced multi slice imaging data. The MRI studies were performed on a 1.5 Tesla MR tomography scanner (Signa®, General Electric, Milwaukee, USA).
Neurological and ophthalmological examinations were performed after admission. General patient data, additional diagnosis, medication at admission and previous study results were noted. Patients underwent endocrinological examination which included blood analysis for pituitary function examining growth hormone (GH), insulin-like growth factor-1 (IGF-1), adrenocorticotropin (ACTH), cortisol, thyroid stimulating hormone (TSH), fT4, fT3, prolactin, luteinising hormone (LH), follicle stimulating hormone (FSH) and human chorionic gonadotropin (hCG); testosterone was measured in male and estradiol in female patients unless a history of recent menstrual bleedings or of contraceptive pill intake was given.
Patients signed written consent for the surgical procedure and general anaesthesia. On the day of surgery, patients with reduced Cortisol plasma levels received 100 mg hydrocortisone (SoluCortef®, Pfizer, New York City, USA) one hour before operation; and another dose of 100 mg hydrocortisone was given perioperatively. After surgery, patients were transferred to intermediate care unit (IMCU). A postoperative CT scan was performed in all patients around six hours after surgery to exclude major bleeding or serious complications. The next morning patients were transferred to the general ward and mobilised. They received 100 mg of hydrocortisone on the first and 50 mg on the second postoperative day. On the morning of the third operative day and/or before discharge, pituitary function was re-examined. If patients' cortisol levels were below 200 nmol/l in early morning blood samples, they received 30 mg of hydrocortisone (Hydrocortone®, Merck, Darmstadt, Germany) per day until endocrinological follow-up as outpatients four weeks after surgery.
All operations were performed in general anaesthesia. Patients were put in supine position on a foldable standard operation table with their head slightly reclined. The head was then fixed in a MRI-compatible head holder after adjusting the radio frequency coil around the patients head. The iMRI scanner used in all patients was a PoleStar™ N20 (0.15 Tesla, Medtronic Navigation, Louisville, CO, USA). Afterwards the intraoperative navigation system (Stealth Station, Medtronic Navigation, Louisville, CO, USA) was referenced with preoperative CT studies. The position of the patient’s head in the scanner was tested by performing a 24 second sagittal e-steady scan (8 mm slices) and adjusted if necessary. Before surgery, a 7-minute, T1-weighted, gadolinium (20 ml Dotarem®, Guerbet, Roissy CdG Cedex, France) enhanced, 4 mm slice, coronal iMRI scan was performed. These images were automatically loaded into the navigation system and merged with the preoperative imaging studies.
All parts of surgical procedures were performed by using an operating microscope (Pentero®, Carl Zeiss, Oberkochen, Germany). At the beginning of operations a self-retaining endonasal speculum was inserted in the nostril chosen for surgical approach. The mucosa was incised and partially removed, the posterior bony part of the septum was removed and the anterior wall of the sphenoid sinus was displayed. The anterior wall of the sphenoid sinus was then opened with punches, the intrasphenoidal mucosa and septum were removed and the inferior and anterior surface of the sella was displayed and opened with a chisel. The dura mater was opened in an x-shaped fashion and the adenoma removed by curettes, grasping forceps and suction devices. Tumour material was sent for frozen sections and neurohistopathological examination. After complete tumour removal according to the surgeon’s impression, a 3.5-minute, T1-weighted, gadolinium- enhanced, 4 mm slice, coronal iMRI scan was performed for resection control in all patients. For better visualisation of possible tumour remnants, a glove-covered ball of bone wax was inserted into the resection cavity for hemostasis and improved interpretation of intraoperative images. The intraoperatively acquired images were automatically merged with the existing preoperative and intraoperative imaging studies. In cases of visible tumour remnant, the resection cavity was re-examined and tumour remnants removed if possible. Analysis of the intraoperative imaging studies were performed by the most experienced surgeon participating in the procedure. Another post-resectional, intraoperative 3.5-minute, T1-weighted, gadolinium- enhanced, 4 mm slice, coronal iMRI scan was performed in those cases. The anterior wall of the sella turcica was reconstructed by using the extracted posterior part of the bony nasal or intrasphenoidal septum. In cases of intraoperative CSF leakage, the sella was packed with abdominal fat and use of fibrin sealant. No nasal packing was used. All operations were performed by the senior author.
All patients were followed-up four weeks postoperatively as outpatients in the endocrinology clinic; hormone levels were analysed and deficiencies replaced if necessary. Three months after surgery, patients received a postoperative MRI study for resection control and patients were seen for neurosurgical follow up and examination.
Patients with non-functioning PA were considered in remission if complete tumour resection could be performed during surgery and neuroradiological follow-up with high-field MRI could not show any signs for tumour remnant of re-growth.
Patients with growth-hormone producing PA were considered in remission if the following criteria were fulfilled: complete tumour resection was possible, symptoms of acromegaly diminished, postoperative high-field MRI showed no signs of tumour remnant or re-growth and postoperative GH level were less than 1.0 µg/l.
