Figure 1
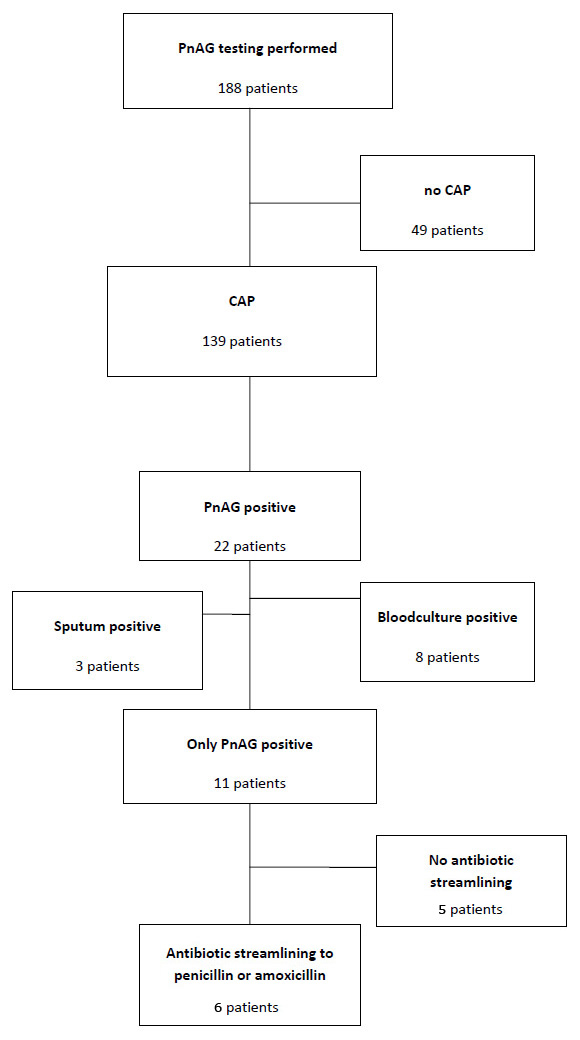
Patients in whom PnAG Test was performed. PnAG = Binax Now® urine Pneumococcal Antigen test.
DOI: https://doi.org/10.4414/smw.2012.13679
Community acquired pneumonia (CAP) is a major cause of morbidity and mortality worldwide. In the United States, it is estimated that 10.5% of hospitalisations are due to CAP in adults ≥65 years, and it seems that this number has doubled in the last 30 years [1]. In Europe, the incidence of CAP varies from 1.9 to 9 cases per 1000 persons with a proportion of hospital admissions that ranges between 8 and 51% [2, 3], with a mean length of stay of 8–10 days [4]. These large differences are mainly due to different study designs in different European countries, as large multinational studies are lacking, and the European Centre of disease prevention and control (ECDC) does not collect these data. Despite many efforts to improve the care of patients with CAP, microbiological pathogen identification and mortality have remained broadly unchanged over the last 30 years [5–7]. About 40% of identified pathogens are pneumococci and up to 57% of CAP with unidentified causative agents are also due to S. pneumonia [8, 9].
Due to the development of resistance to antibiotic treatments, pathogen identification and streamlining of antibiotic treatment is of great importance. In our hospital, the resistance of pneumococci to penicillin is very low, so all patients with a positive test could be streamlined to penicillin or amoxicillin monotherapy. Binax Now® Streptococcus pneumoniae is a rapid immunochromatographic test detecting C polysaccharide antigen in urine. The PnAG test could be a helpful test in discovering pneumococci even if there is no resistance testing, and it is thus recommended by the Infectious Disease Society of America (IDSA) guidelines if clinical and medical history data suggest severe pneumonia or a high probability of pneumococcal pneumonia [10]. Even if these guidelines do not recommend its use in all hospitalised patients with CAP, clinicians often widen the indication. Similarly, almost all our patients with suspected CAP were tested. The aim of our study was to investigate the impact of the PnAG testing on antibiotic prescribing in adult patients admitted to the hospital with suspicion of CAP.
