Natural killer cell immunity after transplantation
DOI: https://doi.org/10.4414/smw.2012.13700
Grzegorz
Terszowski, Jakob R
Passweg, Martin
Stern
Summary
Transplantation immunology has traditionally focused on adaptive, i.e., T- and B-cell reactions. More recently, natural killer (NK) cells were also recognised as playing an important role after transplantation of solid organs and haematopoietic stem cells.
NK cells recognise “cell stress” induced by viral replication and tumour transformation via activating receptors, and are negatively regulated by the interaction between inhibitory molecules and autologous human leukocyte antigens (HLA). The most important inhibitory molecules belong to the family of killer cell immunoglobulin-like receptors (KIR). Differences in the inhibitory KIR/HLA interaction between stem cell donor and patient may lead to beneficial NK cell alloreactivity, resulting in specific graft-versus-tumour reactions, which occur in the absence of graft-versus-host disease. The immaturity of NK cells produced by the stem cell graft early after transplantation has led to different approaches of adoptive transfer of NK cells to further increase tumour control.
The function and role of activating KIR receptors is less clear. Recent data have suggested, that activating KIR may also contribute to anti-tumour immunity after stem cell transplantation, as patients transplanted from donors carrying high numbers of activating KIR receptor genes show reduced relapse rates. In particular, protection from post-transplant disease relapse was demonstrated in transplants carried out from donors carrying the activating KIR2DS1 receptor, if the recipients also expressed the KIR2DS1 ligand HLA-C2.
In conclusion, NK cells have been firmly established in the last two decades as relevant players in transplant immunology, which can critically determine the outcome of haematopoietic stem cell grafts.
Background
Over the past five decades, the transplantation of haematopoietic stem cells after myeloablative radio-/chemotherapy to treat tumours of the lymphohaematopoietic system has developed from an experimental to a standard procedure [1]. Nowadays more than 12,000 allogeneic stem cell transplant procedures are carried out in Europe each year [2]. When the first successful allogeneic transplants were performed in the late 1960s, the function of the transplanted cells was uniquely interpreted as a “replacement tissue” supplanting the patients’ bone marrow, which is destroyed by the supralethal chemo-/radiotherapy preceding the transplant. It was first recognised in the 1980s that patients suffering from graft-versus-host disease (GvHD) – i.e., the immune rejection of healthy recipient tissue by donor derived lymphocytes – showed a lower risk of disease relapse after transplantation compared patients who did not develop GvHD [3]. These observations suggested that – in addition to the cytoreductive properties of the chemo/radiotherapy applied before transplantation – the donor immune system might contribute to the elimination of tumour cells. This concept was confirmed in the 1990s with successful treatment of patients developing disease relapse after stem cell transplantation with donor lymphocyte infusions [4]. The allogeneic recognition of recipient tissue by the donor immune system has since been divided into beneficial effects (if directed against the malignant cells, i.e., graft-versus-tumour, GvT) and detrimental effects (if directed against healthy tissue such as skin, gut and liver, i.e., GvHD). The substantial reduction of both types of reactions associated with T-cell depletion of grafts [5] – i.e., a reduction of GvHD at the cost of increased relapse risk – pointed to a prominent role for T-lymphocytes co-transplanted with the graft in inducing graft-versus-host reactions. There have been a number of unsuccessful attempts to separate T-cell mediated GvT from GvHD, and the induction of an isolated T-cell mediated GvT effect has so far remained the “Holy Grail” of stem cell transplantation. Interestingly, many studies conducted over the past 10–15 years, have shown that another lymphocyte subset, Natural Killer (NK) cells, is also involved in immunity both against residual tumour cells and against pathogens in patients undergoing stem cell transplantation. NK cells appear after stem cell transplantation in quantities similar to those found in healthy donors approximately four weeks post-transplant, whereas numbers of B- and T-lymphocytes take several months to recover [6]. Most importantly, the beneficial effects attributed to NK cells in the post-transplant period appear to occur without the risk of graft-versus-host disease, which makes these cells uniquely suited to adoptive immunotherapy [7].