The statistical analysis was performed using Microsoft Excel (Version 2003) and SPSS Statistic software (Version 16.0). All pre- and postoperative imaging studies were analysed independently and blinded to the clinical outcome using standardised software (picture archiving and communication system, PACS). Tumour volume was calculated based on the diameter method (tumour volume = 4/3 * Pi * ½x * ½y * ½z), where x, y and z are the maximum diameters in the three axis.
On average, 2.51± 0.99 intraoperative imaging studies were acquired per patient; the first at the beginning of the surgical procedure for mapping with the preoperative high-field MRI and a CT study, and another scan was performed before closure to confirm tumour removal and to check for tumour remnant. Based on these images, 44 patients (29.7%) received further tumour removal since tumour remnants were detected. In this case, at least one further imaging study was made to check for further tumour remnant. The other 98 patients (66.2%) showed no sign of tumour remnant (86 patients) unless visible tumour remnant was left in place (12 patients) due to predictably high risk of complications in case of further tumour removal. The rate of patients in remission during the overall follow-up in this study was 75.5% within the group of patients not undergoing further tumour removal and 59.1% in the group undergoing further tumour removal. There were an additional 26 patients (17.6% of study group) in remission during follow-up due to intraoperative imaging that lead to complete adenoma resection.

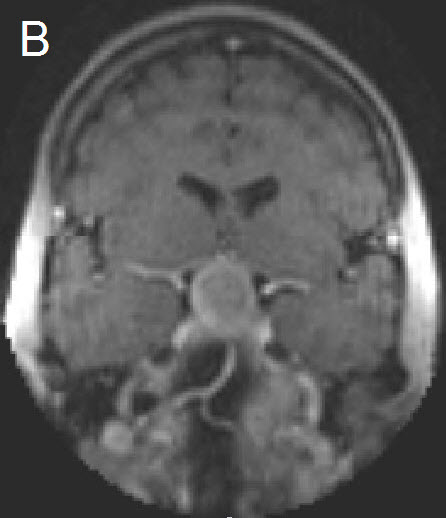
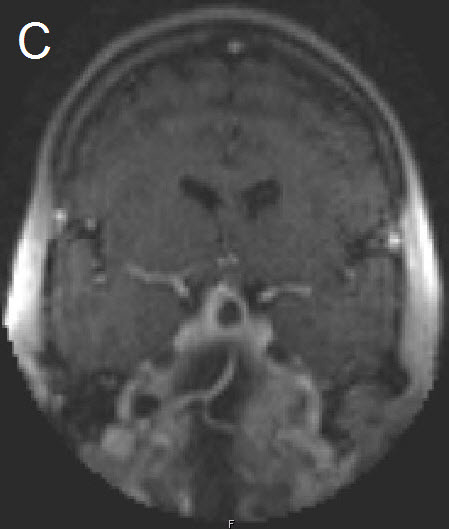
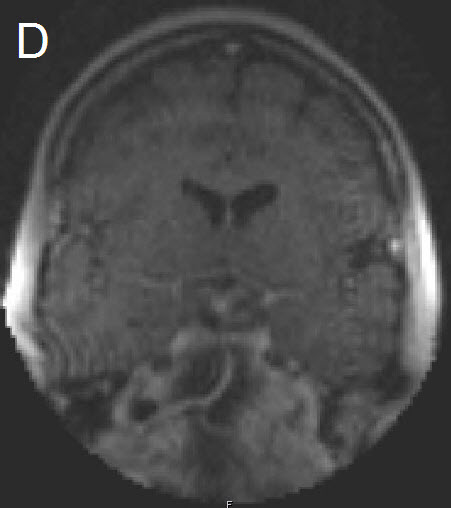
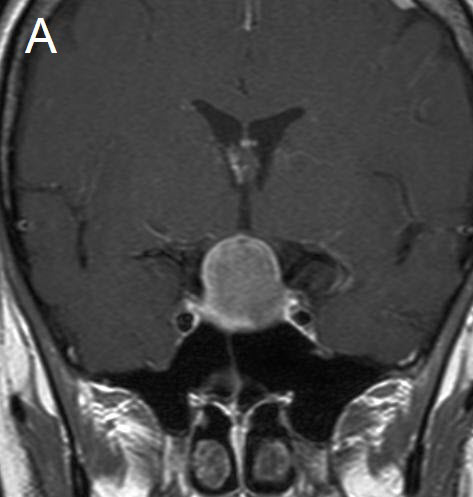
Figure 1 Illustrative case: Preoperative, intraoperative and postoperative imaging. A: Preoperative MRI, coronal view, contrast enhanced. B: Intraoperative MRI, coronal view, contrast enhanced, before skin incision. C: Intraoperative MRI, coronal view, contrast enhanced, during resection. D: Intraoperative MRI, coronal view, contrast enhanced, before closure. E: Postoperative MRI, coronal view, contrast enhanced.
Patients were seen for clinical and neuroradiological follow-up (1.5 Tesla MRI) three months after surgery. There was another MRI performed one year postoperatively, and further imaging studies during overall follow-up in case of visible tumour remnant, tumour regrowth or newly developed symptoms. Overall mean follow-up during this study was 32 months (range 4–62 months).