Kantonsspital Olten is a 300 bed, university affiliated teaching hospital. Our hospital follows the guidelines for antibiotic prescription according to the Swiss society of infectious disease, which are in agreement with the Guidelines of the European Society of Clinical Microbiology and Infectious Disease (ESCMID) and the European Respiratory Society (ERS) [11]. In patients with suspected CAP, amoxicillin-clavulanic acid combined with a macrolide antibiotic in high risk situations is proposed as the first line treatment. All patients with suspected CAP admitted to the emergency department were included in the study. Patients with an alternative definitive diagnosis were included in the analysis of the impact of the PnAG-testing but excluded for the comparison of the PnAg- and control-period. A diagnosis of pneumonia was made according to published guidelines [12]. For the diagnosis of CAP, a new onset of cough and one of the following was required: new focal chest signs, dyspnoea, tachypnoea or fever for at least 4 days. We did not include procalcitonin as a diagnostic criterion. A chest X-ray was performed in all patients, and the presence of pulmonary infiltrates was required for the diagnosis of CAP. Blood cultures, sputum cultures, urinary Binax Now® Legionella antigen testing (LgAG) and Binax Now® pneumococcal antigen testing (PnAG) were performed in all patients when possible. A good quality sputum sample was defined as having >25 polynuclear granulocytes and <10 squamous epithelial cells × 100 field. Gram stain was performed in all specimens, but only good quality samples were cultured. Sputum was considered positive for pneumococci if either culture was positive (definitive) or if gram stain showed gram positive diplococci as the main causative agent and sputum culture results were negative (probable). Pleural puncture, tracheobronchial aspiration and bronchol-alveolar lavage (BAL) were performed when considered clinically necessary. Blood cultures were aerobically and anaerobically cultured (Bactec 9240, Becton Dickinson Diagnostic Instrument Systems, Sparks, Md.). Urine samples were obtained and processed for detection of S. pneumoniae antigen (Binax Now® S.pneumoniae Urinary Antigen Test; Binax) and Legionella pneumophila type I antigen (Biotest Legionella EIA; Biotest, Dreiach, Germany) according to the manufactures’ product instructions.
In the PnAG group, the decision to perform PnAG-testing was made by the treating physician. As the test was very popular, the test was not performed in only 12 patients with CAP. These patients were not included in the analysis.
Our main interest was the antibiotic prescribing policy after microbiological results were available, normally within 48 to 72 hours, and treatment changes beyond this time were not reflected. We did not consider switching from IV to oral treatment, as this switch can be made on clinical parameters only and is not influenced by microbiological data [13].
An infectious disease (ID) specialist performed daily surveillance of all blood culture specimens to determine bacterial growth and collected the results from urinary Legionella pneumophila or S. pneumonia antigen testing. From November 2007 until August 2008 all patients with suspected CAP in whom pneumococcal antigen testing (PnAg) (188/200 patients) was performed were included (PnAg-period). In the second period, we stopped PnAG testing in our institution (control period), but performed blood culture, sputum analysis and Legionella antigen testing on a regular basis. The control period group included all patients with the diagnosis of CAP admitted from September 2008 until March 2009.
The primary outcome in our study was the percentage of antibiotic streamlining from broader spectrum antibiotics to penicillin or amoxicillin between the two periods. Secondary outcomes were differences in overall antibiotic treatment change after microbiological testing between the PnAG and control period. For cost analysis we included every patient tested, even those where testing was not indicated. For comparison of streamlining of antibiotic treatment, only patients with documented CAP were included.
We also calculated the sensitivity of PnAg-testing. Unfortunately, no reference standard for diagnosis of pneumococcal CAP exists. Thus we assumed that patients with a positive PnAG as the sole positive test had pneumococcal pneumonia. We calculated firstly sensitivity for PnAG, secondly sensitivity of blood cultures proven and thirdly sputum proven pneumococcal pneumonia. Due to the low number of alternative microbiological diagnoses, specificity could not be calculated.
