Antidiabetic drugs and kidney disease
DOI: https://doi.org/10.4414/smw.2012.13629
Anne
Zanchi, Roger
Lehmann, Jacques
Philippe
Summary
Patients with diabetes are at risk of early renal function decline. Therefore, kidney function needs monitoring at least once per year. Once the glomerular filtration rate (GFR) is less than 60 ml/min, the pharmacokinetics of antidiabetic drugs may be altered. Sulfonylurea and glinide therapies are associated with a risk of hypoglycaemia which is increased in the presence of renal impairment. Most sulfonylureas must be discontinued once GFR is <60 ml/min. Some glinides may be continued beyond this threshold, in particular repaglinide, which may be used in dialysis patients. In the absence of comorbidities, metformin can be continued at lower doses until a GFR of 45 ml/min, but must be withdrawn in case of dehydration or during the administration of a nephrotoxic drug including dye for radiological investigations. Glitazones may worsen water and sodium retention in patients with renal impairment. The pharmacokinetics of all DPP-IV inhibitors except linagliptin are altered with impaired renal function. Only sitagliptin, saxagliptin and linagliptin may be used in advanced kidney disease, but experience is as yet very limited. GLP-1 agonists are contraindicated in moderate to advanced kidney disease.
Recommendations of the Swiss Society for Endocrinology and Diabetology
Introduction
Diabetes increases the risk of chronic kidney disease by 2.6-fold [1] and the risk of renal death by 3-fold [2]. A large number of patients with diabetes mellitus will at some point have an altered renal function. Once the estimated glomerular filtration rate (eGFR) falls below 60 ml/min, a subject’s antidiabetic therapy needs to be re-evaluated. Some oral antidiabetic drugs are formally contraindicated, and others require dose reduction. In spite of these recommendations, the dose adjustment is seldom performed [3]. The risk of hypoglycaemia increases with increasing renal impairment, and hypoglycemia from antidiabetic drug therapy is among the four leading causes of hospitalisation for adverse drug reactions in the elderly [4]. This paper reviews the use of oral antidiabetic drugs in patients with chronic kidney disease (CKD) and discusses the available treatment options, including recent data on incretins.
Diabetic kidney disease is on the rise in many industrialised nations, and is the leading cause of end-stage renal disease. Overall, the rising incidence of type 2 diabetes, the longer survival of diabetic subjects, the aging population, and demographic expansion explain the significant increase in the number of diabetic subjects who live to reach end-stage renal disease. Recent data from the Swiss Canton of Vaud confirm a substantial increase in the number of diabetic dialysis subjects over an 8-year period [5].
In the Colaus study, a cohort study involving over 6000 subjects between the ages of 35 and 75 living in Lausanne, Switzerland [6], kidney disease, defined by the presence of ≥ stage 3 CKD (estimated MDRD GFR <60 ml/min) and/or the presence of microalbuminuria or proteinuria, was present in approximately 30% of diabetic subjects (personal communication, M. Bochud). In this population study, around 10% of adult diabetic subjects had an eGFR <60 ml/min. The use of oral antidiabetic drugs should be reviewed in all of these subjects.
Renal characterisation of patients with diabetes
At least annual monitoring of renal function is recommended for all adult diabetic subjects with the determination of creatinine levels, calculation of the eGFR using any of the available formulas (MDRD, CKD-EPI, Cockroft-Gault), and determination of the urinary albumin/creatinine ratio [7]. The MDRD Study equation, which requires only the serum creatinine concentration and the patient’s age, is the most commonly used GFR formula and can be automatically calculated by the laboratories. It has been evaluated in patients with a GFR <60 ml/min, and thus should be used for this group. The best formula for higher GFRs is the CKD-Epi formula or for all levels of GFR with a small variation is the Cystatin-C based GFR formula (GFR = (86.7/Cystatin-C)-4.2), if thyroid function is normal.
Since the 1990’s, more intensive hypoglycaemic and antihypertensive treatment has been validated as nephroprotective based on its ability to normalise or slow the progression of proteinuria. Over time, more stringent blood glucose and blood pressure goals have indeed lowered the incidence of proteinuria in the diabetic population [8]. However, US epidemiological data from NHANES show that the incidence of renal impairment, as defined by an eGFR <60 ml/min, has not decreased. In light of these surprising results, world experts are calling for improved renal characterisation of diabetic patients and for the development of new drugs targeting other pathways implicated in the pathogenesis of diabetic kidney disease.