There were 106 patients considered tumour remnant-free, and 101 of them were considered to be in remission. The remaining 5 patients underwent surgery for GHPA and postoperatively complained about persisting symptoms of acromegaly and suffered from elevated GH levels without showing tumour remnant in postoperative high-field MRI.
There were 42 patients with postoperative tumour remnant visible in postoperative high-field follow-up MRI. The mean volume of tumour remnant was 1,191 mm3 (range 4–15,559 mm3). The tumour volume could be reduced to around one sixth of the preoperative size (6,797 mm3 [range 50–65, 450 mm3]) in those patients.
Patients were hospitalised for a mean time of 9 days (range 5–79 days). Six patients needed temporary lumbar drainage and one patient needed temporary external ventricular drainage due to postoperative cerebrospinal fluid (CSF) fistula. Ten patients developed postoperatve transient diabetes insipidus (DI) with the need of temporary treatment with desmopressin during hospitalisation of which one needed permanent medication. Eight patients developed temporary SIADH within the first 24 days after surgical treatment. Three patients needed transsphenoidal revision surgery for CSF fistula with associated meningitis and one for CSF fistula alone.
Two patients died during the study period for reasons considered unrelated to the surgical procedure (one for unknown reasons 11 months after surgery at an age of 87 years, one for pneumonia at an age of 59 years 2 months after surgery).
Patients underwent endocrinological examination pre and postoperatively. The mean hormone levels of cortisol, TSH, fT4, GH, IGF1 and prolactin did not differ significantly between the pre- and the postoperative condition. Mean cortisol level in nmol/l was 393 (3–1640) and 349 (9–808); mean TSH level in mU/l was 1.73 (0.01–6.96) and 1.72 (0.01–6.12); mean fT4 level in pmol/l was 13.80 (5.60–20.30) and 15.35 (4.00–25.40); mean GH level in NFPA patients in ug/l was 0.59 (0.05–4.88) and 1.21 (0.08–5.55); mean IGF1 level in NFPA patients in µg/l was 102 (31–492) and 101 (29–209) and mean prolactin level in µg/l was 25.07 (0.50–109.50) preoperatively and 15.38 (0.50–227.00) postoperatively.
Before surgery, 62 patients (41.9%) were found with partial or total pituitary insufficiency of which only 30 patients (48.4%) received preoperative hormone substitution therapy – in many cases due to the short time between diagnosis and surgery (table 3); GH deficiency was usually not sought by provocative tests and is therefore underreported. 38 (61.3%) of these 62 patients had persisting pituitary insufficiency, with need for ongoing hormone replacement therapy after surgery, but in 25 patients (38.7%) postoperative examinations showed full recovery of pituitary function without signs of insufficiency or further need for substitution therapy. 21 out of these 25 patients (87.5%) were patients with NFPAs without previous pituitary surgery who underwent further tumour removal due to intraoperative imaging. There were statistically no significant differences in Hardys classification of these patients.
Among the 62 patients with preoperative pituitary insufficiency, 22 (35.5%) had a PA invading the cavernous sinus. In the group with postoperatively persisting insufficiency, 14 patients (36.8%) had preoperative tumour infiltration of cavernous sinus. In the group with full recovery of pituitary function the rate was roughly the same with 8 patients (33.3%) showing infiltration of cavernous sinus.
In contrast to the above mentioned group with full recovery of pituitary function, there were 13 patients (8.8%) who developed pituitary insufficiency after surgery and required (at least temporarily) hormone substitution therapy. All these patients had NFPAs. Five of them were in need of permanent hormone substitution therapy. These five patients were all considered not being in remission after surgical procedures during this study. Interestingly there was not one single case with first time pituitary surgery and postoperative newly developed persisting pituitary insufficiency.
Analysis of pituitary function in patients grouped according to the PA’s Hardy classification of their adenoma revealed that those in class D and E were more severely affected than those in classes A, B and C. Before surgery we found pituitary insufficiency in 10 patients (30.3%) of class A, in 24 patients (42.1%) of class B, in 10 patients (30.3%) of class C, in 11 patients (68.7%) of class D and in 7 patients (77.8%) of class E. In all those groups roughly half of the patients were on hormone substitution therapy when admitted to hospital for surgical treatment – 5 patients (15.1%) of class A, 12 patients (21.0%) of class B, 6 patients (18.2%) of class C, 5 patients (31.2%) of class D and 3 patients (33.3%) of class E.
After surgery, improving pituitary function was more often observed than deterioration (i.e. overall, disappearance of pituitary insufficiency was more common than development of pituitary failure). A total of 24 patients with insufficiency at baseline no longer needed hormone substitution therapy after surgery: – 6 (18.2%) of class A, 8 (14.0%) of class B, 4 (12.1%) of class C, 5 (31.2%) of class D and 1 (11.1%) of class E according to Hardys classification.
On the other hand, 13 patients, considered sufficient at baseline, developed pituitary insufficiency after surgery. Five of them suffered from permanent insufficiency and remained dependent on long term hormone replacement therapy – 2 (6.1%) of class A and 3 (5.3%) of class B according to Hardy’s classification.