A statement by the ethical committee was not sought. The first analysis was retrospective and the decision to stop PnAg-testing was part of CAP-treatment policy and not a primary design of the study. We then decided to evaluate the impact of this decision but deemed an ethics committee review as unnecessary.
To compare the “PnAG period” against the “control period” (predictor variable) with respect to antibiotic streamlining (outcome variable), we restricted the unit of analysis to patients with confirmed CAP. In a first step we tested the impact of the period on the outcome streamlining using univariate logistic regression analysis. Streamlining was defined as narrowing antibiotic therapy to penicillin G or amoxicillin. In the univariate analysis, we also compared the follow up treatment after microbiological tests were available for other antibiotic treatments. These (single antibiotics or combination treatment) were chosen according to the different treatment regimes for CAP. In a second step we extended the univariate model to a multivariable logistic regression analysis where we adjusted the effect of the period on the outcome streamlining for the severity of pneumonia (pneumonia severity index, PSI), age, gender and need for intensive care unit (ICU) care. All adjustment variables for the multivariable model were a priori defined and kept in the model irrespective of whether they were significant or not. In both models, the hypothesis test for the effect of period was two-sided and the significance level was set at 5%.
PnAG-Testing was performed in 188 patients with suspicion of CAP. In 49 patients, CAP was not confirmed and they were excluded from further analyses. None of these 49 patients was positive for PnAG. In 22 of the remaining 139 patients, PnAG was positive. In 8 of these 22 patients the streptococcus origin was additionally confirmed by blood culture positivity and in 3 of 22 patients S. pneumoniae were also identified in sputum. In the remaining 11/22 patients, PnAG was the only confirmatory diagnosis for pneumococcal pneumonia. In five patients, antibiotic treatment was not streamlined despite the availability of the results (fig. 1). So in only 6/188 (3.2%) of patients in whom the test was performed could an impact on antibiotic prescription be found.

Figure 1
Patients in whom PnAG Test was performed. PnAG = Binax Now® urine Pneumococcal Antigen test.
Of all cases of pneumococcal pneumonia (PnAG positive or/and blood culture positive and/or sputum positive), 22/39 were PnAG positive, resulting in a sensitivity of 56% (95%CI: 41–72%). The sensitivity of blood culture or sputum confirmed pneumococcal pneumonias were 8/13 (61.5%; 95%CI: 35–88%) and 3/15 (20%; 95%CI: 0–40%) (table 2).
For the evaluation of the antibiotic use, we compared all 139 confirmed cases with CAP with a PnAG test available (PnAG-Group) with all 147 cases of CAP, in whom PnAG was not performed (Control-Group). Patients of group 1 were slightly younger, but the two groups were equally matched according to pneumonia severity index (PSI) and co-morbidities (table 1: Baseline characteristics).
In the PnAG group, 39/139 pneumococcal pneumonias were ascertained compared to 15/147 in group 2 (p <0.001). This difference was also present in positive sputum cultures (22 vs 7 cases (p = 0.002)) but not in blood culture (13 vs 9 cases (p = 0.3)).
There was no difference in first line antibiotic treatment. Most patients were treated with amoxicillin clavulanic acid, with or without a macrolide in both groups (61 vs 70%), or a third generation cephalosporin plus a macrolide (14% vs. 11%). Slightly less often, a third generation cephalosporin alone was prescribed (8 vs 11% , table 3).
Amoxicillin/clavulanic acid remained the most often used antibiotic (52 vs 72%) in the PnAG group and control group respectively (p <0.001) after microbiological results were available. Third generation cephalosporins were used in 15% and 8% for a second treatment (p = 0.04). Quinolones are rarely used in our institution, and there were no significant differences in the two groups. In empiric treatment, they were prescribed in 1 and 0% respectively, and in 4 and 1% respectively as a second treatment.
