Pituitary surgery: experience from a large network in Central Switzerland
DOI: https://doi.org/10.4414/smw.2012.13680
Sven
Berkmann, Javier
Fandino, Beat
Müller, Karl F
Kothbauer, Christoph
Henzen, Hans
Landolt
Summary
PRINCIPLES: During the past years our group built a care network for patients with pituitary tumours with referrals from the midlands and the central part of Switzerland, comprising about 1.6 million inhabitants. The purpose of this retrospective observational study with longitudinal data is to review the experience of pituitary surgery and the operative outcome within this Swiss-wide largest network.
METHODS: A total of 182 patients operated at the Neurosurgical Department of the Kantonsspital Aarau 2005–2010 were included in this study. The follow-up was 3.6±1.6 years.
RESULTS: The following lesions were found: non-functioning adenoma (n = 114; 63%); macroprolactinoma (n = 18; 10%); microprolactinoma (n = 11; 6%); acromegaly (n = 11; 6%), Cushing’s disease (n = 7; 4%); Rathke’s cleft cyst (RCC; n = 9; 5%); others (n = 12; 7%). Intraoperative MRI (iMRI) was used in 115 (63%) patients. Preoperatively, hypopituitarism was found in 105 (58%) patients. Postoperative recovery of defunct axes was detected in 48%. Visual field and visual acuity deficits due to optic pathway compression by tumour were detected in 48% and 41% of the patients, respectively. Postoperative recovery of visual function was seen in 89%. The increase of total resection rate by iMRI was statistically significant (p = 0.0007). Recurrent tumour growth was seen in 5 (3%) patients during follow-up.
CONCLUSIONS: Transsphenoidal surgery is the primary treatment for most sellar lesions. The use of iMRI may lead to higher gross total resection rates. In Switzerland close cooperation between specialised centres is a very positive experience both to support operative case loads and to optimise patient follow-up.
Introduction
The goals of pituitary tumour surgery are the cure of endocrine syndromes like acromegaly, recovery from neurological deficits, for example visual impairment resulting from chiasmal compression, and long-term tumour remission [1]. A multidisciplinary approach within a well-structured network is mandatory. While transsphenoidal resection by the specialised neurosurgeon is the primary therapy in the majority of cases, endocrinologists, ophthalmologists, otorhinolaryngologists, neuropathologists, and general practitioners are involved in the perioperative, as well as in the long-term treatment and follow-up of these patients.
During the past six years our group built a network of care with referrals from the central part of Switzerland, comprising about 1.6 million inhabitants of the cantons: Aargau, Lucerne, Nidwalden, Obwalden, Solothurn, Schwyz, Uri, and Zug. The purpose of this retrospective observational study with longitudinal data is to review the experience of pituitary surgery and the operative outcome within this Swiss-wide largest network.
Patients and Methods
Patients’ characteristics and indications for surgery
A total of 182 patients with sellar lesions who underwent surgery at the Neurosurgical Department of the Kantonsspital Aarau from 1 January 2005 to 31 December 2010 were included in this study. Demographic data of these patients, details about preoperative work-up, surgical technique, and follow-up controls were collected from hospital records (table 1).
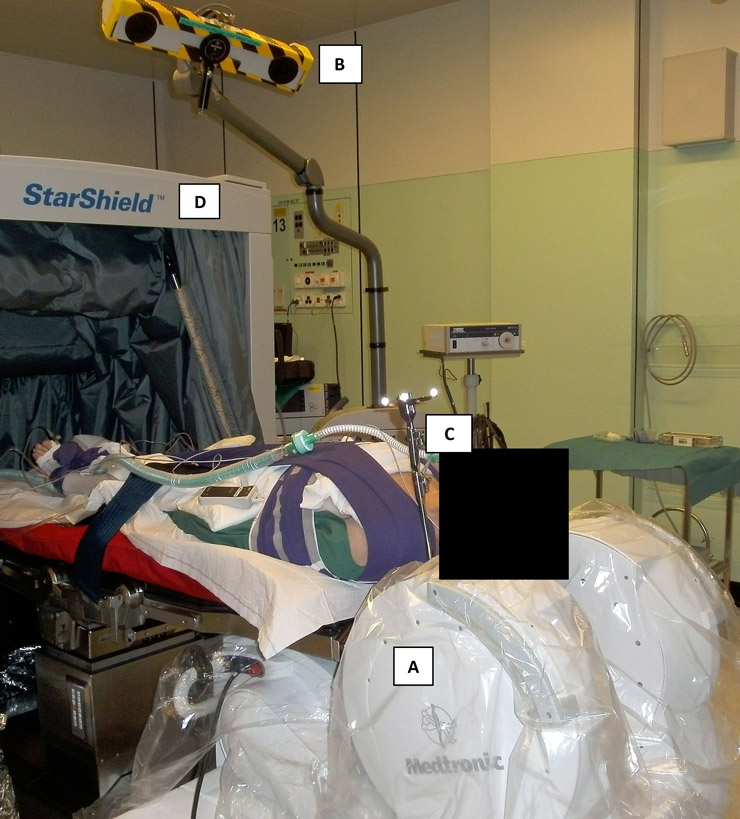
Figure 1
Setup for iMRI-guided transsphenoidal surgery.