The remission rates in the different patient groups were 72.7% for class A, 77.2% for class B, 69.7% for class C, 25.0% for class D and 66.6% for class E according to Hardy’s classification.
Overall, there were 62 patients with pituitary insufficiency before surgery; 30 of them were on hormone replacement treatment. Overall, there were 43 patients with postoperative pituitary insufficiency and need for ongoing hormone substitution therapy – 5 with newly developed insufficiency postoperatively and 38 with persisting insufficiency. Therefore, surgical treatment resulted in a net decrease of 19 patients who were in need for medical treatment in the postoperative as compared to the preoperative condition; –30.6% of the patients with preoperative pituitary insufficiency or 12.8% of the complete study population.
Patients were seen at three months follow-up for clinical-neurological examination. Out of 56 patients complaining about headache before surgery, 38 patients (67.8%) reported complete regression, 5 patients (8.9%) improvement and 13 patients (23.2%) unchanged pain. Regarding visual field deficits there was a complete regression found in 55 patients (69.6%), an improvement in 14 patients (17.7%) and unchanged symptoms in 10 patients (12.6%).
A forty-two year old female was admitted for resection of a large pituitary adenoma. She complained about headache, amenorrhea and galactorrhea. Endocrinological diagnosis revealed a panhypopituitarism. The patient underwent surgery and the non-functioning pituitary adenoma could be totally removed. Postoperative follow-up showed normal pituitary function and the patient has now been in remission for 48 months. The figures show preoperative imaging, intraoperative imaging with various stages of the tumour resection, and postoperative follow-up MRI.
| Table 2: Pre-, intra- and postoperative imaging. | |
| Preoperative tumour size in mm3 | 6,797 (50–65,450) |
| Infiltration of cavernous sinus | 51 (35.7%) |
| Intraoperative imaging p.pt. | 2.51 ± 0.99 |
| Intraoperative additional tumour removal | 56 (37.8%) |
| Mean follow up in months | 32 (4–62) |
| Postoperative tumour remnant in mm3 | 42 (28.4%) |
| Mean size of tumour remnant in mm3 | 1,191 (4–15,559) |
| Patients being in remission | 101 (68.2%) |
| Table 3: Characteristics of 62 patients with preoperative hypopituitarism: type of deficiencies and replacement therapy before and after surgery. | |||||||||||
| Pt | Sex | Age | Size | HC | CS | Prev surg. | Add res intraOP | Deficiency preOP | Substitution preOP | Defiency and substitution postOP | Remis. |
| 1 | f | 38 | 1,319 | B | CT, ADH | CT, ADH | CT | y | |||
| 2 | f | 19 | 13,722 | D | 1 | CT, THT, GT | CT, THT | CT, THT, ADH | n | ||
| 3 | f | 20 | 308 | B | 1 | 1 | 1 | CT, THT, GT | CT, THT, GT | CT, THT, GT | n |
| 4 | m | 58 | 132 | A | 1 | CT, GT | CT | CT | n | ||
| 5 | m | 48 | 50 | A | GT | GT | GT | y | |||
| 6 | f | 44 | 2,356 | C | 1 | ADH, GT | ADH | - | n | ||
| 7 | m | 36 | 65,450 | D | 1 | CT, THT, GT, ST | - | CT, THT, GT, ST | n | ||
| 8 | m | 51 | 41,167 | D | 1 | 1 | CT, GT | - | - | n | |
| 9 | m | 34 | 23,637 | D | 1 | GT | - | CT, THT | y | ||
| 10 | f | 70 | 2,356 | B | 1 | GT | - | - | y | ||
| 11 | f | 42 | 603 | A | 1 | CT, GT | - | - | n | ||
| 12 | m | 58 | 6,807 | B | 1 | 1 | 1 | CT, THT, GT | CT, THT, GT | CT, THT, GT | n |
| 13 | m | 48 | 9,817 | C | 1 | 1 | CT, THT | CT, THT | - | n | |
| 14 | m | 68 | 4,273 | E | 1 | 1 | GT, CT | - | - | n | |
| 15 | f | 80 | 7,037 | E | 1 | THT | THT | THT | y | ||
| 16 | m | 73 | 5,909 | B | 1 | 1 | CT, THT, GT | CT, THT | CT | y | |
| 17 | f | 47 | 3,142 | B | 1 | CT, ADH, GT | - | - | y | ||
| 18 | m | 43 | 4,536 | B | 1 | 1 | CT, THT, GT | CT, THT, GT | CT, THT, GT, ADH | y | |
| 19 | f | 63 | 4,147 | B | 1 | 1 | CT, THT, GT | CT, THT, GT | CT, THT | n | |
| 20 | f | 66 | 3,064 | B | 1 | 1 | 1 | CT, THT | CT, THT | - | y |