Overall, in 63 and 54% of the patients the treatment was changed (p = 0.12). In most instances, antibiotic treatment was narrowed (47 vs 50%, ns), mainly due to withdrawing the macrolide (40 vs 37%, p = 0.53). Broadening of the treatment occurred more often in the PnAG-group than in controls (15 vs 4 %, p = 0.002). Streamlining to aqueous penicillin or amoxicillin was somewhat higher in group 1 (13 vs 8%) but a significant difference could not be observed with either a univariate analysis or after multivariate adjustment. In the control group, third generation cephalosporin was more often changed to amoxicillin/clavulanic acid (6 vs 14%, p = 0.02, table 4).
| Table 1: Baseline characteristics. | |||||
| PnAG group | Control | p-value | |||
| Absolute | % | Absolute | % | ||
| 139 | 147 | ||||
| Age (mean ± SD) | 66.9 ± 16.9 | 72.3 ± 13.2 | 0.004 | ||
| Female gender | 47 | 34 | 61 | 41 | ns |
| PSI-score | |||||
| I | 8 | 6 | 5 | 3 | |
| II | 25 | 18 | 21 | 14 | |
| III | 24 | 17 | 28 | 19 | |
| IV | 45 | 32 | 59 | 40 | |
| V | 37 | 27 | 34 | 23 | |
| ICU admission | 36 | 27 | 41 | 27 | ns |
| Co-morbitities | |||||
| Diabetes | 33 | 24 | 34 | 23 | ns |
| Coronary heart disease | 50 | 36 | 57 | 38 | ns |
| Acute coronary syndrome | 4 | 3 | 5 | 3 | ns |
| Peripheral arterial occlusive disease | 9 | 7 | 7 | 5 | ns |
| Alcohol abuse | 9 | 7 | 9 | 6 | ns |
| Chronic obstructive lung disease | 46 | 33 | 44 | 30 | ns |
| Renal insuffiency | 26 | 19 | 36 | 25 | ns |
| Lung tumour | 5 | 4 | 9 | 6 | ns |
| Other tumour | 12 | 9 | 12 | 8 | ns |
| PnAG = Binax Now® urine Pneumococcal Antigen test. | |||||
| Table 2: Sensitivity assays. | |||
| Blood culture pos | Sputum pos/blood culture neg | Blood culture neg/sputum neg or not done | |
| PnAg pos (n) | 8 | 3 | 11 |
| PnAg neg (n) | 5 | 12 | 100 |
| Sensitivity % (95%CI) | 61.5 (35–82) | 20 (7–45) | Not applicable |
| PnAG = Binax Now® urine Pneumococcal Antigen test Due to low number of alternative microbiological diagnoses, specificity could not be calculated. | |||
| Table 3: Antibiotic treatment. | |||||
| PnAG group (n = 139) | Control group (n = 147) | ||||
| Initial treatment | Follow-up treatment | Initial treatment | Follow-up treatment | p-value follow-up treatment | |
| Penicillin/amoxicillin | 18 (13%) | 12 (8%) | 0.24 | ||
| Amoxicillin-clavulanic acid or cefuroxim | 50 (36%) | 46 (46%) | 54 (37%) | 96 (65%) | 0.006 |
| Amoxicillin-clavulanic acid plus macrolide | 43 (31%) | 8 (6%) | 49 (33%) | 10 (7%) | 0.81 |
| 3. Generation cephalosporin | 11 (8%) | 13 (9%) | 16 (11%) | 9 (6%) | 0.42 |
| 3. Generation cephalosporin plus macrolide | 20 (14%) | 8 (6%) | 16 (11%) | 2 (1%) | 0.054 |
| Macrolide monotherapy | 3 (2%) | 6 (4%) | 1 (1%) | 2 (1%) | 0.16 |
| Macrolide total | 70 (50%) | 22 (15%) | 67 (45%) | 14 (10%) | 0.11 |
| Wide spectrum betalactam | 5 (4%) | 10/7%) | 5 (3%) | 6 (4%) | 0.31 |
| Quinonolone | 2 (2%) | 6 (4%) | 1 (1%) | 7 (5%) | 1.0 |
| Other/no antibiotic | 9 (7%) | 4 (3%) | 7 (5%) | 7 (5%) | 1.0 |
| PnAG = Binax Now® urine Pneumococcal Antigen test. | |||||
| Table 4: Treatment changes. | |||||
| PnAG Group n = 139 | Control n = 147 | ||||
| Absolute | % (95%CI) | Absolute | % (95%CI) | ||
| No change | 51 | 37 (30–45) | 67 | 46 (38–54) | 0.15 |
| Narrowing therapy | 66 | 47 (39–55) | 73 | 50 (42–58) | 0.73 |
| Streamlining to penicillin or amoxicillin | 17 | 13 (8–19) | 13 | 8 (5–15) | 0.44 |
| Stopp macrolide | 56 | 40 (32–49) | 54 | 36 (29–45) | 0.54 |