The patient is positioned in supine position with the head fixed in a MRI-compatible head clamp. Since the PoleStar® device (A; Medtronic, Inc.) needs to be placed very close to the shoulders, gentle traction is applied by soft bandages. Two LED cameras (B) record the position of a tracking frame (C) which is fixed to the head clamp and referenced to the surface of the patient’s face for frameless stereotactic navigation by the StealthStation TREON® system (Medtronic, Inc.). During the scans, the PoleStar® device is moved into scanning position and the StarShield Portable RF Shield® (D; Medtronic, Inc.) is closed. Thereby, electronic devices may remain turned on during scanning and there’s no need for full room radio frequency shielding.
The resulting data were analysed using GraphPad® statistical software. The results were evaluated using contingency table analysis by Fisher’s exact test. P-values <0.05 were regarded as statistically significant.
Surgery was indicated as primary treatment in patients with endocrinologically non-functioning lesions with local mass effects, acromegaly, and Cushing’s disease according to the consensus statements formulated by the Endocrine Society [2, 3]. In the case of asymptomatic non-functioning adenomas (NA) incidentally detected by cranial MRI, defined as incidentaloma, resection was indicated if further growth on follow-up imaging was found, if development of mass lesions was impending (e.g., in the case of an elevated optic chiasm), and/or if histological assessment was mandatory (e.g., history of extracranial malign tumour). As proposed in the literature [4], drug therapy with cabergoline (Cabaser® and Dostinex®, Pfizer AG, Zurich, Switzerland), bromocriptine (Parlodel®, Novartis Pharma Schweiz AG, Berne, Switzerland), or Quinagolid (Norprolac®, Ferring AG, Baar, Switzerland) is the primary treatment option for patients with prolactinoma at our institutions. Tumour resection was an option in the following cases: intolerable side effects due to long-term medication; persisting symptomatic hyperprolactinaemia during drug therapy; rapid decline of visual acuity because of intratumoural haemorrhage; patients objecting to long-term medication; tumour regrowth after tapering of long-term drug therapy in patients objecting to be on medication again.
Pre- and postoperative workup
Each patient underwent a comprehensive preoperative screening for ophthalmologic and endocrinological symptoms. The ophthalmologic examination included testing of visual acuity (VA) and colour vision, visual field (VF) testing with the Goldmann perimeter and fundus examination. The endocrinological evaluation included the following tests: morning serum cortisol and dynamic testing with tetracosactid-hexaacetate (Synacthen®); free thyroxine (T4) and total trijodthyronine (T3) and/or free T3, thyroid-stimulating hormone (TSH); luteinising hormone; follicle-stimulating hormone; estradiol; testosterone; insulin-like growth factor (IGF-1); human growth hormone (hGH); prolactin (PRL); urine osmolality; serum and urine sodium.
The radiological workup included a preoperative CT and MRI scan for tumour assessment and frameless stereotactic navigation. In patients with occult adenoma causing Cushing’s disease we used sinus petrosus sampling and 11C-methionine positron emission tomography as described by Tang et al. [5]. The tumour volume was calculated using the formula proposed by Lundin et al. [6]. In addition, the extension of the lesions was classified by the modified version of Hardy’s grading system [7, 8].
Only patients with a follow-up of at least 12 months were included in this study. Every patient had neurosurgical, endocrinological and ophthalmological tests within eight weeks and a high-field MRI-scan three months after surgery. Depending on the results, further follow up checks were scheduled at least once each year for at least five years.
Surgical technique for transsphenoidal tumour resection and criteria for using intraoperative MRI (iMRI)
The patients were operated by a microsurgical transnasal, transsphenoidal approach; the surgical technique used at the Kantonsspital Aarau was published [9]. Since April 2006, iMRI (PoleStar N20®, Medtronic Navigation, Louisville, CO, USA) with a permanent magnetic field of 0.15 Tesla was used for 115 patients (63% of the total study group) (fig. 1 and table 1). Routinely, 11-min 2 mm-slice gadolinium-enhanced T1-weighted scans were made to assess tumour resection and obtain datasets for further intraoperative navigation by StealthStation TREON®. The iMRI was used to guide resection whenever possible; however, due to the following reasons iMRI was not used in the whole study group: (1.) contraindications by the patient (i.e. cardiac pacemaker); (2.) small tumour not visible on preoperative high-field MRI; (3.) restricted availability for emergency cases. The non-iMRI cases were operated on using fluoroscopy and frameless image-guidance based on preoperative MRI and CT scans.
