
DOI: https://doi.org/10.4414/smw.2012.13701
The epidemiologic relevance of heart failure has been reported on extensively [1, 2]. Many factors affect the incidence and prevalence as well as the evolution of advanced heart failure, such as improvements in drug therapy and anti-arrhythmic strategies. The increased severity of the disease with age and time requires repeated hospitalisations: this represents substantial health care costs for society and a decrease in quality of life for the patient. However, patients with advanced heart failure are not necessarily transplant candidates, since there is some evidence that patients on a waiting list for a cardiac allograft may survive in up to 60% of the cases for 5 years [3].
Heart transplantation currently represents a well accepted and successful treatment for patients suffering from end-stage heart failure with more than 85% one-year survival and approximately 70 to 75% survival at 5 years, according to the registry of the International Society for Heart and Lung Transplantation [4]. This survival is superior to the dismal prognosis of 75% mortality at one year with optimal medical therapy as reported in the REMATCH trial [5]. In the INTrEPID trial, which evaluated the impact of left ventricular assist device (LVAD) support on survival and quality of life in inotrope-dependent heart failure patients ineligible for cardiac transplantation, a high short-term mortality rate was observed in drug-treated patients whereas a significant survival advantage was found following "destination" mechanical circulatory support [6].
Ventricular assist devices (VAD) have been used for more than 30 years to support patients who develop refractory heart failure as a “bridge to transplant” option. The success of these devices has markedly improved over the past decade [7], although a substantial proportion of patients suffer from numerous co-morbidities. Data from multiple publications suggest that approximately 20 to 30% of patients who received a VAD as a bridge to transplantation will not survive the bridging period, regardless of the device used [8, 9]. The most frequent causes of death are multi-organ failure, bleeding, infection and right ventricular failure. These data suggest that patient selection and pre-operative management are important factors to achieve successful therapy, specially for destination therapy [10, 11].
In 2003, the pulsatile HeartMate® XVE LVAD received FDA approval for destination therapy and the centres for Medicare and Medicaid Services approved full coverage in the USA. These were milestone decisions that allowed introduction of LVAD in the treatment of advanced heart failure, and helped to resolve regulatory and reimbursement issues.
Approval of LVAD as a destination therapy was based on results from the REMATCH trial which showed a significant increase in survival and improved quality of life in those patients who received LVAD support versus patients who received optimised medical treatment only [5]. However, the comparison of drug-treated patients of the REMATCH as well as of the INTrEPID trial indicated that patients who received LVAD were generally sicker than those with optimised medical treatment.
As destination therapy may expand in the future, the clinical and economic outcomes of LVAD have to be analysed critically and compared to those of HTx in adults [12].
With the simultaneously rising numbers of HTx candidates and improving outcome with VADs, destination therapy will become a reasonable new option for well selected patients [13, 14]. There is so far no survey in Switzerland which has analysed the need for LVAD as a destination therapy but a general estimation of 50 to 100 cases/year seems reasonable. Unfortunately, destination therapy has yet not been approved definitively by the national health authorities in Switzerland. An application to the Swiss Federal Health Office as well as the inclusion in the Swiss-DRG list are pending. A major question will be, how many and which centres should receive approval for destination therapy. Outcome after LVAD implantation as destination therapy depends on the centre volume [15]. Hence, it is most probably justified that implantation of LVAD as a destination therapy should be performed in centres which also perform cardiac transplant surgery. Approval by the Swiss authorities should ideally be restricted to these centres.
In general, VAD support is indicated in patients with end-stage acute and chronic heart failure with imminent additional organ failure in whom all conventional medicament, anti-arrhythmic and surgical options have been exhausted.


Figure 1
The Heart Mate II left ventricular assist device (reprinted with permission from Thoratec corporation). A: Housing with vascular prothesis to the ascending aorta. B: The impeller which is located within the housing. (© With courtesy by Thoratec Corporation).