| PnAG = Binax Now® urine Pneumococcal Antigen test | |||||
In Switzerland, there is a low prevalence of penicillin resistant pneumococci [14]. Therefore, the increased identification of S. pneumonia could lead to more prescriptions of narrow spectrum antibiotics. Pneumococcal antigen testing could be an ideal candidate for having an impact on antibiotic treatment, being not invasive, rapid and specific. However we do not recommend this test in the setting of CAP, mainly for two reasons: Firstly, the sensitivity in blood culture negative CAP was low in our study, and secondly, the impact was too small, as CAP treatment was changed in only 6/139 patients. Binax now® Pneumococcal Ag test costs CHF 42, and penicillin treatment is more expensive than amoxicillin/clavulanic acid. Thus no cost savings by detection of S. pneumonia can be expected.
Compared to older studies, we found a lower sensitivity of the test. All tests were performed according to the manufacturer’s instructions by specially trained employees in the microbiological laboratory, so we can exclude errors in handling technique. In the study of Marcos et al., the sensitivity of blood culture proven pneumococcal pneumonias was 100% [15]. We found only 61.5% positive PnAg of blood culture positive pneumococcal pneumonias, and the overall sensitivity was 56.4%. This is similar to a more recent retrospective study in patients with pneumococcal bacteraemia with a sensitivity of 64.5% [16]. The aim of PnAG-test should be to improve the overall detection of S. pneumoniae in CAP patients. However, especially in blood culture negative patients, the sensitivity of the test is too low, as PnAG was positive in only 3/15 patients with S. pneumoniae found in sputum and in 11/100 patients with negative sputum and blood culture. A limitation of sensitivity assays in microbiological testing is the lack of a gold standard. In CAP, we must accept that no gold standard exists. Blood culture proven pneumococcal pneumonias remain the minority of all CAP. In our study 10% were positive, thus the sensitivity of blood culture is too low to serve as a reference standard. However, in up to 57% of patients, in whom no pathogen could be identified by conventional methods, serological assays for pneumococci were positive [8]. As the PnAg test is known to be highly specific in adult patients [17], we assumed that the 11/139 patients with postive PnAg as the sole microbiological finding had pneumococcal pneumonia. The low sensitivity especially for blood culture negative CAP was disappointing, as sputum was obtained in only 138/286 (48%) patients, and routine sputum testing is not recommended by the guidelines [10]. All our patients had community acquired pneumonia and we had a low number of alternative microbiological diagnoses, so the calculation of specificity was not possible. It is known that the test can be positive if the upper respiratory tract is colonised by S. pneumoniae, which is the case in approximately 10% of healthy adults and 20–40% of children [18, 19]. The difference in detection of pneumococcal pneumonias in sputum culture between PnAg- and control groups was unexpected. There was no difference in the number of sputum examinations performed, but a higher amount of oral flora in the control group. As the examinations were done by the same staff, we believe that differences in quality of examination are highly unlikely. A change in the prevalence of pneumococcal disease was also considered unlikely.