| 21 | m | 49 | 6,545 | B | 1 | 1 | CT, THT, GT, ADH | CT, THT, GT; ADH | CT, THT, GT, ADH | n | |
| 22 | m | 58 | 25,970 | D | 1 | CT-GT | - | - | n | ||
| 23 | m | 65 | 12,566 | D | 1 | GT | GT | CT, GT | y | ||
| 24 | m | 49 | 43,982 | D | 1 | 1 | CT, THT, GT | - | CT, THT, GT | n | |
| 25 | m | 67 | 7,854 | E | 1 | CT, GT | - | CT, THT, GT | y | ||
| 26 | m | 63 | 2,513 | B | 1 | 1 | CT, THT, GT | CT, THT | CT, THT | y | |
| 27 | f | 42 | 5,720 | B | 1 | GT | - | - | y | ||
| 28 | m | 61 | 3,940 | B | 1 | 1 | CT, THT, GT | CT, THT | THT | y | |
| 29 | m | 53 | 10,891 | E | 1 | 1 | CT, GT | - | CT, THT, GT | y | |
| 30 | m | 61 | 4,985 | B | 1 | 1 | CT, GT | - | - | y | |
| 31 | m | 36 | 10,179 | E | 1 | CT | CT | - | y | ||
| 32 | f | 54 | 17,153 | D | 1 | 1 | CT | CT | - | n | |
| 33 | m | 46 | 8,382 | B | 1 | 1 | GT | - | ST | n | |
| 34 | m | 61 | 6,283 | C | 1 | CT, GT | - | - | y | ||
| 35 | m | 56 | 3,393 | B | 1 | GT | - | CT, THT, GT | y | ||
| 36 | f | 48 | 9,020 | C | 1 | THT | THT | THT | y | ||
| 37 | f | 50 | 4,629 | B | 1 | CTH | - | - | y | ||
| 38 | m | 52 | 829 | A | 1 | 1 | CT, GT | - | - | y | |
| 39 | m | 54 | 9,161 | B | 1 | CT, THT, GT | - | GT | n | ||
| 40 | m | 76 | 3,223 | B | 1 | GT | - | - | y | ||
| 41 | m | 41 | 180 | A | 1 | 1 | GT | - | - | y | |
| 42 | m | 65 | 2,419 | B | 1 | 1 | CT, GT | - | GT | n | |
| 43 | f | 55 | 2,507 | A | 1 | 1 | CT, THT, GT | CT, THT, GT | CT, THT, GT | y | |
| 44 | m | 65 | 6,744 | C | 1 | 1 | 1 | CT, GT | - | GT | n |
| 45 | m | 59 | 6,267 | C | 1 | CT, THT, GT | CT, THT, GT | CT, THT, GT | y | ||
| 46 | m | 27 | 18,385 | C | 1 | CT, GT | - | CT, THT | n | ||
| 47 | f | 69 | 11,140 | C | 1 | CT, THT | - | CT, THT | n | ||
| 48 | m | 61 | 7,257 | E | 1 | 1 | CT, THT, GT | CT, THT, GT | CT, THT, GT | y | |
| 49 | m | 24 | 16,965 | D | 1 | CT, GT | - | - | n | ||
| 50 | m | 50 | 26,005 | D | 1 | CT, THT, GT | - | - | n | ||
| 51 | m | 62 | 8,482 | C | 1 | CT, THT, GT | CT, THT | THT, GT | y | ||
| 52 | f | 64 | 4,477 | B | 1 | 1 | THT | THT | THT | y | |
| 53 | m | 71 | 942 | A | 1 | GT | - | - | y | ||
| 54 | f | 59 | 1,994 | A | 1 | 1 | THT | THT | THT | n | |
| 55 | m | 57 | 18,322 | D | 1 | 1 | CT, GT | CT, GT | CT, THT, GT, ADH | y | |
| 56 | m | 46 | 17,157 | C | 1 | 1 | CT | - | - | n | |
| 57 | f | 56 | 4,320 | B | 1 | 1 | THT | THT | THT | y | |
| 58 | f | 56 | 8,972 | E | 1 | 1 | 1 | GT | GT | GT | n |
| 59 | m | 25 | 503 | A | 1 | GT | - | - | y | ||
| 60 | m | 79 | 3,079 | A | 1 | CT, THT, GT, ST | - | - | y | ||
| 61 | m | 68 | 3,958 | B | 1 | 1 | GT | - | - | y | |
| 62 | m | 74 | 3,233 | B | 1 | 1 | GT | GT | GT | y | |
| Pt = Patient; HC = Hardys classification; CS = cavernous sinus infiltration of PA; Prev. surg = history of previous surgery; Remis. = remission; Add Res intraOP = additional resection due to intraoperative MRI; CT = corticotroph; ThT = thyrotroph; GT = gonadotroph; ST = somatotroph. | |||||||||||
| Table 4: Endocrine outcome of patients grouped according to Hardy’s classification. | |||||||
| HardyClass. | Patient number | Remission | PostOP new hypopituitarism [temporary] | Hypopituitarism | Permanent substitution | ||
| preOP | postOP | preOP | postOP | ||||
| A | 33 (22.3%) | 24 (72.7%) | 2 (6.1%) + [3 (9.1%)] | 10 (30.3%) | 9 (27.3%) | 4 (12.1%%) | 6 (18.2%) |
| B | 57 (38.5%) | 44 (77.2%) | 3 (5.3%) + [2 (3.5%)] | 24 (42.1%) | 21 (36.8%) | 12 (21.0%) | 19 (33.3%) |
| C | 33 (22.3%) | 23 (69.7%) | [2 (6.1%)] | 10 (30.3%) | 8 (24.2%) | 6 (18.2%) | 6 (18.2%) |
| D | 16 (10.8%) | 4 (25.0%) | [1 (6.2%)] | 11 (68.7%) | 7 (43.7%) | 5 (31.2%) | 6 (37.5%) |
| E | 9 (6.1%) | 6 (66.6%) | 7 (77.8%) | 6 (66.7%) | 3 (33.3%) | 6 (66.7%) | |
In this largest study to date we analysed the postoperative outcome, neurological symptoms and postoperative pituitary function in 148 patients undergoing iMRI-assisted transsphenoidal surgery for GH-producing- and NFPAs.