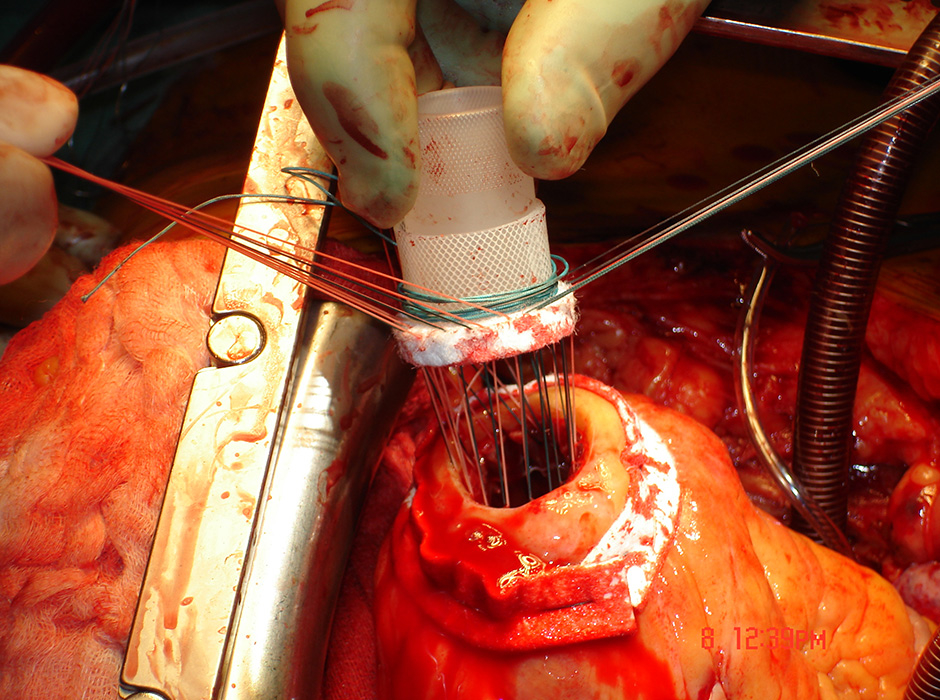
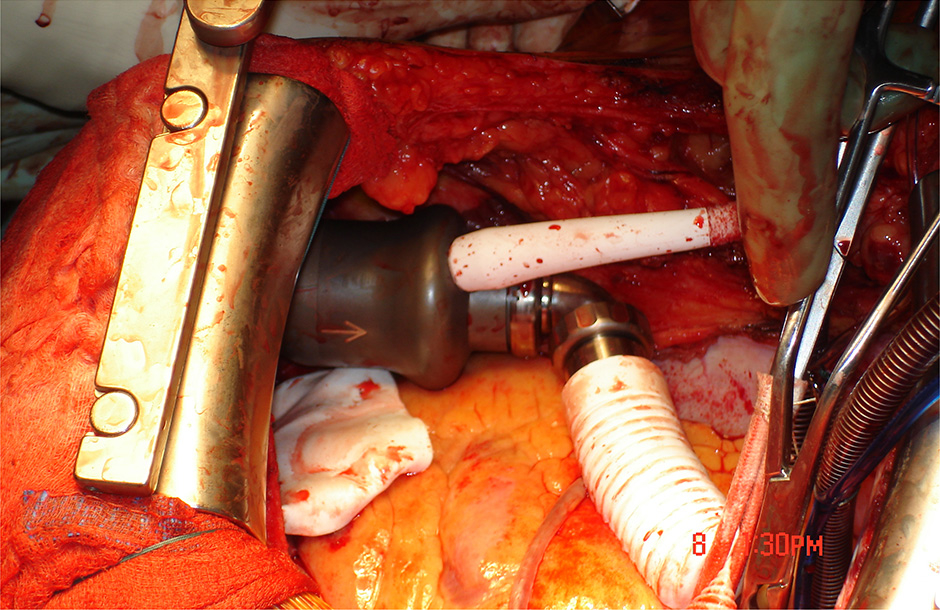
Figure 2:
A: Fixation of the sewing ring for further insertion of the device wihtin the left ventricular apex. B: Device in situ (intrapericardial).
In summary, the following potential goals of mechanical circulatory support can be defined according to the intention and the clinical situation:
1. Bridge to decision;
2. Bridge to recovery;
3. Bridge to candidacy;
4. Bridge to transplant;
5. Destination therapy.
Short-term MCS-systems (any ECMO system or TANDEM-Heart®, Impella® LP 5.0, DeltaStream® (MEDOS) and Centri-Mag® (Levitronics)) are important in emergency cardiogenic shock situations due to acute myocardial infarction, fulminant myocarditis or postoperative heart failure. These systems allow immediate stabilisation of the circulation and give time to evaluate the function of other organ systems (brain, lung, liver, kidney) and to discuss further options in case of non-recovery. The majority of these patients are in severe cardiogenic shock and/or the implantation of a mechanical cardiac support occurs under reanimation [16]. These patients primarily need efficient short-term support within the emergency situation and therefore a device that is easy and quick to implant. At implantation, there is often uncertainty concerning the neurological condition. Typically a short term MCS allows support from a few days to 2–3 weeks. Ideally, the situation can be discussed with the patient and/or the relatives. These systems should be available in every heart centre with extensive experience in interventional cardiology and/or cardiac surgery.