Natural killer (NK) cells reside in the bone marrow, spleen and peripheral blood, where they comprise approximately 10% of peripheral blood lymphocytes. Unlike B- and T-lymphocytes, NK cells do not express clonally rearranged receptors to detect antigens. Instead, activation is regulated by integration of signaling from germ-line encoded activating and inhibitory cell surface receptors. These include inhibitory receptors for HLA class I antigens, and activating receptors such as the immunoglobulin gamma Fc-region receptor IIIa (CD16), DNAM-1, NKG2D and natural cytotoxicity receptors (NCRs) NKp30, NKp44, and NKp46.
Inhibitory receptors for self-HLA include Killer cell Immunoglobulin-like Receptors (KIR), the lectin-like receptor NKG2A, and LILRB1/ILT-2 (table 1). The ligands to activating receptors are self-proteins, which are usually rare on normal cells, but are up-regulated at the cell surface during either infection or malignant transformation. Upon interaction with target cells expressing activating ligands, a lack of engagement of inhibitory receptors results in the predominance of activating signalling and target cell lysis. This process is initiated by the release of small granules from the NK cell cytoplasm that contain proteins such as perforin and proteases known as granzymes. Upon release in close proximity to a cell slated for killing, perforin forms pores in the cell membrane of the target cell, which creates an aqueous channel through which the granzymes and associated molecules can enter, inducing either apoptosis or osmotic cell lysis [8]. A second pathway involves the engagement of death receptors (e.g., Fas/CD95) on target cells by their cognate ligands (e.g., FasL) on NK cells, resulting in classical caspase-dependent apoptosis [9]. These systems form the basis of the “missing self” recognition and exemplify the mechanisms that the immune system uses to counteract HLA down-regulation induced by tumours and viral infection to escape T-cell recognition.
Apart from their lack of rearranged receptors, NK cells also differ from T- and B-lymphocytes in that they do not undergo clonal expansion after activation. A long-held dogma in the field of immunology is that immunological memory is restricted to adaptive immune cells undergoing clonal expansion. However, recent experiments in mice have shown that murine NK cells display properties of memory such as a lower threshold for activation after re-exposure to an antigen, which is transplantable to naïve animals by adoptive transfer of NK cells [10]. Whether such immunological memory is also a property of human NK cells remains so far unknown and is the topic of intensive research.
|
Table 1: Main natural killer cell surface receptors and their ligands. |
|
Inhibitory receptors
|
Ligands
|
| Inhibitory L-KIR
- KIR2DL1
- KIR2DL2/3
- KIR2DL5
- KIR3DL1
- KIR3DL2 |
HLA A/B/C
HLA C group 2 (e.g. Cw2, 4, 5, 6)
HLA C group 1 (e.g. Cw1, 3, 7, 8)
Unknown
HLA Bw4 (e.g. B5, 13, 17, 27)
HLA A3/A11 |
| NKG2A
LILRB1 |
HLA-E
HLA-A/B/C |
|
Activating receptors
|
Ligands
|
| Natural cytotoxicity receptors
(NKp30, NKp44, NKp46) |
Some bacterial and viral antigens, endogenous ligands mostly unknown |
| KIR2DL4 |
HLA-G |
| Activating S-KIR |
Unknown (HLA-C group 2 for KIR2DS1, some HLA-A and -C for KIR2DS4) |
| NKG2D |
MIC-A/B, ULBPs |