The overall remission rate during a mean follow-up of 32 months (4–62) was 101 out of 148 patients (68.2%) applying the remission criteria of the latest international consensus conference regarding the GH-producing PAs and not detectable tumour remnant or re-growth in neuro-radiological follow-up with high-field MRI in patients with NFPA. These results are at least as good as previously published series reporting remission rates between 25 and 70% [5, 27, 34–41]. Comparison remains difficult as most of the series included all kind of PAs but did not include giant adenomas, and remission criteria differed widely.
Our results show that pituitary function was preserved in most of the patients as there were only 5 patients (3.4%) showing postoperative pituitary insufficiency and need for permanent medical treatment, which is in accordance with the literature showing rates ranging from 5.6% to 30.0% [42]. Mean hormone values showed no significant differences between pre- and postoperative endocrinological examinations in this study.
Adenoma resections even led to complete recovery of pituitary function in 24 patients so that overall, 19 patients (12.8%) less remained dependent on permanent substitution therapy. Comparison with other published series is difficult as patient groups, type of included adenoma and other study parameters differ widely.
Interestingly, remission rates were not related to postoperative need for further hormone substitution, which has already been shown in the literature [42]. Among the patients with preoperative pituitary insufficiency, 36 patients were in remission while 26 patients were not. There was no need for further hormone substitution in 14 out of these 36 patients (38.9%) and in 10 out of those 26 patients (38.5%).
The surgical method used appeared safe as only a minor number of patients suffered complications. There were only four patients with need for re-operation because of CSF fistula and no patients with neurological deterioration due to surgery. The two deaths (one due to pneumonia) within the study population were considered unrelated to the surgical procedure. This all adds up to a remarkably low rate of complications compared to the literature; particularly considering that 15 patients with giant adenomas were included [24, 43–45].
Our results show that previous pituitary surgery elevates the risk of postoperative newly diagnosed pituitary insufficiency as all patients with new need for postoperative hormone substitution therapy had revision surgery for tumour re-growth or remnant. Interestingly those 5 patients (3.4%) had tumours of Hardy classification A or B only, which means that tumour size seems to be less of a risk factor for newly developing postoperative pituitary insufficiency. Out of 124 patients with first time pituitary surgery there was not a single case with postoperative newly developed pituitary insufficiency. Another factor explaining these results is that patients with Hardy classification D and E had a higher percentage of pituitary insufficiency even before surgery (72.2%) compared to Hardy classification A, B and C patients (24.4%).
Cavernous sinus infiltration of PAs seems to be a sign of greater tumour expansion but interestingly did not lead to higher rates of pituitary insufficiency, possibly since this does not mean more compression of healthy pituitary tissue in all cases. There were only 35.5% of patients with preoperative pituitary insufficiency showing cavernous sinus infiltration. In the complete study population there were 50 patients (33.8%) with cavernous sinus infiltration as well. Out of the 38 patients with postoperative persisting pituitary insufficiency there were 14 patients (36.8%) with cavernous sinus infiltration and out of the 24 patients with no further pituitary insufficiency there were 8 patients (33.3%) with cavernous sinus infiltration. These results suggest that cavernous sinus infiltration is not a risk factor for pituitary insufficiency.
There were some general limitations in this study that need to be considered. iMRI was thought to be helpful in surgery and to improve outcome so that it seemed unethical to perform surgery without iMRI in part of the study population. Pure endoscopic treatment of pituitary adenomas was not performed during the time of this study so there is no group of patients for comparison of the results within the same centre. This is especially a drawback as comparison to previously published series is complicated as not only the surgical method but also pre- and postoperative study protocols differ widely.
Although the iMRI was useful and lead to improved remission rates and improved endocrinological- and neurological outcome there are some drawbacks in ultra-low field iMRI to consider as well. Even though studies have failed to show better results in surgical series using intraoperative high field MRI, image quality and resolution is – at least theoretically – better than in ultra-low field MRI [23, 41]. Costs for intraoperative imaging are still high due to installation, maintenance and time loss during surgery. Studies with larger patient numbers and cost/risk analysis should address the question of whether better clinical and endocrinological outcome can compensate for these increasing costs.