Patients suffering from advanced heart failure secondary to myocardial infarction, myocarditis, intoxication, graft failure immediately after transplantation as well as females with peripartum cardiomyopathy may qualify for a “bridge to recovery” indication. Reverse remodelling can occur under long term support with complete unloading of the left ventricle. Dandel and co-authors showed that VAD removal in chronic cardiomyopathy patients was feasible in a series of 47 patients with dilated cardiomyopathy and could be successful even after incomplete cardiac recovery. Parameters of pre-explantation cardiac function, LV size and geometry, their stability during final off-pump trials, and HF duration allowed detection of those patients with a potential to remain stable for more than 5 years following explantation of the device [17]. Post-weaning 5 year freedom from HF recurrence reached 66%. However, there is no general consensus on which biomarkers may be reliable to predict the recovery process. Krabatsch investigated if bridge to recovery was more likely to happen with pulsatile or non pulsatile devices: in 34 patients, LVAD removal due to myocardial recovery was performed with long-term stable cardiac function (weaning rate, 8.8%). Patients with a pulsatile-flow LVAD had an almost threefold chance for myocardial recovery than patients who received continuous-flow devices. Younger patients had significantly higher recovery rates than older patients [18]. He concluded that further studies should investigate whether pulsatility in itself or the different degrees of left ventricular unloading by the two types of systems played the major role in myocardial recovery. A recent publication reported on the continuous assessment of cardiac function during rotary blood pump support by using a contractility index derived from the pump flow [19].
In patients with pulmonary artery hypertension precluding direct transplantation, more time is needed to be able to make a final recommendation for heart transplantation. In this case, patients are supported with a long-term VAD until, for instance, pulmonary hypertension decreases to normal or only slight elevated values and is no longer considered as an absolute contraindication to transplantation. Beyersdorf showed that mechanical support using an implantable LVAD was a very efficient approach with an acceptable risk to treat severe pulmonary hypertension in end-stage heart failure patients before HTX. Adequate reduction of PVR can be expected within 3–6 months and subsequent HTX is associated with a good outcome [20]. Another rare example is a tumour diagnosed during the heart transplant evaluation which must be treated first. If there is any uncertainty regarding recurrence of the tumour, observational time can be gained by implanting a VAD.
Left ventricular assist devices are increasingly used as a bridge to transplantation. It remains unclear whether the use of pre-transplant left ventricular assist devices adversely affects short-term survival after cardiac transplantation. A retrospective review of 317 consecutive patients undergoing cardiac transplantation at an academic centre between 1986 and 2006 was undertaken [21]. Left ventricular assist devices were used pre-transplant in 23 of these 317 patients, and 294 patients did not require left ventricular assist device support. The main information from this paper was that LVAD – when used as a bridge to transplantation – do not compromise 1-year survival after cardiac transplantation. Of the patients who died after transplantation, those bridged with LVAD were at higher risk for death within 30 days of transplant. Patlolla came to different results, at least in stable heart failure patients: extracorporeal VADs were associated with higher mortality within 6 months and again beyond 5 years after transplantation. Intra-corporeal VADs were associated with a small increase in mortality in the first 6 months and a clinically significant increase in mortality beyond 5 years. These data do not provide evidence supporting VAD implantation in stable United Network for Organ Sharing status I patients awaiting heart transplantation [22].
In Switzerland, “bridge to transplantation” is the most frequent indication for long-term circulatory support. It is performed for patients in end-stage heart failure irrespective of the aetiology when the indication for HTx is confirmed, and if medical therapy has been fully optimised and there is still an imminent risk for secondary organ failure (liver, kidneys, lungs). The INTERMACS-levels have been introduced to help assess the ideal time-point for VAD implantation [23, 24]. Other pre-operative parameters for pre-operative prediction of post-VAD implant mortality have been published recently [25].
Target patients for destination therapy are those with an existing “bridge to transplant” indication but who are not considered for transplantation for one of the following reasons: the patient does not wish transplantation, age (i.e., over 65–70 years), immunological contraindication for transplantation (highly pre-sensitised patient), incompatibility to immunosuppressive therapy or significant co-morbidity which complicates or precludes transplantation. Long-term success is essentially dependent on the technical progress of these systems. However no level of evidence and data on long-term survival with MCS treatment exist so far in the current literature.
Recent results show an increasing reliability of the pump systems with a one-year survival rate of up to 86% [11, 13]; these results are comparable with those after heart transplantation [26], making this treatment a real alternative to HTx for selected patients in the near future.