|
Table 1: Patients’ characteristics. |
| |
All
|
NA
|
MacroPRL
|
MicroPRL
|
Acromegaly
|
M. Cushing
|
RCC
|
| N |
182 |
114 (63%) |
18 (10%) |
11 (6%) |
11 (6%) |
7 (4%) |
9 (5%) |
| Mean age (yrs) |
52.7±17.6 |
59.5±14.5 |
37.2±17.1 |
27.4±8.0 |
51.5±16.5 |
35.9±11.9 |
49.3±16.4 |
| Age range (yrs) |
16–88 |
22–88 |
16–65 |
18–41 |
18–66 |
18–49 |
21–72 |
| Male patients |
107 (59%) |
79 (69%) |
10 (56%) |
2 (18%) |
9 (82%) |
|
4 (44%) |
| Transsph. approach |
179 (98%) |
114 (100%) |
18 (100%) |
11 (100%) |
11 (100%) |
7 (100%) |
8 (89%) |
| Use of iMRI |
115 (63%) |
79 (69%) |
7 (39%) |
6 (55%) |
6 (54%) |
4 (57%) |
5 (56%) |
| NA = non-functioning adenoma; macroPRL = macroprolactinoma; microPRL = microprolactinoma; RCC = Rathke’s cleft cyst; transsph = transsphenoidal; iMRI = intraoperative MRI; yrs = years. |
Results
Patients’ characteristics and preoperative findings
Endocrinological and histological analysis confirmed the following diagnoses: NA (n = 114; 63%); macroprolactinoma (n = 18; 10%); microprolactinoma (n = 11; 6%); acromegaly (n = 11; 6%), Cushing’s disease (n = 7; 4%); Rathke’s cleft cyst (RCC)(n = 9; 5%); others (n = 12; 6%; i.e., craniopharyngioma, n = 3; Tuberculum sellae meningioma with intrasellar extension, n = 2; metastases, n = 2; necrotic adenoma, n = 1; pituizytoma, n = 1; granular cell tumour, n = 1; thrombosed aneurysm, n = 1; colloidal cyst, n = 1). Thirteen patients (7%) presented with incidentalomas. Details of patients’ characteristics are shown in table 1.
In patients’ suffering from prolactinomas, the surgical indications were as follows: intolerable side effects during long term medication, n = 8 (42%); persisting symptomatic hyperprolactinaemia during drug therapy, n = 7 (37%); rapid decline of visual acuity due to intratumoural haemorrhage, n = 5 (26%); patients objecting to long term medication, n = 4 (21%); tumour recurrence after tapering of medication, n = 4 (21%). One patient [10] with tumour induced erosion of the sellar floor suffered from meningitis during therapy with dopamine agonists. In this case, tumour shrinkage permitted intracranial bacterial spread from endonasal sinusitis. During antibiotic therapy the tumour was resected and the CSF fistula closed.
Macroadenomas, defined as tumours with a maximal diameter >10 mm were detected as follows: NA, n = 113 (99%); acromegaly n = 8 (73%); Cushing’s disease, n = 1 (14%). Details of tumour sizes and extensions are shown in table 2. In 3 patients with Cushing’s disease the tumour could not be detected by preoperative MRI.
The incidences of preoperative hypopituitarism are shown in table 3. Dysfunctions of the individual pituitary axes were detected as follows: pituitary-adrenal axis, n = 47 (27%); pituitary-thyroid axis, n = 55 (30%); somatotroph axis, n = 56 (33%); pituitary-gonadal axis, n = 78 (49%). There were single cases of preoperative diabetes insipidus (DI) (57 year-old patient with a sellar metastasis of ductal breast cancer), and preoperative syndrome of inadequate antidiuretic hormone secretion (SIADH; 86 year-old patient with a NA).
The results of the preoperative ophthalmological workup are shown on table 4. Diplopia due to nerve palsies was found in 10 patients with palsies of the following nerves: oculomotor nerve, n = 5 (3%); trochlear nerve, n = 1 (0.5%); abducent nerve, n = 8 (5%).
|
Table 2: Radiological tumour characteristics. |
|
|
All
|
NA
|
MacroPRL
|
MicroPRL
|
Acromegaly
|
M. Cushing
|
RCC
|
| Mean ap (mm) |
18±8 |
19±7 |
19±6 |
7±2 |
16±10 |
6±3 |
14±3 |
| Mean lateral (mm) |
19±8 |
21±6 |
23±11 |
7±2 |
18±12 |
6±3 |
17±5 |
| Mean cc (mm) |
23±11 |
25±11 |
24±8 |
7±2 |
19±15 |
6±3 |
18±5 |
| Mean volume (cm³) |
6.0±7.4 |
7.1±8.0 |
6.6±5.4 |
0.18±0.1 |
6.1±8.7 |
0.2±0.2 |
2.6±2.1 |
| Mean Hardy's grade |
2.5±0.8 |
2.7±0.7 |
2.7±0.6 |
1±0 |
2.1±1.0 |
1.2±0.4 |
2.4±0.5 |
| NA = non-functioning adenoma; macroPRL = macroprolactinoma; microPRL = microprolactinoma; RCC = Rathke’s cleft cyst; ap = anterior-posterior diameter; lateral = lateral diameter; cc = cranio-caudal diameter.