Data from this largest study to date analysing general postoperative outcome and pituitary function in patients undergoing ultra-low-field iMRI-assisted microsurgical treatment of PAs show that the presented method is a safe and highly effective treatment option. The results are at least as good as those reported in previously published series – even in those with use of high-field iMRI or endoscopy. iMRI appeared to increase the rate of remission while keeping the complication rate low. Postoperative pituitary function was usually preserved and even improved in around 17% of the patients – possibly due to more exact and more extended iMRI-assisted tumour removal. This is not only a great achievement for each individual patient but also an important socioeconomic factor as hormone substitution therapy is expensive and health care systems worldwide face increasing budget deficits.
1 Kreutzer J, Fahlbusch R. Diagnosis and treatment of pituitary tumors. Curr Opin Neurol. 2004;17:693–703.
2 Monson J. The epidemiology of endocrine tumours. Endocr Relat Cancer. 2000;7:29–36.
3 Wöhrer A, Waldhör T, Heinzl H, Hackl M, Feichtinger J, Gruber-Mösenbacher U, et al. The Austrian Brain Tumour Registry: a cooperative way to establish a population-based brain tumour registry. J Neurooncol. 2009;95:401–11.
4 Comtois R, Beauregard H, Somma M, Serri O, Aris-Jilwan N, Hardy J. The clinical and endocrine outcome to trans-sphenoidal microsurgery of nonsecreting pituitary adenomas. Cancer. 1991;68:860–6.
5 Gondim J, Schops M, de Almeida J, de Albuquerque L, Gomes E, Ferraz T, et al. Endoscopic endonasal transsphenoidal surgery: surgical results of 228 pituitary adenomas treated in a pituitary center. Pituitary. 2010;13:68–77.
6 Jaffe C. Clinically non-functioning pituitary adenoma. Pituitary. 2006;9:317–21.
7 Losa M, Mortini P, Barzaghi R, Franzin A, Giovanelli M. Endocrine inactive and gonadotroph adenomas: diagnosis and management. J Neurooncol. 2001;54:167–77.
8 Bengtsson BA, Eden S, Ernest I, Oden A, Sjogren B. Epidemiology and long-term survival in acromegaly. A study of 166 cases diagnosed between 1955 and 1984. Acta Med Scand. 1988;223:327–35.
9 Nabarro JD. Acromegaly. Clin Endocrinol. (Oxf) 1987;26:481–512.
10 Wright AD, Hill DM, Lowy C, Fraser TR. Mortality in acromegaly. Q J Med. 1970;39:1–16.
11 Schloffer H. Erfolgreiche Operation eines Hypophysentumors auf nasalem Wege. Wein Klein Wochenschr. 1907;20:621–4.
12 Alexander J, Biller B, Bikkal H, Zervas N, Arnold A, Klibanski A. Clinically nonfunctioning pituitary tumors are monoclonal in origin. J Clin Invest. 1990;86:336–40.
13 Saunders S, Vora J. Endocrine evaluation of pituitary tumours. Br J Neurosurg. 2008;22:602–8.
14 Nomikos P, Ladar C, Fahlbusch R, Buchfelder M. Impact of primary surgery on pituitary function in patients with non-functioning pituitary adenomas – a study on 721 patients. Acta Neurochir. (Wien) 2004;146:27–35.
15 Cappabianca P, Alfieri A, Colao A, Ferone D, Lombardi G, de Divitiis E. Endoscopic endonasal transsphenoidal approach: an additional reason in support of surgery in the management of pituitary lesions. Skull Base Surg. 1999;9:109–17.
16 Ezzat S, Serri O, Chik CL, Johnson MD, Beauregard H, Marcovitz S, Nyomba BL, Ramirez JR, Ur E. Canadian consensus guidelines for the diagnosis and management of acromegaly. Clin Invest Med. 2006;29:29–39.
17 Melmed S, Casanueva FF, Cavagnini F, Chanson P, Frohman L, Grossman A, et al. Guidelines for acromegaly management. J Clin Endocrinol Metab. 2002;87:4054–8.
18 Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D, et al. Guidelines for acromegaly management: an update. J Clin Endocrinol Metab. 2009;94:1509–17.
19 Jane JJ, Laws EJ. The management of non-functioning pituitary adenomas. Neurol India. 2003;51:461–5.
20 Laws E, Jane JJ. Neurosurgical approach to treating pituitary adenomas. Growth Horm IGF Res. 2005;15(Suppl A):S36–41.
21 Nimsky C, von Keller B, Ganslandt O, Fahlbusch R. Intraoperative high-field magnetic resonance imaging in transsphenoidal surgery of hormonally inactive pituitary macroadenomas. Neurosurgery. 2006;59:105–14; discussion 105–14.
22 Cushing H. III. Partial hypophysectomy for acromegaly: with remarks on the function of the hypophysis. Ann Surg. 1909;50:1002–17.
23 Hardy J. The transsphenoidal surgical approach to the pituitary. Hosp Pract. 1979;14:81–9.
24 Baumann F, Schmid C, Bernays RL. Intraoperative magnetic resonance imaging-guided transsphenoidal surgery for giant pituitary adenomas. Neurosurg Rev. 2010;33:83–90.