Rogers and co-workers reported on functional capacity and quality of life of patients under long-term LVAD support. Data from advanced heart failure patients enrolled in the HeartMate II LVAD bridge to transplantation (BTT) (n = 281) and destination therapy (DT) (n = 374) trials were analysed. NYHA functional class, 6-min walk distance, patient activity scores as well as quality of life (Minnesota Living With Heart Failure [MLWHF] and Kansas City Cardiomyopathy Questionnaires [KCCQ]) were collected before and after LVAD implantation.
Compared with baseline, LVAD patients from both groups demonstrated early and sustained improvements in functional status and quality of life. Most patients had NYHA functional class IV symptoms at baseline. Following implant, 80% of destination treatment patients at 6 months and 79% at 24 months improved to NYHA functional class I or II. Mean 6-min walk distance in these patients was 204 m in patients able to ambulate at baseline, which improved to 350 and 360 m at 6 and 24 months. There were also significant and sustained improvements from baseline in both quality of life scores. The authors concluded that the use of a continuous flow LVAD in advanced heart failure patients resulted in clinically relevant improvements in functional capacity and heart failure-related quality of life [27]. Other reports confirmed these findings [28].
Years ago, the first LVAD systems were powered by large, extracorporeal units, and patients could only be partially mobilised: most often they remained hospitalised until transplantation. In contrast to the first generation of pulsatile, mostly pneumatic pumps, substantial development has led to the construction of non-pulsatile, miniaturised rotational pumps. Among them, the HeartMate II LVAS received FDA approval for the destination indication in January 2010, after obtaining FDA approval for “bridge to transplantation” in April 2008. This system is an intracorporeal pump with an axial flow pattern. The size of this pump has been significantly reduced compared to the previous pulsatile systems. The pump is driven by a rotating magnetic levitated impeller and has a capacity of up to 15,000 rotations/min, resulting in a theoretical maximal blood flow of 8–10 l/min. The pump has a weight of 300 g and is connected to the apex of the left ventricle and to the ascending aorta through a vascular graft return. This type of system is considerably quieter than the pulsatile one. It allows a fast mobilisation of patients and almost always a subsequent long term ambulatory rehabilitation.

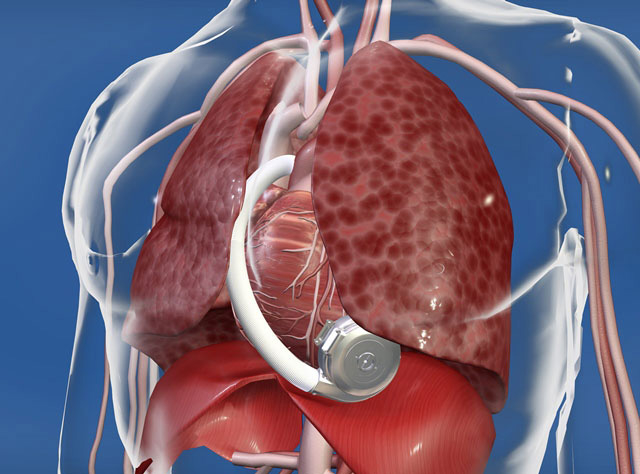
Figure 3:
A: The HeartWare LVAD device with the tip of the device to be inserted into the LV apex (blue arrow) and the exit of the pump to be connected to the vascular prosthesis (ascending aorta; red arrow) (HVAD® pump. © HeartWare. Reprinted with permission). B: In situ position of the device in the thoracic cavity (HVAD® pump in pericardial space. © HeartWare. Reprinted with permission).
One year ago, European results with the HM II LVAD were published, including 571 patients (19% female, 70% suffering from ischemic heart disease, age ranging from 14 to 75 years and body surface area from 1.3 to 2.5 m2) at 64 European institutions [11]. The intention of support was to provide bridge to transplantation in 73%, destination therapy in 21% and a bridge for potential recovery in 6%. The mean support duration was 236 ± 214 days, ranging from 0 to 1019 days. In 12 patients, support was effective for more than 2 years. Overall survival to transplantation, recovery or ongoing support at the end of the study was 69% with a 30 day mortality of 17.5%. The most important adverse events were bleeding (42%) infections (percutaneous lead or pocket infection) varying from 0.2 to 0.7% events per patient year, ischemic and hemorrhagic stroke between 0.04 and 0.16% per patient year.
Currently, third generation support devices are already in clinical use. These are smaller rotational pumps, designed with a magnetic levitating rotor (similar to a propeller). These pumps are simple in maintenance and at the same time less harmful to blood cells, thereby reducing haemolysis.