Hypoglycemic treatments and the kidney
Glycaemic control is the only effective therapeutic intervention for the primary prevention of diabetic kidney disease in normoalbuminuric and normotensive subjects. In type 2 diabetic subjects, the UKPDS study has demonstrated that intensive hypoglycaemic treatment decreases the incidence and progression of microalbuminuria and is independent of the type of antidiabetic treatment used (metformin, sulfonylurea, or insulin). To date, it has not been possible to demonstrate that any particular antidiabetic drug is more effective than any other in terms of nephroprotection. The main objective is to achieve an HbA1C of <7% while ensuring that the conditions of antidiabetic drug use are compatible with renal function [9, 10].
Oral antidiabetic drugs in chronic kidney disease
The ideal antidiabetic drug lowers blood glucose without increasing the risk of hypoglycaemia or weight gain. Among the available oral antidiabetic drugs, sulfonylureas and glinides are associated with a risk of hypoglycaemia. These drugs stimulate the release of insulin from pancreatic β-cells following the interaction with ATP-dependent potassium channels, independently from glucose levels. When sulfonylureas are combined with insulin, the risk of hypoglycaemia may increase more than 14-fold [11]. Renal impairment is a major risk factor for hypoglycaemia. Patients taking drugs associated with a risk of hypoglycaemia (sulfonylureas, glinides, insulin) need to undergo systematic blood glucose monitoring when driving to make sure that blood glucose is >5 mmol/l [12]. Legal issues regarding these recommendations are currently being revised in Switzerland.
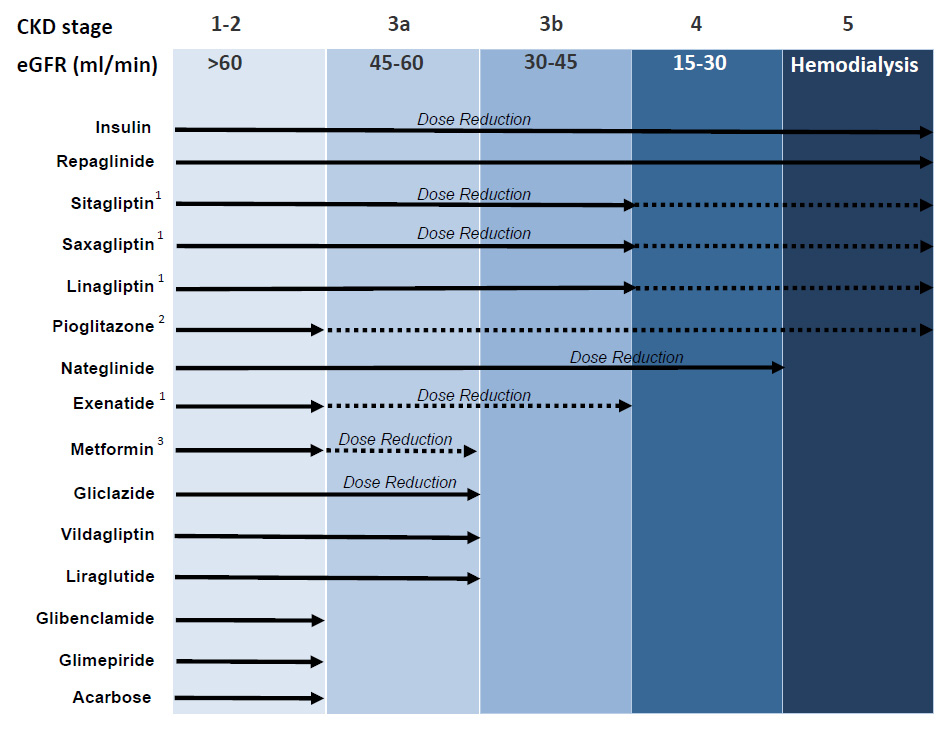
Figure 1
Antidiabetic treatment in chronic renal failure according to the KDIGO staging of CKD. Adapted from: Schernthaner G, Ritz E, Schernthaner GH. Strict glycaemic control in diabetic patients with CKD or ESRD: beneficial or deadly? Nephrol Dial Transplant. 2010;25:2044–7 [25] (© 2010, Oxford University Press. Reprinted with permission).
Recommendations adapted to stages of CKD based on a consensus from pharmacological data, Swiss and international recommendations.
1 Limited experience (dotted line); 2 monitor for fluid retention; 3 avoid introducing as new therapy or if conditions increasing lactic acidosis risk.