| DNAM-1 |
PVR, Nectin-2 |
| CD16 |
IgG |
| 2B4 |
CD48 |
| KIR = killer cell Immunoglobulin-like receptors; HLA = human leukocyte antigen; NKG2A/D = natural killer cell group 2A/D; MIC = major histocompatibility complex class I chain-related; ULBP = UL16 binding proteins; DNAM-1 = DNAX accessory molecule 1; PVR = polio virus receptor, IgG = immunoglobulin G. |
KIR ligand mismatching in haploidentical haematopoietic stem cell transplantation
Haploidentical stem cell transplantation is the transfer of haematopoietic stem cells from a donor that shares half of the HLA antigens with the patient, and is typically a first-degree relative (i.e., father, mother, sibling, or child). Haploidentical HSCT is only carried out if a fully or almost fully matched related or volunteer unrelated donor is not available. Due to the multiple HLA mismatches between donor and patients, patients are at high risk of T-cell mediated graft-versus-host disease. Therefore, this procedure is always accompanied by rigorous depletion of T-cells ex vivo (by magnetic sorting), or in vivo(by co-administration of a T-cell depleting antibody such as alemtuzumab, or by post-transplant administration of a lymphodepleting agent such as cyclophosphamide). T-cell depletion leads to the loss of beneficial T-cell mediated graft-versus-tumour effects, which in some cases can be compensated for by NK cells [11]. Especially in the case of a KIR ligand mismatch, i.e. in the setting when a donor but not a patient carries a KIR ligand, a subset of NK cells produced from the graft after transplantation will become alloreactive and eliminate residual leukaemic cells (fig. 1). Therefore, donor selection algorithms in haploidentical transplantation give priority to a donor carrying a KIR ligand, which the patients lacks: e.g., for a patient carrying HLA-Cw antigens Cw2 and Cw4 (which are both ligands to KIR2DL1), the preferred a haploidentical donor will carry a KIR ligand to the KIR2DL2/3 receptor (e.g., HLA-Cw1 or HLA-Cw3, table 1), as the patient lacks a ligand to this KIR. NK cells produced from the graft after transplantation and expressing KIR2DL2/3 will not be inhibited by recipient leukaemia cells and therefore can exert beneficial anti-leukaemic effects. It is worth noting that NK cell associated alloreactivity may not only result from direct lysis of target cells by NK cells, but may also be mediated by an indirect pathway, i.e., through the production of pro-inflammatory cytokines such as IFN-γ and TNF-α which may stimulate T-cell responses. There is increasing evidence indicating that NK cells can also prevent and limit adaptive auto- or allo-immune responses via elimination of antigen presenting cells [12].
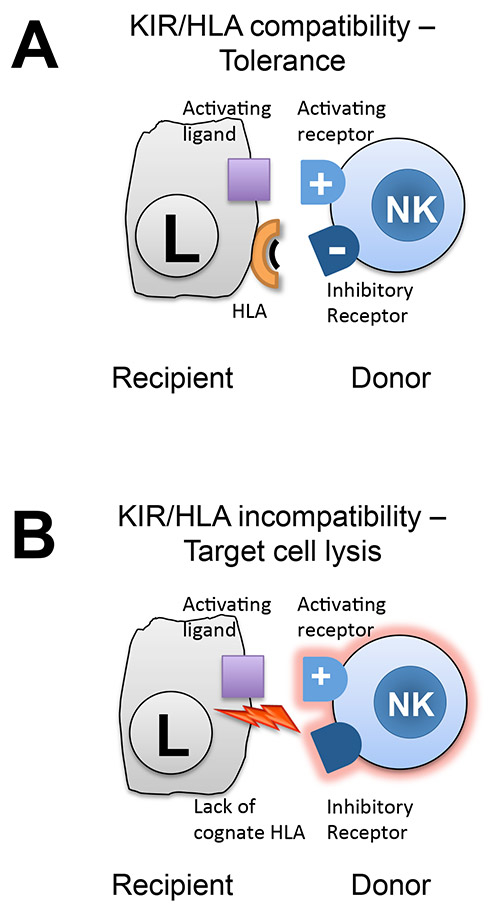
Figure 1
NK cell alloreactivity after haploidentical stem cell transplantation is determined by the HLA class I antigen/KIR constellation of donor and recipient. Donor NK cell activating and inhibitory receptors interact with ligands on recipient target (leukaemia) cells. The activation state of NK cells is determined by the integration of both types of signals. In the case of KIR/HLA compatibility, the inhibitory signal dominates and induces tolerance (panel A). In the absence of a cognate HLA ligand, activating signals dominate and lead to target cell lysis (panel B).