Hardy’s grading system: grade I: diameter <10 mm; grade II: diameter 10–20 mm and suprasellar extension within 10 mm of the sphenoidal plane; grade III: diameter 20–40 mm and suprasellar extension of up to 30 mm; grade IV: diameter greater than 40 mm and extension far beyond the sellar space. |
|
Table 3: Pre- and postoperative endocrinological function. |
|
|
All
|
NA
|
MacroPRL
|
MicroPRL
|
Acromegaly
|
Cushing
|
RCC
|
| N |
182 |
114 |
18 |
11 |
11 |
7 |
9 |
| Hypopit preop |
105 (58%) |
83 (73%) |
6 (33%) |
|
2 (18%) |
1 (14%) |
3 (33%) |
| Hypopit postop |
100 (55%) |
72 (63%) |
9 (50%) |
1 (9%) |
2 (18%) |
1 (14%) |
3 (33%) |
| Recovery |
50 (48%) |
42 (51%) |
3 (50%) |
N/A |
2 (100%) |
1 (100%) |
2 (66%) |
| Loss |
35 (19%) |
28 (25%) |
3 (18%) |
1 (9%) |
1 (9%) |
|
1 (11%) |
| Transient DI |
16 (9%) |
6 (5%) |
1 (5%) |
3 (27%) |
1 (9%) |
1 (14%) |
1 (11%) |
| Permanent DI |
8 (4%) |
5 (4%) |
|
|
1 (9%) |
|
2 (22%) |
| SIADH |
10 (5%) |
5(4%) |
1 (5%) |
1 (9%) |
|
1 (14%) |
|
| NA = non-functioning adenoma; macroPRL = macroprolactinoma; microPRL = microprolactinoma; RCC = Rathke’s cleft cyst; hypopit preop = preoperative hypopituitarism; hypopit post = postoperative hypopituitarism; recovery = recovery of any pituitary axis; loss = loss of any pituitary axis; DI = diabetes insipidus; SIADH = syndrome of inadequate antidiuretic hormone secretion. |
|
Table 4: Pre- and postoperative ophthalmological function. |
|
|
All
|
NA
|
MacroPRL
|
MicroPRL
|
Acromegaly
|
Cushing
|
RCC
|
| N |
182 |
114 |
18 |
11 |
11 |
7 |
9 |
| VFD preop |
88 (48%) |
68 (60%) |
8 (44%) |
|
3 (27%) |
|
3 (33%) |
| VFD postop recovery |
78 (89%) |
64 (94%) |
6 (75%) |
N/A |
3 (100%) |
N/A |
3 (100%) |
| VFD postop stable |
8 (9%) |
4 (5%) |
2 (25%) |
N/A |
|
N/A |
|
| VFD postop worse |
2 (2%) |
1 (1%) |
|
N/A |
|
N/A |
|
| VAD preop |
75 (41%) |
59 (52%) |
6 (33%) |
|
3 (27%) |
|
3 (33%) |
| VAD postop recovery |
65 (87%) |
55 (93%) |
5 (83%) |
N/A |
3 (100%) |
N/A |
2 (67%) |
| VAD postop stable |
8 (11%) |
4 (8%) |
1 (17%) |
N/A |
|
N/A |
1 (33%) |
| VAD postop worse |
2 (3%) |
1 (2%) |
|
N/A |
|
N/A |
|
| NA = non-functioning adenoma; macroPRL = macroprolactinoma; microPRL = microprolactinoma; RCC = Rathke’s cleft cyst; VFD = visual field deficit; VAD = visual acuity deficit; preop = preoperative; postop = postoperative. |
Resection rate with intraoperative MRI guidance
Intraoperative MRI was used in 115 (63%) patients. The majority of the iMRI-cases were patients with NA (n = 79, 69%). The results are summarised in table 5. Tumour remnants were detected by iMRI in 45 (39%) patients of the total iMRI-group and in 34 (43%) of the patients with NA. Additional resection was possible in 36 (80%) and 31 (91%) patients, respectively. This resulted in a gross total resection (GTR) rate on postoperative MRI of 82% in both groups. Due to additional resections of unexpected remnants detected by iMRI a significant difference of GTR rates was accomplished (total iMRI group, 82% instead of 61%, p = 0.0007; NA, 82% instead of 57%, p = 0.0009).
|
Table 5: Results of intraoperative MRI. |
|
|
All
|
NA
|
| N |
115 |
79 |
| GTR on 1st iMRI |
70 (61%) |
45 (57%) |
| Tumour remnant on 1st iMRI |
45 (39%) |
34 (43%) |
| Additional resection |
36 (80%) |
31 (91%) |
| GTR on postop MRI |
94 (82%) |
65 (82%) |
| Tumour remnant on postop MRI |
21 (18%) |
14 (18%) |
| iMRI-sensitivity for GTR |
0.81 (0.72–0.88) |
0.87 (0.76–0.93) |
| iMRI-specifity for GTR |
1.0 (0.47–1.0) |
1.0 (0.25–1.0) |
| Increase of GTR by iMRI, p
|
0.0007 |
0.0009 |
| NA = non-functioning adenoma; GTR = gross total resection; 1st iMRI = first intraoperative MRI scan after initial resection. |
Postoperative results and follow-up
A summary of the endocrinological results is shown in table 3. Overall recovery rate for at least one axis was 48%. Recovery of the individual axes after surgery was detected as follows: pituitary-adrenal axis, n = 15 (32%); pituitary-thyroid axis, n = 18 (33%); somatotroph axis, n = 56 (32%); pituitary-gonadal axis, n = 24 (31%). The rates for new hypopituitarism were as follows: pituitary-adrenal axis, n = 26 (19%); pituitary-thyroid axis, n = 18 (14%); somatotroph axis, n = 13 (11%); pituitary-gonadal axis, n = 15 (20%).