Almost 25% of the patients initially tested with PnAG did not have CAP. This number seems relatively high, but the number was constant, also for the second group in which only Legionella antigen was tested. Despite clear guidelines for the diagnosis of pneumonia, it remains a challenge for physicians in an emergency department. None of these patients had a positive PnAG, although 27/49 patients had either an exacerbation of a chronic obstructive bronchitis or upper respiratory tract infection.
The impact of the PnAG test in the adjustment of antibiotic treatment was low. There was no difference in the group with antigen testing done compared to the control group. The low impact of the test was also demonstrated in a recent publication [20].
We particularly looked at the adjustment of empirical treatment after microbiological results were obtained, as the goal of microbiological diagnostic is improvement of antibiotic treatment. Despite more pneumococcal pneumonias diagnosed, partly due to the PnAG-test and partly due to more positive sputum results, there was no difference in narrow spectrum antibiotic use as second-line treatment in the two groups. The use of amoxicillin/clavulanic acid as well as the change from third generation cephalosporin to amoxicillin/clavulanic acid was higher in the group without PnAG-Testing. We believe that this is related to constant education that there is little need for use of third generation cephalosporin in the setting of CAP. Third generation cephalosporins may have their value in countries with higher prevalence of intermediate penicillin resistant pneumococci. However direct comparisons have not been published, and the need of antibiotics other than penicillin in PRSP have been questioned in non-invasive pneumococcal disease [21].
Most studies only describe the empirical treatment. However, it is of interest that in almost 60% of our patients, antibiotic treatment was changed. In only 10% was treatment broadened. The proportion of patients with changed treatment was higher than in a recent study, which found 33% of antibiotic treatment changed in respiratory tract infections. Interestingly, they found a higher proportion of inadequate treatment in adjusted than in empirical treatment [22]. While blood culture and Legionella-AG have a high impact, sputum results and pneumococcal antigen testing have a relatively low impact [23]. The low impact of PnAg is not only due to the low sensitivity, as despite a positive result in 5/11 patients treatment was not changed. It could be argued that the power of the analysis was too small to show differences between the two groups. However, we think that a test with a number needed to test of 31 patients to streamline antibiotic treatment without further advantage is not justified as a routine diagnostic procedure. The reasons why clinicians do not streamline according to results are mainly due to the attitude of “never change a winning team” [24]. S. pneumoniae remains the major cause of community acquired pneumonias and the major cause of death in these patients [25]. Despite rising prevalence of penicillin resistant pneumococci, treatment is relatively simple. A high sensitive and specific test could improve treatment and reduce costs of antibiotic treatment. However, PnAG-testing has an insufficient sensitivity and clinicians overestimate the possibility of missing other microorganisms involved when streamlining antibiotic treatment.
1 National center of health Statistics. health, united states, 2008. available at: www.cdc.gov/nchs/data/hus/hus08.pdf [Internet]; 2008.
2 Woodhead M. Community-acquired pneumonia in europe: Causative pathogens and resistance patterns. Eur Respir J Suppl. 2002;36:20s-7s.
3 Woodhead M. The european vision of community-acquired pneumonia. Semin Respir Cr Care Med. 2009;30(2):136–45.
4 Schuetz P, Albrich WC, Suter I, Hug BL, Christ-Crain M, Holler T, et al. Quality of care delivered by fee-for-service and DRG hospitals in switzerland in patients with community-acquired pneumonia. Swiss Med Wkly. 2011;141:w13228.
5 File TM,Jr, Marrie TJ. Burden of community-acquired pneumonia in north american adults. Postgrad Med. 2010;122(2):130–41.
6 Ramirez JA, Anzueto AR. Changing needs of community-acquired pneumonia. J Antimicrob Chemother. 2011;66(Suppl 3):iii3–9.
7 Feikin DR, Schuchat A, Kolczak M, Barrett NL, Harrison LH, Lefkowitz L, et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995–1997. Am J Public Health. 2000;90(2):223–9.