The blood flow is not axial because inflow and outflow axes are arranged in a 90° angle. Moreover this pump runs with lower rotation speed of 1000 to 2500/min. The small, wearless pump weighs 140 grams. The pump has one moving part, called an impeller, which spins blood to generate up to 10 l/min. flow. The small size of the pump allows intra-corporeal implantation within the pericardial cavity. The pump is connected to the controller via a thin driveline which is tunnelled through the abdominal muscle and leaves the body in the upper right quadrant of the abdomen. While active patients will always use two batteries, patients sleeping or relaxing should use power from an electrical outlet (AC adapter).
Despite all efforts to optimise cardiac support devices, substantial complications still occur throughout the whole period of support [29–35]. The most frequent are:
– Bleeding, particularly in the peri-operative period.
– Cerebral and peripheral thrombo-embolic complications.
– Infection is the most frequent complication in patients with a mechanical circulatory support system. Infection is often linked to the percutaneous driveline infection.
– Right ventricular failure and life-threatening arrhythmias.
– Haemolysis is most probably dependent on the pump design, whose shear forces affect the red blood cells when they pass through the pump.
The presence of intra-cardiac shunt such as patent foramen ovale or atrial septal defect must be investigated with echocardiography using a bubble study prior to cardiopulmonary bypass. It is important to close a patent foramen before LVAD support because significant right-to-left shunting may occur postoperatively, leading to severe hypoxemia under certain conditions. Likewise, co-existing other intra-cardiac problems can compromise long-term LVAD function: significant aortic and tricuspid regurgitation should be corrected. In appropriate cases, myocardial revascularisation may optimise the outcome (for instance bypass of the right coronary artery in case of right ventricular ischemia).
Following implantation, the patient is returned to the intensive care unit. Fluids are given to maintain pump flow index over 2 l/min. with left and right heart filling pressure below 20 mm Hg. Inotropic support for the right ventricle is important in the first few days following LVAD implantation. Anticoagulation is individualised for each patient. Low-dose intravenous heparin is started on pod 1 to a target PTT of 40–50 seconds. As soon as patients tolerate oral medication, warfarin is started very carefully and titrated to obtain an INR value between 2.5 and 3.5. In addition platelet activity is monitored to obtain a platelet inhibition of 50%.
Right ventricular failure may be one of the most important causes of peri-operative and early postoperative mortality and morbidity following LVAD implantation. Therefore, it is important to evaluate the RV risk-profile preoperatively.
Changes that occur immediately after initiating left ventricular support should be followed very closely during early post-implant management in the intensive care unit. Echocardiography has shown that an acute fall in LV pressure with a simultaneous increase in venous return to the right atrium results in alteration of the geometry of cardiac cavities and valvular function. In particular the interventricular septum is pulled to the left and the RV free wall is distended.
Matthews and co-authors analysed 68 of 197 patients who received LVAD with a postoperative outcome complicated by RV failure [36]. Pre-operative clinical, laboratory, echocardiographic, and hemodynamic predictors of RV failure were collected. Right ventricular failure was defined as the need for post-operative intravenous inotrope support for <14 days, inhaled nitric oxide for >48 h, right-sided circulatory support, or hospital discharge on an inotrope. An RV failure risk score (RVFRS) was created from multivariable logistic regression model coefficients. A vasopressor requirement (4 points), aspartate aminotransferase >80 IU/l (2 points), bilirubin >2.0 mg/dl (2.5 points), and creatinine >2.3 mg/dl (3 points) were independent predictors of RV failure. The odds ratio for RV failure for patients with an RVFRS 3.0, 4.0 to 5.0, and >5.5 were 0.49, 2.8 and 7.6 respectively, and 180-day survivals were 90 ± 3%, 80 ± 8%, and 66 ± 9%, respectively. The authors concluded that the RVFRS, composed of routinely collected, non-invasive pre-operative clinical data, effectively stratifies the risk of RV failure and death after LVAD implantation.
Before LVAD implantation, it is therefore very important to optimise RV function and lower atrial pressure to around 10 mm Hg. Those patients who show resistance to diuretics or only modest response in right atrial pressure to dilators and diuretics may benefit from methods of ultra-filtration in order to achieve lower intravascular volume and right atrial pressure.
While pulmonary hypertension and the risk of severe RV failure are some of the major concerns during the assessment of candidates for “bridge to transplantation” these factors are only of modest importance in the setting of destination therapy. Implantation of a LVAD will typically lead to a significant decrease of pulmonary pressure and secondarily of right atrial pressure [20].
Conventional anti-microbial prophylaxis is administered according to the institution’s profile for cardiac surgery (usually cephalosporines) and continued for 48 hours. The mediastinal and pleural drainages are removed as soon as possible. Early extubation, removal of monitoring lines and expedient patient ambulation are recommended, as well as restoration of oral nutrition. The sutures of the percutaneous driveline are removed after 2–3 weeks, as soon as the tissue in-growth is stable and there is no sign of skin infection at the exit site.