Early research had indicated that the NK cell KIR repertoire after transplantation immediately resembles that of the stem cell donor. However, more recent investigations have shown that the potentially alloreactive NK cell subset after transplantation is in fact much smaller than in the donor [13]. An additional observation that emerged from these recent studies was that the reconstitution of alloreactive NK cell clones was dependent of the type of KIR involved, with alloreactive NK cells expressing the KIR2DL2/3 receptors recovering at a much earlier time-point than the remaining KIR (fig. 2A). When correlating these data with the survival of acute leukemia patients transplanted from a haploidentical donor, we observed that patients transplanted from a donor who was mismatched for the ligand of a KIR with early reconstitution showed significantly better survival and less leukaemia relapse than patients transplanted from a donor mismatched for a KIR ligand with late reconstitution or from a KIR ligand matched donor (fig. 2B) [13]. These data clearly point to the importance of NK cells in this type of transplantation and to the relative benefit associated with the early occurrence of alloreactive NK cells.
More recently, attention has been also given to KIR ligand mismatching after transplantation of haematopoietic stem cells derived from cord blood, an emerging source of stem cells for patients lacking a fully HLA-identical donor. As cord blood cells are immunologically naïve, HLA mismatches can be tolerated without excessive risk of graft-versus-host disease. The majority of cord blood products are therefore not fully HLA matched with the patient to whom they are administered. Interestingly, a retrospective study suggested that KIR ligand mismatching between cord blood and patient led to a reduced relapse risk and increased survival [14]. However, this finding will need to be confirmed in a prospective study. Data regarding KIR/HLA matching in solid organ transplantation are more controversial. Some smaller scale studies have suggested that NK cells may participate in graft rejection in the case of a KIR ligand mismatch between recipient and transplanted organ [15, 16], an effect that could not be confirmed in larger registry studies [17, 18].
Adoptive immunotherapy with NK cell donor lymphocyte infusions
In order to improve the repertoire of NK cells early after stem cell transplantation, administration of purified donor NK cell products has been proposed for several years. The University Hospital Basel was among the first to explore this approach and showed in a prospective trial that production of highly purified NK cell products is feasible and safe, as long as stringent T-cell depletion is guaranteed [19]. However, the outcomes of patients treated with NK DLI were not improved compared to those of patients treated with haploidentical HSCT alone [20]. Therefore, an NK cell expansion protocol was developed, which allows the expansion of NK cell products up to 100-fold, along with a strong increase in cytolytic potential [21]. Studies are currently underway in which these expanded NK cell products are administered after haploidentical transplantation for patients with leukaemia or after autologous transplantation to patients with multiple myeloma.
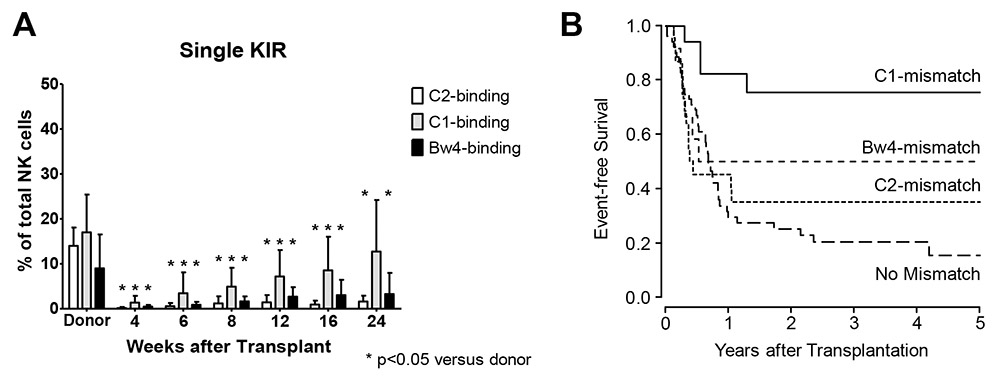
Figure 2
Temporal evolution of the frequencies single KIR expressing NK cells as percentage of total NK cells shows a skewed repertoire with slow reconstitution of single KIR expressing subsets. Reconstitution is fastest for C1-binding KIR, followed by Bw4 binding KIR and C1-binding KIR. This is mirrored in survival curves of patients stratified by type of KIR ligand mismatch, with best survival seen in patients transplanted from a donor KIR ligand mismatched for a receptor that reconstitutes early after transplantation (adapted from Stern M, de Angelis C, Urbani E, Mancusi A, Aversa F, Velardi A, L Ruggeri L. Natural killer-cell KIR repertoire reconstitution after haploidentical SCT. Bone Marrow Transplantation. 2010;45,1607–10).