The postoperative outcome of ophthalmological function is shown in table 4. While about 90% of the patients recovered from chiasmal syndrome, one patient with an invasive non-functioning giant pituitary adenoma and one patient with a large craniopharyngioma suffered from decreased visual function after surgery. Both patients showed tumour remnants on immediate postoperative imaging and were reoperated; however, they did not fully recover.
Data on follow-up times, remission rates and additional therapies are summarised on table 6. The mean follow-up of the total study group was 3.6±1.6 years. Remission of local compression symptoms was reached in 109 (96%) patients with non-functioning adenoma. Three (2%) patients in this subgroup showed persistence of ophthalmologic deficits; in two cases suprasellar remnants had to be reoperated by subfrontal approaches. One case was treated by Gamma Knife radiosurgery, as were two patients with asymptomatic growth of smaller remnants detected by follow-up imaging. Eleven (61%) cases with macroprolactinoma suffered from persistence of PRL excess. These patients were treated by dopamine agonists; however, radiosurgery and surgery was indicated in single cases due to limited response of PRL levels to drug therapy. Six (55%) patients with microprolactinoma showed long-term remission for 4.1±1.5 years after selective adenomectomy. In the remaining 5 (45%) patients therapy with dopamine agonists was successful in normalising PRL levels. There were no symptoms due to side-effects of drug therapy after surgery.
Three patients with invasive hGH-secreting macroadenomas had to be treated with drug therapy while long-term remission rate after surgery was 73%.
Remission rate during follow-up of 3.2±1.6 years in patients with Cushing’s disease was 57%. In 3 cases the microadenoma could not be visualised by preoperative MRI, and resection was guided by the results of sinus petrosus sampling. Two of these later patients suffered from recurrent ACTH-excess during the first year of follow-up and were reoperated. One patient with persisting disease had bilateral adrenalectomy because of persisting Cushing’s disease after two transsphenoidal tumour resections.
|
Table 6: Follow-up and additional therapies. |
|
|
All
|
NA
|
MacroPRL
|
MicroPRL
|
Acromegaly
|
Cushing
|
RCC
|
| N |
182 |
114 |
18 |
11 |
11 |
7 |
9 |
| Mean follow-up |
3.6±1.6 |
3.4±1.7 |
4.0±1.8 |
4.1±1.5 |
3.8±1.9 |
3.2±1.6 |
4.4±1.6 |
| Remission |
151 (83%) |
109 (96%) |
5 (28%) |
6 (55%) |
8 (73%) |
4 (57%) |
9 (100%) |
| Symptomatic persistence |
26 (14%) |
3 (2%) |
11 (61%) |
4 (36%) |
3 (27%) |
1 (14%) |
|
| Recurrent tumour growth |
5 (3%) |
2 (2%) |
1 (6%) |
1 (17%) |
|
2 (28%) |
|
| Recurrence latency (yrs) |
2.5±0.9 |
2.9±0.5 |
3.0 |
2.0 |
N/A |
2.1±1.4 |
N/A |
| Longterm drug therapy |
17 (9%) |
|
11 (72%) |
5 (45%) |
3 (27%) |
|
|
| Additional surgery |
6 (3%) |
2 (2%) |
1 (6%) |
|
|
3 (43%) |
|
| Adjuvant radiotherapy |
5 (3%) |
3 (3%) |
1 (6%) |
|
|
|
|
| NA = non-functioning adenoma; macroPRL = macroprolactinoma; microPRL = microprolactinoma; RCC = Rathke’s cleft cyst; remission = number of patients with recovery of local mass effect and/or cure of endocrinological syndromes. |
Discussion
This study is a comprehensive compilation of the characteristics of patients with surgical diseases of the pituitary gland in the central part of Switzerland. In pituitary surgery, as in other specialities in medicine, a minimum number of cases is necessary to sustain the best possible outcome [11, 12]. This case load was reached by a close interaction between the different specialists within our network. The oncological, endocrinological, and ophthalmological results of the network established to treat these patients compares favourably to international series [13–16].
During the observed time interval low-field iMRI was used whenever possible. It allowed assessment of residual tumours, updating the images for intraoperative navigation tools, and exclusion of imminent complications such as haemorrhage before the site was closed. Intraoperative MR imaging has been validated in transsphenoidal surgery during the past decade [17–20]. As shown by others [9, 21, 22] as well as in the present study, it may significantly increase gross total resection rates.
Recovery rates of visual field deficits and impaired visual acuity were >90% in the present study groups. Several studies have analysed ophthalmological outcome after transsphenoidal surgery [23–27]. Improvement of vision was noted in 50% to 90% of the patients in the literature. Due to different methods in assessing ophthalmological function, as well as variable grades of severity and duration of deficits comparisons of the various studies may be biased. Nevertheless, the use of iMRI may be an important factor to increase visual recovery rates. It was reported that iMR imaging seems to correlate with the prognosis of visual deficits and revision surgery in the case of unexpected symptomatic suprasellar remnants [9].