Risk factors present before device implantation include preoperative infection at remote sites, malnutrition, immunosuppressive medications, mechanical ventilation and the presence of central venous catheters [33]. Predisposing factors for local wound infection are age, diabetes, tension on the wound edges, localised haematoma in the device pocket followed by bacterial colonisation. Aseptic techniques must be used at all times for exit site care, regardless of when, where and by whom the care is provided. To prevent this nasty complication, training in proper driveline care given to patients and their relatives before discharge should be reinforced as part of the long-term care.
The extent of hematologic effects of continuous flow devices has not been studied in depth yet. The majority of clinical studies have demonstrated that haemolysis and thrombosis are not common during support, but bleeding remains a concern. The rate of postoperative bleeding is similar to the previous generation of pulsatile devices but gastrointestinal bleeding due to angiodysplasia or arteriovenous malformations is more common and appears to be related to the different flow characteristics of these devices [37–40].
Crow and colleagues reported on a prospective multi-centre study including 37 patients to characterise the von Willebrand factor profiles in patients under continuous flow LVAD support [37]. All 37 patients exhibited significant loss of high-molecular-weight von Willebrand factor multimers within 30 days of continuous flow LVAD implantation. A total of 10 of the 37 patients experienced bleeding complications. Since not all patients had bleeding events, the authors concluded that loss of von Willebrand factor multimers alone cannot predict bleeding risk. The shear stress of continuous flow devices may cause proteolysis of the high-molecular-weight multimers of the von Willebrand factor. In addition, there is a prolonged activation of the fibrinolytic system, and a loss of platelets and some additional dysfunction during circulatory support. In order to decrease the incidence of such events, screening for von Willebrand disease and gastrointestinal pathologies may be indicated before implantation of such LVAD systems.
An emergency situation happens when the LVAD cannot pump enough blood flow or when the patient has some other device-related acute problems. The implanting centre should be notified of any emergencies. In case of emergency, the patient and his relative should not be separated because the latter usually has vital knowledge and skills that not-specialised medical teams might not have. For instance, the relative may need to perform hand pumping. If the LVAD is pumping but produces low flow, clot formation may occur. In such situations, first responders should organise immediate transfer of the patient and ask about whether heparin should be administered.
Device longevity is most probably increased by minimising the force loads on pump components as well as by reducing the number of pump rotations (for instance it might be beneficial to run the device at a lower speed during the night when less flow is required). Reducing the pump workload is possible by decreasing the afterload (systolic arterial pressure of the patient).
As destination treatment enters the clinical routine, it is important to remember that patients will be supported with the device until the end of their life and doctors should be prepared for potential end-of-life issues in order to promote medically and ethically sound decisions for termination of LVAD support. The following aspects should be respected: 1./ any medical care should serve the patient’s goals, 2./ minimise the possibility of under- or over-treatment, 3./ reduce the potential for conflicts between family members, the healthcare providers and the patient. For this matter, it is important to document those circumstances in which the patient would not like to continue LVAD support or other life sustaining therapy (e.g., dialysis, artificial nutrition, mechanical ventilation).
Switzerland has not yet accepted the destination therapy unlike other countries like Germany, Austria, USA or Canada [41]. In these countries, the opinion is that patients have the right to receive the most adequate and individualised treatment and destination therapy therefore clearly represents one of the valid alternatives.
The financing of long-term VAD (including destination therapy) and heart transplantation has to be considered in the global context of heart failure treatment. The payees seem to fear that with the establishment of alternative cardiac replacement procedures, a new considerable cost-push will be expected from the healthcare premium payers. However, the costs of rarely performed cardiac replacement procedures with LVAD are very small compared to the costs generated by patients with advanced heart failure that need several hospitalisations a year. A calculation from the USA shows that a patient with a long term LVAD, leaving the hospital after 40 days already costs less than a patient waiting for a heart transplant in an intensive care environment [42].
Cost-effectiveness associated with continuous flow LVADs for destination therapy has improved significantly compared to the costs of pulsatile flow devices. The incremental cost-effectiveness ratio (ICER) of the continuous flow device was $198.184 per quality-adjusted life year and $167.208 per life year [43].
One essential technical progress needed to optimise the destination option and simplify the daily life of such patients is the so-called “Transcutaneous Energy Transfer System” (TETS) [44, 45]. With the aid of TETS, the percutaneous energy driveline, which is the main source of infection, would be avoidable. In addition, certain inconveniences in the quality of life of such patients could be resolved. Parallel to the introduction of TETS further improvement of the battery technology is expected.