Sulfonylureas
Sulfonylureas have been used for the management of type 2 diabetes for over 50 years. First-generation sulfonylureas (chlorpropamide, tolbutamide) have been abandoned because of the risk of prolonged hypoglycaemia. Second-generation sulfonylureas (glibenclamide = glyburide, glimepiride, gliclazide) have shorter half-lives (5–15 hours), but their duration of action may be as long as 24 hours. The hypoglycaemic effect of sulfonylureas is faster and more potent compared to metformin or rosiglitazone, but may diminish over time [13]. The risk of hypoglycaemia induced by sulfonylureas in CKD is due to the accumulation of active metabolites. Other factors may contribute to the risk of hypoglycaemia, such as dose, omission of carbohydrates during meals, malnutrition, excessive alcohol intake, hepatic dysfunction, heart failure, advanced age, and interactions with some drugs (aspirin, sulfonamides, gemfibrozil, warfarin, etc.) which may shift sulfonylureas from their plasma protein binding sites.
Glibenclamide (= glyburide)(Daonil®; 2.5–10 mg/day) is metabolised by the liver, and is eliminated equally in bile and urine. Some of its metabolites are active and may accumulate in CKD although hepatobiliary elimination may partially compensate for the decrease in renal elimination. Hypoglycaemic episodes may be severe and last longer than 24 hours in the presence of renal impairment. Glibenclamide should be used with caution in patients with mild CKD (eGFR 60–90 ml/min) and is contraindicated in ≥3 CKD stages (eGFR <60 ml/min).
Glimepiride(Amaryl®, 1–8 mg/day) is metabolised by the liver to two main metabolites, one of which has hypoglycaemic activity. In patients with renal impairment, these metabolites can accumulate. Although its half-life is 5–7 hours, the drug may produce hypoglycaemic episodes that are severe and may persist for more than 24 hours in patients with CKD. As with glibenclamide, the use of glimepiride is contraindicated in patients with a GFR of <60 ml/min. Dosage adjustment is required in mild CKD.
Gliclazide (Diamicron® 80–320 mg/day; DiamicronMR® 30–120 mg/day) is metabolised by the liver to inactive metabolites, which are eliminated mainly in the urine (80%). Various studies have shown that among the sulfonylureas available in Switzerland, gliclazide poses a lower risk for severe hypoglycaemia than glibenclamide and glimepiride. Although no data are available in patients with severe renal impairment, studies have shown neither pharmacokinetic modifications of the drug nor a risk of hypoglycaemia in patients with a GFR >40 ml/min. In Switzerland, gliclazide is the only sulfonylurea that can be used in subjects with a GFR of 40–60 ml/min. However, it must be stopped once GFR falls below 40 ml/min.
Glinides
Two glinides are available: repaglinide and nateglinide. These drugs are characterised by a rapid onset and a short duration of action. They are however less potent than sulfonylureas. They are only taken prior to meals that contain carbohydrates, and efficiently reduce postprandial blood glucose levels. The risk of hypoglycaemia is lower than with sulfonylureas.
Repaglinide (Novonorm®, 0.5–12 mg/day) is mainly metabolised by the liver, and less than 10% is recovered in urine. Although renal impairment may slightly prolong its half-life, the use of repaglinide is not contraindicated in patients with renal impairment or dialysis patients. A preprandial dose of 0.5–4 mg should be titrated according to the postprandial blood glucose response.
Nateglinide(Starlix®, 60–360 mg/day) is metabolised by the liver with a half-life of 1.2–1.8 hours. Parent drug and certain active metabolites may accumulate in case of renal impairment. Therefore, the drug should be used with caution in patients with kidney disease.
Metformin (Glucophage®, 500-2,550 mg/day)
International recommendations consider metformin as the first choice for the treatment of type 2 diabetes. Metformin has been available for over 50 years and has the advantage of effectively lowering blood glucose levels and favours weight loss, without increasing the risk of hypoglycaemia. The principal action of metformin is the inhibition of hepatic glucose production. It also facilitates the insulin-mediated peripheral glucose uptake. Unchanged drug is eliminated via the kidneys by glomerular filtration and tubular secretion. Therefore, there is a risk of accumulation of the drug in case of renal impairment. This situation has been linked with a risk of lactic acidosis (estimated at 5/100000 patient-years) which may carry a mortality of up to 40%. However, it should be emphasised that this risk is lower than the mortality/morbidity risk associated with severe hypoglycaemia during sulfonylurea or insulin treatment in patients with CKD. Lactic acidosis is often accompanied by other predisposing conditions such as heart failure, hepatic dysfunction, advanced age, excessive alcohol intake, administration of iodinated contrast dye, hypoxaemia, and shock. A causal relationship between lactic acidosis and the accumulation of metformin has never been clearly demonstrated [14, 15]. In fact, the major cause of severe lactic acidosis appears to be rather linked to the risk of hypoxia. However, many of us have shared the clinical experience of an elderly patient taking metformin and suffering from dehydration, acute renal failure and severe acidosis. Furthermore, extreme overdose of metformin (e.g., in the case of suicidal attempt) has been associated with severe, lethal lactic acidosis, even in otherwise healthy individuals [16]. Therefore, it is important to re-emphasise the need to discontinue metformin in case of vomiting, diarrhea, other causes of dehydration as well as investigation with iodinated dye. We suggest exercising great caution and dose adjustment with the evaluation of renal function more than once per year in patients already on metformin, whose GFR is 45–60 ml/min and stopping treatment once GFR is <45 ml/min. This is more strict than the limit of 30 ml/min as proposed by Lipska, but also safer [15]. Gastric bypass increases the bioavailability of metformin by 50% [17] and the dose should be therefore reduced with milder degrees of CKD.