In our study, recovery of defunct pituitary axes was seen in 50% of the patients with hypopituitarism. A significant factor for postoperative recovery of hypopituitarism is the detection of gross total resection on postoperative imaging [28]. As in the present study, the rate of new onset of hypopituitarism is commonly below 20% [11, 13, 29–31]. Higher rates have been described in the literature, especially in the case of larger and hormonally inactive tumours [13, 28, 32], pituitary apoplexy [33, 34], and transcranial surgery [31]. While the impact of iMRI on the gross total resection rate in the present subgroup was significant, the correlation to postoperative endocrinological function is unclear; however, it was reported in a controlled cohort study that the use of iMRI does not provoke higher rates of hypopituitarism or lower recovery rates [22].
Although virtually always benign, hGH-expressing tumours are associated with cardiovascular, respiratory, endocrine and metabolic syndroms [35, 36]. The weighted mean of the standardised mortality ratio from 16 published studies of patients with acromegaly was reported to be 1.72 [95% confidence interval (CI), 1.62–1.83] [37]. Therefore, radical tumour resection is mandatory. Additional therapies, such as radiotherapy and long-term drug therapy are commonly supposed to be second-line treatments in the case of persistent hormonal excess, which is often caused by invasive growth of a residual tumour [3]. Depending on the tumour size and growth pattern long-term remission was reported in 50%–90% of the cases in the literature. The remission rate in our series of 11 patients during a mean follow up of 3.8 ± 1.9 years was 73%. The remaining three (27%) patients needed additional drug therapy. The use of iMRI in surgery for acromegaly was analysed by Fahlbusch et al.; they concluded that with regard to preoperative GH levels and tumour size, intraoperative MRI can help to achieve endocrine remission in patients who are normally considered not to be curable. Furthermore, iMRI increased the rate of endocrine remission by 10%. In the present study, two (40%) patients in the non-iMRI group and one (16%) patient in the iMRI group did not show remission; however, the significance of this difference in this uncontrolled series remains unclear.
Hypercortisolism caused by Cushing’s disease leads to diabetes mellitus, arterial hypertension, obesity, osteoporosis, and immunosuppression; therefore, five year survival rates of untreated Cushing’s disease were reported to be below 50% [38]. The first-line treatment is transsphenoidal resection; however, visualisation and targeted removal of these often rather small tumours can be demanding. In up to 40% of these cases the neoplasm cannot be detected on preoperative high-field MRI. In these cases preoperative localisation of the tumours is done by sinus petrosus sampling [39, 40]. As an adjunct to this invasive method we used 11C-methionine positron emission tomography as described by Tang et al. [5]. In 18 retrospective reports published since 1995 involving more than 3,000 patients, all with a minimum of 40 patients and a minimum 6-month follow-up, the remission rate ranged from 69% to 98% (simple average 79%) [41]. Recurrence after primary successful remission occurs in about 20% of patients [41, 42]. In the present study, six (86%) of seven patients showed postoperative remission. During the follow-up of 3.2±1.6 years, two (29%) patients showed recurrence. Intraoperative MRI was helpful to assess the extent of the approach and the site of the resection; however, due to the often restricted visibility of the tumour, remnants could not be visualised.
As proposed in the literature [4], surgical treatment for prolactinoma was restricted within our network to a few patients with the following indications: (1.) intolerable side effects due to long-term medication, (2.) persisting symptomatic hyperprolactinaemia during therapy, (3.) rapid decline of visual acuity because of intratumoural haemorrhage, (4.) patients objecting to long-term medication and (5.) tumour regrowth after tapering of long-term medication in patients objecting to be on medication again. Long-term drug therapy was necessary in 11 (72%) patients with macroprolactinoma and in 5 (42%) cases with microprolactinoma. In medically resistant prolactinoma controlled studies regarding surgical outcomes are lacking. Recurrence rates range from 7%–75%, depending on tumour volume, extension and experience of the neurosurgeon [43, 44]. Postoperative remission in microprolactinomas was reported to be as high as 90%, and 84% of these patients may show normal PRL levels during long-term follow-up [45]. Reported rates of immediate (35%) as well as long-term remission (25%) are generally much worse for macroadenomas [46]. Any series analysing the impact of iMRI in prolactinoma surgery is lacking in the literature, and the small number of patients in the present series does not allow analysis as to whether iMRI would lead to a better outcome.
Conclusion
Transsphenoidal surgery is the primary treatment for most sellar lesions. The use of iMRI may lead to higher gross total resection rates. In Switzerland close cooperation between specialised centres is a very positive experience both to support operative case loads and to optimise patient follow-up.