Although the discussion is a critical one, it would clearly make sense to limit the centres that are authorised to implant long-term VAD to those dealing with cardiac transplantation in Switzerland. Otherwise the numbers of implantations per centre required to generate sufficient experience (including intensive care and ward nursing staff) will not be reached.
1 Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;1:30–41.
2 Mohacsi P, Moschovitis G, Tanner H, Hess OM, Hullin R. Prevalence, increase and costs of heart failure. Heart and Metabolism. 2001;14:9–16.
3 Lietz K, Miller LW. Improved survival of patients with end-stage heart failure listed for heart transplantation: analysis of organ procurement and transplantation network/U.S. United Network of Organ Sharing data, 1990 to 2005. J Am Coll Cardiol. 2007;50:1282–90.
4 Stehlik J, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplantation report-2010. J Heart Lung Transplant. 2010;10:1089–103.
5 Rose EA, Gelijns AC, Moskowitz AJ, Group RS. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345;1435–43.
6 Rogers JG, Butler J, Lansman SL, Gass A, Portner PM, Pasque MK, Pierson RN 3rd. Chronic mechanical circulatory support for intrope-depenent heart failure patients who are not transplant candidates: results of the INTrEPID Trial. J Am Coll Cardiol. 2007;50:741–7.
7 Jeevanandam V. The evolution of cardiac assist device technology. J Heart Lung Transplant. 2011;29:11–2.
8 Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, et al. Third INTERMACS annual report: the evolution of destination therapy in the United States. J Heart Lung Transplant. 2011;30:115–23.
9 Lietz K, Miller LW. Patient selection for left-ventricular assist device. Curr Opin Cardiol. 2009;24:246–51.
10 Lietz K. Destination therapy: patient selection and current outcomes. J Card Surg. 2010;25:462–71.
11 Lahpor J, Khaghani A, Hetzer R, Pavie A, Friedrich I, Sander K, et al. European results with a continuous-flow ventricular assist device for advanced heart failure patients. Eur J Cardiothorac Surg. 2010;37:357–61.
12 Slaughter MS, Bostic R, Tong K, Russo M, Rogers JG. Temporal changes in hospital costs for left ventricular assist device implantation. J Card Surg. 2011;26:535–41.
13 Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–51.
14 Strueber M, O’Driscoll G, Khaghani A, Levy WC, Wieselthaler GM, Investigators H. Multicenter evaluation of an intrapericardial left ventricular assist system. J Am Coll Cardiol. 2011;57:1375–82.
15 Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MS, Milano CA, et al. Impact of center volume on outcomes of left ventricular assist device implantation as destination therapy: analysis of the Thoratec HeartMate Registry, 1998–2005. Circ Heart Fail. 2009;2:3–10.
16 Froesch P, Martinelli M, Meier P, Cook S, Hullin R, Windecker S, et al. Clinical use of temporary percutaneous left ventricular assist devices. Catheter Cardiovasc Interv. 2011;78:304–11.
17 Dandel M, Weng Y, Siniawski H, Stepanenko A, Krabatsch T, Potapov E, et al. Heart failure reversal by ventricular unloading in patients with chronic cardiomyopathy: criteria for weaning from ventricular assist devices. Eur Heart J. 2011;32:1148–60.
18 Krabatsch T, Schweiger M, Dandel M, Stepanenko A, Drews T, Potapov E, et al. Is bridge to recovery more likely with pulsatile left ventricular assist devices than with nonpulsatile flow systems? Ann Thorac Surg. 2011;91:1335–40.
19 Naiyanetr P, Moscato F, Vollkron M, Zimpfer D, Wiesenthaler G, Schirma H. Continuous assessment of cardiac function during rotary blood pump support: a contractility index derived from pump flow. J Heart Lung Transplant. 2011;29:37–44.
20 Beyersdorf F, Schlensak C, Berchtold-Herz M, Trummer G. Regression of “fixed” pulmonary vascular resistance in heart transplant candidates after unloading with ventricular assist devices. J Thorac Cardiovasc Surg. 2010;140:747–9.
21 Cleveland JC Jr, Grover FL, Fullerton DA, Campbell DN, Mitchell MB, Lindenfeld J, et al. Left ventricular assist device as bridge to transplantation does not adversely affect one-year heart transplantation survival. J Thorac Cardiovasc Surg. 2008;136:774–7.
22 Patlolla V, Patten RD, Denofrio D, Konstam MA, Krishnamani R. The effect of ventricular assist devices on post-transplant mortality an analysis of the United network for organ sharing thoracic registry. J Am Coll Cardiol. 2009;53:264–71.