Finally, the question arises whether allogeneic transplantation is required as a prerequisite for successful therapy with allogeneic NK cells. Exciting data have shown that the infusion of a purified NK cell product into patients treated with cyclophosphamide, fludarabine and interleukin-2 may lead to transient engraftment of transfused NK cells and induction of remission in refractory AML patients [22]. NK cell chimerism was detectable for several months after infusion, although ultimately all patients rejected the transferred NK cells and relapsed. Nonetheless, the data provide an excellent demonstration of the powerful effects of NK cells in vivo and may argue for a combination of (haploidentical) allogeneic transplantation and NK DLI, as a transplant preceding NK infusion will prevent rejection of NK cells and ultimately serves as a source of alloreactive NK cells on its own.
New drugs targeting NK cells that could provide more practicability for NK cell manipulation are also being developed. The most advanced compound targeting NK cell population is a blocking anti-KIR monoclonal antibody (IPH2101) [23]. This antibody recognises KIR2DL1/L2/L3 (a subset representing half of the overall NK cell population) and therefore blocks the inhibition imposed by all HLA-C alleles, allowing it to be used in all patients, regardless of their KIR and HLA genotypes. It remains to be seen whether the administration of this blocking antibody to patients will provide efficacy in clinical trials without affecting NK cell development.
Activating KIR genes and their contribution to immunity against leukemia
In contrast to the well-defined biology of inhibitory KIR, the function of activating KIR has remained largely elusive. Activating KIR probably recognise HLA molecules or modified HLA molecules, but only the specificity of KIR2DS1 for C2 alleles has been firmly established both biochemically and functionally (activating function against C2 bearing cells, see table 1).
While all individuals carry the genes for inhibitory KIR receptors – which indicates that they are necessary for NK cell function – KIR haplotypes are highly variable in terms of activating KIR gene content. Approximately 25% of healthy Caucasians do not carry activating KIR genes. Recent analyses correlating donor KIR genotype with the outcome of HSCT for acute leukaemia have shown that patients grafted from a donor carrying a high number of activating KIR genes have a survival advantage [24]. This improved survival was mainly due to a reduced risk of disease relapse, which suggests that activating KIR genes could contribute to post-transplant immunity against residual leukaemic cells. Indeed, NK cells expressing the KIR2DS1 receptor have been shown in vitro to contribute to protection from disease relapse after haploidentical HSCT in patients carrying the KIR2DS1 ligand HLA-C2 [25]. Recently a large analysis of patients undergoing unrelated donor stem cell transplantation confirmed a reduced relapse rate in the “Donor carrying KIR2DS1 – Patient carrying HLA-C2” constellation [26], and this is likely to have an impact on donor selection algorithms in this type of transplant. Elaborate analyses of KIR gene content and relapse risk after HSCT for acute leukemia suggest that other activating KIR genes may also be involved in anti-tumour immunity after HSCT [27], however no individual KIR genes other than KIR2DS1 have yet identified to have played such a role.
Outlook and conclusions
Over the past 20 years, NK cells have attracted substantial interest in the context of haematopoietic stem cell transplantation. While many aspects of NK cell biology in this setting remain poorly understood, donor selection criteria in HLA-mismatched transplantation include the potential of a donor to generate alloreactive NK cells post-transplant. Retrospective studies showing a survival advantage for patients transplanted from an HLA-identical donor carrying activating KIR receptors [27] have led to large prospective trials that aim to preferentially recruit stem cell donors with a beneficial activating KIR gene profile. While these are exciting times for researchers interested in NK cells, much work remains to be performed to unravel the many ways in which NK cells influence the transplant outcome.