Acknowledgement: The authors are obliged to all the referring practitioners for their daily cooperation, and especially to the following colleagues: Dr. med. J. Lareida, Division of Endocrinology, Hirslanden Klinik, Aarau; Prof. Dr. med. J. H. Beer, Department of Internal Medicine, Kantonsspital, Baden; Dr. med. B. Schwegler, Division of Endocrinology, Kantonsspital, Zug; Dr. med. M. Stahl, Division of Endocrinology, Kantonsspital, Olten; Prof. Dr. med. F. Metternich, Clinic of Otorhinolaryngology, Kantonsspital, Aarau; Dr. med. Ch. Schlegel, Division of Otorhinolaryngology, Luzerner Kantonsspital; Prof. Dr. med. H. Killer, Division of Neuroophthalmology, Kantonsspital, Aarau; Dr. med. O. Job, Department of Ophthalmology, Luzerner Kantonsspital.
References
1 Buchfelder M, Schlaffer S. Surgical treatment of pituitary tumours. Best Pract Res Clin Endocrinol Metab. 2009;23(5):677–92.
2 Freda PU, Beckers AM, Katznelson L, Molitch ME, Montori VM, et al. Pituitary incidentaloma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(4):894–904.
3 Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D, et al. Guidelines for acromegaly management: an update. J Clin Endocrinol Metab. 2009;94(5):1509–17.
4 Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273–88.
5 Tang BN, Levivier M, Heureux M, Wikler D, Massager N, Devriendt D, et al. 11C-methionine PET for the diagnosis and management of recurrent pituitary adenomas. Eur J Nucl Med Mol Imaging. 2006;33(2):169–78.
6 Lundin P, Pedersen F. Volume of pituitary macroadenomas: assessment by MRI. J Comput Assist Tomogr. 1992;16(4):519–28.
7 Hardy J, Wigser SM. Transsphenoidal surgery of pituitary fossa tumors with televised radiofluoroscopic control. J Neurosurg. 1965;23(6):612–9.
8 Shou XF, Li SQ, Wang YF, Zhao Y, Jia PF, Zhou LF. Treatment of pituitary adenomas with a transsphenoidal approach. Neurosurgery. 2005;56(2):249–56; discussion 249–56.
9 Berkmann S, Fandino J, Zosso S, Killer HE, Remonda L, Landolt H. Intraoperative magnetic resonance imaging and early prognosis for vision after transsphenoidal surgery for sellar lesions. J Neurosurg. 2011;115(3):518–27.
10 Berkmann S, Landolt H. Sinugene Meningitis bei Makroprolaktinom unter Dopamin-Agonisten. Forum Med Suisse. 2010;10(11):216–7.
11 Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40(2):225–36; discussion 236–7.
12 Ahmed S, Elsheikh M, Stratton IM, Page RC, Adams CB, Wass JA. Outcome of transphenoidal surgery for acromegaly and its relationship to surgical experience. Clin Endocrinol (Oxf). 1999;50(5):561–7.
13 Fatemi N, Dusick JR, Mattozo C, McArthur DL, Cohan P, Boscardin J,, et al. Pituitary hormonal loss and recovery after transsphenoidal adenoma removal. Neurosurgery. 2008;63(4):709–18; discussion 18–9.
14 Murad MH, Fernández-Balsells MM, Barwise A, Gallegos-Orozco JF, Paul A, Lane MA, et al. Outcomes of surgical treatment for nonfunctioning pituitary adenomas: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2010;73(6):777–91.
15 Kreutzer J, Buslei R, Wallaschofski H, et al. Operative treatment of prolactinomas: indications and results in a current consecutive series of 212 patients. Eur J Endocrinol. 2008;158(1):11–8.
16 Hofmann BM, Hlavac M, Martinez R, Buchfelder M, Muller OA, Fahlbusch R. Long-term results after microsurgery for Cushing disease: experience with 426 primary operations over 35 years. J Neurosurg. 2008;108(1):9–18.
17 Bohinski RJ, Warnick RE, Gaskill-Shipley MF, et al. Intraoperative magnetic resonance imaging to determine the extent of resection of pituitary macroadenomas during transsphenoidal microsurgery. Neurosurgery. 2001;49(5):1133–43; discussion 43–4.
18 Fahlbusch R, Ganslandt O, Buchfelder M, Schott W, Nimsky C. Intraoperative magnetic resonance imaging during transsphenoidal surgery. J Neurosurg. 2001;95(3):381–90.
19 Hirschl RA, Wilson J, Miller B, Bergese S, Chiocca EA. The predictive value of low-field strength magnetic resonance imaging for intraoperative residual tumor detection. Clinical article. J Neurosurg. 2009;111(2):252–7.
20 Nimsky C, Ganslandt O, Hofmann B, Fahlbusch R. Limited benefit of intraoperative low-field magnetic resonance imaging in craniopharyngioma surgery. Neurosurgery. 2003;53(1):72–80; discussion 80–1.
21 Nimsky C, von Keller B, Ganslandt O, Fahlbusch R. Intraoperative high-field magnetic resonance imaging in transsphenoidal surgery of hormonally inactive pituitary macroadenomas. Neurosurgery. 2006;59(1):105–14; discussion 105–14.
22 Berkmann S, Fandino J, Muller B, Remonda L, Landolt H. Intraoperative MRI and endocrinological outcome of transsphenoidal surgery for non-functioning pituitary adenoma. Acta Neurochir (Wien). 2012;154(4):639-47.