23 Stevenson LW, Pagani FD, Young JB, Jessup M, Miller LW, Kormos RL, et al. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant. 2009;28:535–41.
24 Alba AC, Rao V, Ivanov J, Ross HJ, Delgado DH. Usefulness of the INTERMACS scale to predict outcomes after mechanical assist device implantation. J Heart Lung Transplant. 2009;28:827–33.
25 Klotz S, Valhaus C, Riehl C, Reitz C, Sindermann JR, Scheld HH. Preoperative prediction of post-VAD implant mortality using easily accessible clinical parameters. J Heart Lung Transplant. 2011;29:45–52.
26 Williams ML, Trivedi JR, McCants KC, Prabhu SD, Birks EJ, Oliver L, Slaughter MS. Heart transplant vs left ventricular assist device in heart transplant-eligible patients. Ann Thorac Surg. 2011;91:1330–3.
27 Rogers JG, Aaronson KD, Boyle AJ, Russell SD, Milano CA, Pagani FD, et al.; HeartMate II Investigators. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826–34.
28 Osorio J. Device therapy: continuous flow LVAD improves quality of life. Nature Review Cardiology. 2010;7:360.
29 Backes D, van den Bergh WM, van Duijn AL, Lahpor JR, van Dijk D, Slooter AJ. Cerebrovascular complications of left ventricular assist devices. Eur J Cardiothorac Surg. 2012 Jun 1. [Epub ahead of print]
30 Krishan K, Nair A, Pinney S, Adams D, Anyanwu AC. Low incidence of bleeding-related morbidity with left ventricular assist device implantation in the current era. Artif Organs. 2012;36:746–51.
31 Felix SE, Martina JR, Kirkels JH, Klöpping C, Nathoe H, Sukkel E, et al. Continuous-flow left ventricular assist device support in patients with advanced heart failure: points of interest for the daily management. Eur J Heart Fail. 2012;14:351–6.
32 Kurien S, Hughes KA. Anticoagulation and bleeding in patients with ventricular assist devices: walking the tightrope. AACN Adv Crit Care. 2012;23:91–8.
33 Califano S, Pagani FD, Malani PN. Left ventricular assist device-associated infections. Infect Dis Clin North Am. 2012;26:77–87.
34 Boyle A. Arrhythmias in patients with ventricular assist devices. Curr Opin Cardiol. 2012;27:13–8.
35 Mangi AA. Right ventricular dysfunction in patients undergoing left ventricular assist device implantation: predictors, management, and device utilization. Cardiol Clin. 2011;29:629–37.
36 Matthews JC, Koelling TM, Pagani FD, Aaronson KD. The right ventricular failure risk score. A pre-operative tool for assessing the risk of right ventricualr failure in left ventricular assist device candidates. J Am Coll Cardiol. 2008;51:2163–72.
37 Crow S, Ranjit J, Boyle A, Shumway S, Liao K, Colvin-Adams M, et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;137:208–15.
38 Uriel N, Pak S-W, Jorde UP, Jude B, Susen S, Vincentelli A, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–13.
39 Slaughter M. Hematologic effects of continuous flow left ventricular assist devices. J Cardiovasc Transl Res. 2010;3:618–24.
40 Crow S, Chen D, Milano C, Thomas W, Joyce L, Placentino Vr, et al. Acquired von-Willebrandt syndrome in continuous flow ventricular assist device recipients. Ann Thorac Surg. 2010;90:1263–9.
41 Slaughter MS. Long-term continuous flow left ventricular assist device support and end organ function: prospects for destination therapy. J Cardiac Surg. 2010;25:490–4.
42 Mishra V, Geiran O, Fiane AE, Sørensen G, Andresen S, Olsen E, et al. Costs and reimbursement gaps after implementation of third-generation left ventricular assist devices. J Heart Lung Transplant. 2011;29:72–8.
43 Rogers JG, Bostic RR, Tong KB, Adamson R, Russo M, Slaughter MS. Cost effectiveness analysis of continuous flow left ventricular assist devices as destination therapy. Circ Heart Fail 2011, Epub ahead of print.
44 Lahpor JR. State of the art: implantable ventricular assist devices. Curr Opin Organ Transplant. 2009;14:554–9.
45 Potapov E, Krabatsch T, Ventura HO, Hetzer R. Advances in mechanical circulatory support: year in review. J Heart Lung Transplant. 2011;30:487–93.
Funding / potential competing interests: P. Mohacsi is member of the European Advisory Board (Thoratec Europe Ltd.). No financial support and no other potential conflict of interest relevant to this article were reported.