8 van Mens SP, Meijvis SC, Endeman H, van Velzen-Blad H, Biesma DH, Grutters JC, et al. Longitudinal analysis of pneumococcal antibodies during community-acquired pneumonia reveals a much higher involvement of streptococcus pneumoniae than estimated by conventional methods alone. Clin Vaccine Immunol. 2011;18(5):796–801.
9 Johansson N, Kalin M, Tiveljung-Lindell A, Giske CG, Hedlund J. Etiology of community-acquired pneumonia: Increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50(2):202–9.
10 Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious diseases society of America/American thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72.
11 Woodhead M, Blasi F, Ewig S, Huchon G, Ieven M, Ortqvist A, et al. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2005;26(6):1138–80.
12 Hoffken G, Lorenz J, Kern W, Welte T, Bauer T, Dalhoff K, et al. Epidemiology, diagnosis, antimicrobial therapy and management of community-acquired pneumonia and lower respiratory tract infections in adults. guidelines of the paul-ehrlich-society for chemotherapy, the german respiratory society, the german society for infectiology and the competence network CAPNETZ germany. Pneumologie. 2009;63(10):e1–68.
13 Mertz D, Koller M, Haller P, Lampert ML, Plagge H, Hug B, et al. Outcomes of early switching from intravenous to oral antibiotics on medical wards. J Antimicrob Chemother. 2009;64(1):188–99.
14 Kronenberg A, Zucs P, Droz S, Muhlemann K. Distribution and invasiveness of streptococcus pneumoniae serotypes in switzerland, a country with low antibiotic selection pressure, from 2001 to 2004. J Clin Microbiol. 2006;44(6):2032–8.
15 Marcos MA, Jimenez de Anta MT, de la Bellacasa JP, Gonzalez J, Martinez E, Garcia E, et al. Rapid urinary antigen test for diagnosis of pneumococcal community-acquired pneumonia in adults. Eur Respir J. 2003;21(2):209–14.
16 Selickman J, Paxos M, File TM,Jr, Seltzer R, Bonilla H. Performance measure of urinary antigen in patients with streptococcus pneumoniae bacteremia. Diagn Microbiol Infect Dis. 2010 Mar 23.
17 Smith MD, Sheppard CL, Hogan A, Harrison TG, Dance DA, Derrington P, et al. Diagnosis of streptococcus pneumoniae infections in adults with bacteremia and community-acquired pneumonia: Clinical comparison of pneumococcal PCR and urinary antigen detection. J Clin Microbiol. 2009;47(4):1046–9.
18 Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144–54.
19 van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009;374(9700):1543–56.
20 Matta M, Kerneis S, Day N, Lescat M, Hoi AB, Varon E, et al. Do clinicians consider the results of binax NOW streptococcus pneumoniae urinary antigen to adapt antibiotic regimen in pneumonia patients? Clin Microbiol Infect. 2009 Oct 20.
21 Feldman C. Clinical relevance of antimicrobial resistance in the management of pneumococcal community-acquired pneumonia. J Lab Clin Med. 2004;143(5):269–83.
22 Mettler J, Simcock M, Sendi P, Widmer AF, Bingisser R, Battegay M, et al. Empirical use of antibiotics and adjustment of empirical antibiotic therapies in a university hospital: A prospective observational study. BMC Infect Dis. 2007;7:21.
23 Signori LG, Ferreira MW, Vieira LC, Muller KR, Mattos WL. Sputum examination in the clinical management of community-acquired pneumonia. J Bras Pneumol. 2008;34(3):152–8.
24 Schouten JA, Hulscher ME, Natsch S, Kullberg BJ, van der Meer JW, Grol RP. Barriers to optimal antibiotic use for community-acquired pneumonia at hospitals: A qualitative study. Qual Saf Health Care. 2007;16(2):143–9.
25 Cutts FT, Zaman SM, Enwere G, Jaffar S, Levine OS, Okoko JB, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in the gambia: Randomised, double-blind, placebo-controlled trial. Lancet. 2005;365(9465):1139–46.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.