23 Powell M. Recovery of vision following transsphenoidal surgery for pituitary adenomas. Br J Neurosurg. 1995;9:367–73.
24 Cohen AR, Cooper PR, Kupersmith MJ, Flamm ES, Ransohoff J. Visual recovery after transsphenoidal removal of pituitary adenomas. Neurosurgery. 1985;17(3):446–52.
25 Findlay G, McFadzean RM, Teasdale G. Recovery of vision following treatment of pituitary tumours; application of a new system of assessment to patients treated by transsphenoidal operation. Acta Neurochir (Wien). 1983;68(3–4):175–86.
26 Harris PE, Afshar F, Coates P, Doniach I, Wass JA, Besser GM, et al. The effects of transsphenoidal surgery on endocrine function and visual fields in patients with functionless pituitary tumours. Q J Med. 1989;71(265):417–27.
27 Laws ER Jr, Trautmann JC, Hollenhorst RW Jr. Transsphenoidal decompression of the optic nerve and chiasm. Visual results in 62 patients. J Neurosurg. 1977;46(6):717–22.
28 Webb SM, Rigla M, Wagner A, Oliver B, Bartumeus F. Recovery of hypopituitarism after neurosurgical treatment of pituitary adenomas. J Clin Endocrinol Metab. 1999;84(10):3696–700.
29 Barker FG, 2nd, Klibanski A, Swearingen B. Transsphenoidal surgery for pituitary tumors in the United States, 1996–2000: mortality, morbidity, and the effects of hospital and surgeon volume. J Clin Endocrinol Metab. 2003;88(10):4709–19.
30 Comtois R, Beauregard H, Somma M, Serri O, Aris-Jilwan N, Hardy J. The clinical and endocrine outcome to trans-sphenoidal microsurgery of nonsecreting pituitary adenomas. Cancer. 1991;68(4):860–6.
31 Nomikos P, Ladar C, Fahlbusch R, Buchfelder M. Impact of primary surgery on pituitary function in patients with non-functioning pituitary adenomas – a study on 721 patients. Acta Neurochir (Wien). 2004;146(1):27–35.
32 Colao A, Cerbone G, Cappabianca P, Ferone D, Alfieri A, Di Salle F, et al. Effect of surgery and radiotherapy on visual and endocrine function in nonfunctioning pituitary adenomas. J Endocrinol Invest. 1998;21(5):284–90.
33 Randeva HS, Schoebel J, Byrne J, Esiri M, Adams CB, Wass JA. Classical pituitary apoplexy: clinical features, management and outcome. Clin Endocrinol. (Oxf) 1999;51(2):181–8.
34 Semple PL, Webb MK, de Villiers JC, Laws ER Jr. Pituitary apoplexy. Neurosurgery. 2005;56(1):65–72; discussion 72–3.
35 Melmed S. Medical progress: Acromegaly. N Engl J Med. 2006;355(24):2558–73.
36 Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004;25(1):102–52.
37 Dekkers OM, Biermasz NR, Pereira AM, Romijn JA, Vandenbroucke JP. Mortality in acromegaly: a metaanalysis. J Clin Endocrinol Metab. 2008;93(1):61–7.
38 Freda PU, Wardlaw SL. Clinical review 110: Diagnosis and treatment of pituitary tumors. J Clin Endocrinol Metab. 1999;84(11):3859–66.
39 Boscaro M, Arnaldi G. Approach to the patient with possible Cushing’s syndrome. J Clin Endocrinol Metab. 2009;94(9):3121–31.
40 Jagannathan J, Sheehan JP, Jane JA Jr. Evaluation and management of Cushing syndrome in cases of negative sellar magnetic resonance imaging. Neurosurg Focus. 2007;23:E3.
41 Kelly DF. Transsphenoidal surgery for Cushing’s disease: a review of success rates, remission predictors, management of failed surgery, and Nelson’s Syndrome. Neurosurg Focus. 2007;23:E5.
42 Bochicchio D, Losa M, Buchfelder M. Factors influencing the immediate and late outcome of Cushing’s disease treated by transsphenoidal surgery: a retrospective study by the European Cushing’s Disease Survey Group. J Clin Endocrinol Metab. 1995;80(11):3114–20.
43 Losa M, Mortini P, Barzaghi R, Gioia L, Giovanelli M. Surgical treatment of prolactin-secreting pituitary adenomas: early results and long-term outcome. J Clin Endocrinol Metab. 2002;87(7):3180–6.
44 Serri O, Rasio E, Beauregard H, Hardy J, Somma M. Recurrence of hyperprolactinemia after selective transsphenoidal adenomectomy in women with prolactinoma. N Engl J Med. 1983;309(5):280–3.
45 Tyrrell JB, Lamborn KR, Hannegan LT, Applebury CB, Wilson CB. Transsphenoidal microsurgical therapy of prolactinomas: initial outcomes and long-term results. Neurosurgery. 1999;44(2):254–61; discussion 61–3.
46 Molitch MT, Thorner MO, Wilson C. Management of prolactinomas. J Clin Endocrinol Metab. 1997;82(4):996–1000.