References
1 Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26.
2 Passweg JR, Baldomero H, Gratwohl A, Bregni M, Cesaro S, Dreger P, et al. The EBMT activity survey: 1990–2010. Bone Marrow Transplant. 2012;47:906–23.
3 Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62.
4 Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–50.
5 Maraninchi D, Gluckman E, Blaise D, Guyotat D, Rio B, Pico JL, et al. Impact of T-cell depletion on outcome of allogeneic bone-marrow transplantation for standard-risk leukaemias. Lancet. 1987;2:175–8.
6 Buhlmann L, Buser AS, Cantoni N, Gerull S, Tichelli A, Gratwohl A, et al. Lymphocyte subset recovery and outcome after T-cell replete allogeneic hematopoietic SCT. Bone Marrow Transplant. 2011;46:1357–62.
7 Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100.
8 Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9.
9 Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, et al. Activation of NK cell cytotoxicity. Molecular Immunology. 2005;42:501–10.
10 Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–61.
11 Stern M, Ruggeri L, Mancusi A, Bernardo ME, de Angelis C, Bucher C, et al. Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood. 2008;112:2990–5.
12 Villard J. The role of natural killer cells in human solid organ and tissue transplantation. J Innate Immun. 2011;3:395–402.
13 Stern M, de Angelis C, Urbani E, Mancusi A, Aversa F, Velardi A, et al. Natural killer-cell KIR repertoire reconstitution after haploidentical SCT. Bone Marrow Transplant. 2010;45:1607–10.
14 Willemze R, Rodrigues CA, Labopin M, Sanz G, Michel G, Socie G, et al. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia. 2009;23:492–500.
15 van Bergen J, Thompson A, Haasnoot GW, Roodnat JI, de Fijter JW, Claas FH, et al. KIR-ligand mismatches are associated with reduced long-term graft survival in HLA-compatible kidney transplantation. Am J Transplant. 2011;11:1959–64.
16 Hanvesakul R, Spencer N, Cook M, Gunson B, Hathaway M, Brown R, et al. Donor HLA-C genotype has a profound impact on the clinical outcome following liver transplantation. Am J Transplant. 2008;8:1931–41.
17 Tran TH, Middleton D, Dohler B, Scherer S, Meenagh A, Sleator C, et al. Reassessing the impact of donor HLA-C genotype on long-term liver transplant survival. Am J Transplant. 2009;9:1674–8.
18 Tran TH, Mytilineos J, Scherer S, Laux G, Middleton D, Opelz G. Analysis of KIR ligand incompatibility in human renal transplantation. Transplantation. 2005;80:1121–3.
19 Passweg JR, Tichelli A, Meyer-Monard S, Heim D, Stern M, Kuhne T, et al. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia. 2004;18:1835–8.
20 Stern M, Passweg JR, Meyer-Monard S, Esser R, Tonn T, Soerensen J, et al. Pre-emptive immunotherapy with purified natural killer cells after haploidentical SCT: a prospective phase II study in two centers. Bone Marrow Transplant. 2012.
21 Siegler U, Meyer-Monard S, Jorger S, Stern M, Tichelli A, Gratwohl A, et al. Good manufacturing practice-compliant cell sorting and large-scale expansion of single KIR-positive alloreactive human natural killer cells for multiple infusions to leukemia patients. Cytotherapy. 2010;12:750–63.
22 Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7.
23 Romagne F, Andre P, Spee P, Zahn S, Anfossi N, Gauthier L, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–77.
24 Cooley S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113:726–32.
25 Pende D, Marcenaro S, Falco M, Martini S, Bernardo ME, Montagna D, et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood. 2009;113:3119–29.
26 Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367:805–16.
27 Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116:2411–9.