25 Bellut D, Hlavica M, Schmid C, Bernays RL. Intraoperative magnetic resonance imaging-assisted transsphenoidal pituitary surgery in patients with acromegaly. Neurosurg Focus. 2010;29:E9.
26 Bohinski RJ, Warnick RE, Gaskill-Shipley MF, Zuccarello M, van Loveren HR, Kormos DW, et al. Intraoperative magnetic resonance imaging to determine the extent of resection of pituitary macroadenomas during transsphenoidal microsurgery. Neurosurgery. 2001;49:1133–43; discussion 1143–34.
27 Fahlbusch R, Ganslandt O, Buchfelder M, Schott W, Nimsky C. Intraoperative magnetic resonance imaging during transsphenoidal surgery. J Neurosurg. 2001;95:381–90.
28 Gerlach R, du Mesnil de Rochemont R, Gasser T, Marquardt G, Reusch J, Imoehl L, et al. Feasibility of Polestar N20, an ultra-low-field intraoperative magnetic resonance imaging system in resection control of pituitary macroadenomas: lessons learned from the first 40 cases. Neurosurgery. 2008;63:272–84; discussion 284–75.
29 Martin CH, Schwartz R, Jolesz F, Black PM. Transsphenoidal resection of pituitary adenomas in an intraoperative MRI unit. Pituitary. 1999;2:155–62.
30 Nimsky C, Ganslandt O, Von Keller B, Romstöck J, Fahlbusch R. Intraoperative high-field-strength MR imaging: implementation and experience in 200 patients. Radiology. 2004;233:67–78.
31 Schwartz TH, Stieg PE, Anand VK. Endoscopic transsphenoidal pituitary surgery with intraoperative magnetic resonance imaging. Neurosurgery 58:ONS44-51; discussion ONS44-51, 2006.
32 Theodosopoulos PV, Leach J, Kerr RG, Zimmer LA, Denny AM, Guthikonda B, et al. Maximizing the extent of tumor resection during transsphenoidal surgery for pituitary macroadenomas: can endoscopy replace intraoperative magnetic resonance imaging? J Neurosurg. 2010;112:736–43.
33 Wu JS, Shou XF, Yao CJ, Wang YF, Zhuang DX, Mao Y, Li SQ, Zhou LF. Transsphenoidal pituitary macroadenomas resection guided by PoleStar N20 low-field intraoperative magnetic resonance imaging: comparison with early postoperative high-field magnetic resonance imaging. Neurosurgery. 2009;65:63–70; discussion 70–61.
34 Beauregard C, Truong U, Hardy J, Serri O. Long-term outcome and mortality after transsphenoidal adenomectomy for acromegaly. Clin Endocrinol. (Oxf) 2003;58:86–91.
35 Laws ER, Vance ML, Thapar K. Pituitary surgery for the management of acromegaly. Horm Res. 2000;53(Suppl 3):71–5.
36 Nomikos P, Buchfelder M, Fahlbusch R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical “cure”. Eur J Endocrinol. 2005;152:379–87.
37 Cappabianca P, Cavallo LM, Colao A, Del Basso De Caro M, Esposito F, Cirillo S, et al. Endoscopic endonasal transsphenoidal approach: outcome analysis of 100 consecutive procedures. Minim Invasive Neurosurg. 2002;45:193–200.
38 Dehdashti AR, Ganna A, Karabatsou K, Gentili F. Pure endoscopic endonasal approach for pituitary adenomas: early surgical results in 200 patients and comparison with previous microsurgical series. Neurosurgery. 62:1006-1015.
39 Frank G, Pasquini E, Farneti G, Mazzatenta D, Sciarretta V, Grasso V, et al. The endoscopic versus the traditional approach in pituitary surgery. Neuroendocrinology. 2006;83:240–8.
40 Jho HD. Endoscopic transsphenoidal surgery. J Neurooncol. 2001;54:187–95.
41 Santos AR, Fonseca Neto RM, Veiga JC, Viana Jr J, Scaliassi NM, Lancellotti CL, et al. Endoscopic endonasal transsphenoidal approach for pituitary adenomas: technical aspects and report of casuistic. Arq Neuropsiquiatr. 2010;68:608–12.
42 Kristof RA, Schramm J, Redel L, Neuloh G, Wichers M, Klingmüller D. Endocrinological outcome following first time transsphenoidal surgery for GH-, ACTH-, and ORL-secreting pituitary adenomas. Acta Neurochir. 2002;11:555–61.
43 Basso A. Commentary to giant pituitary tumors: a study based on surgical treatment of 118 cases. Surgical Neurology. 2004;61:445.
44 Goel A, Nadkarni T, Muzumdar D, Desai K, Phalke U, Sharma P. Giant pituitary tumors: a study based on surgical treatment of 118 cases. Surg Neurol. 2004;61:436–45; discussion 445–36.
45 Losa M, Mortini P, Barzaghi R, Ribotto P, Terreni MR, Marzoli SB, et al. Early results of surgery in patients with nonfunctioning pituitary adenoma and analysis of the risk of tumor recurrence. J Neurosurg. 2008;108:525–32.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.