Glitazones
Glitazones are potent insulin sensitisers which have been available for the management of type 2 diabetes for over a decade. The metabolic effects result from the improvement in insulin sensitivity in the liver, adipose tissue, and muscle, leading to durable glycaemic control. Their arrival on the U.S. market was received with enthusiasm, leading to the widespread prescription although evidence of long-term safety was still lacking. The risk of water and sodium retention, increased by renal impairment and insulin therapy, was identified soon after their market entry. This risk, an issue with rosiglitazone (Avandia®) and pioglitazone (Actos®, 15–45 mg/day), affects 5–15% of patients treated. Seven years after its market entry, concerns were raised about the cardiovascular safety of rosiglitazone, eventually leading to its removal in 2010. The cardiovascular safety studies of pioglitazone are reassuring for the time being. Glitazones are also associated with a risk of osteoporosis, especially in postmenopausal women. The recent identification of a possible risk of bladder cancer with pioglitazone has resulted in the recommendation to avoid its use in patients with a history of bladder cancer and to evaluate the risk factors of bladder cancer (age, tobacco use, exposure to certain chemotherapy agents). Neither the European nor the U.S. regulatory authorities have suggested or requested that pioglitazone be removed from the market. Pioglitazone continues to be approved for the treatment of type 2 diabetes in patients who do not tolerate or are inadequately controlled by metformin. However, both France and Germany have removed pioglitazone from the market and recently Swissmedic limits the use of pioglitazone to two years and recommends continuing therapy only if the benefits of pioglitazone overcome the risk of bladder cancer. The pharmacokinetics of pioglitazone are not altered by renal impairment, and there is no need for dose adjustment in this setting. Despite this advantage and the fact that pioglitazone does not induce hypoglycaemia, the drug should be used with caution in CKD because of the risk of water and sodium retention and heart failure. Data regarding its safety in dialysis patients are as yet very limited. We recommend great caution when using pioglitazone in patients whose eGFR is <60 ml/min.
Alpha-glucosidase inhibitors (Glucobay®, 50–300 mg/day)
These drugs slow the intestinal carbohydrate absorption through the inhibition of the intestinal alpha-glucosidase enzyme. Their hypoglycaemic efficacy is low, and side effects of flatulence and diarrhea limit their use. These medications are occasionally prescribed for reactive hypoglycaemia in patients who have undergone gastric bypass surgery. The use of acarbose is not recommended in patients with renal impairment because of the risk of accumulation and lack of experience.
GLP-1 receptor agonists and DPP4 Inhibitors
The mechanism of action of GLP-1 receptor agonists and DPP4 enzyme inhibitors is based on the physiology of incretin hormones. They may occasionally be considered for use in patients with renal impairment.
The GLP-1 hormone plays a central role in glucose homeostasis. It is secreted by intestinal mucosal L-cells in response to food intake. GLP-1 reduces postprandial blood glucose levels by stimulating insulin production and secretion in a glucose-dependent manner. This stimulation occurs only in the presence of elevated blood glucose levels. The very low risk of hypoglycemia associated with these treatments is an important aspect when considering their use in patients with renal impairment. Apart from stimulating insulin secretion, GLP-1 reduces glucagon secretion and hepatic glucose production. GLP-1 receptor agonists, in particular the short acting ones (Exenatide) slow gastric emptying considerably, and also, increase satiety with a decrease in food intake like with the longer acting agonists (liraglutide or extended-release exenatide)
GLP-1 receptor agonists
GLP-1 receptor agonists are a treatment option for patients who fail to achieve adequate blood glucose control with metformin alone or with multiple oral antidiabetic drugs. These drugs are particularly useful when the goal is not only to improve diabetes treatment, but also to favour weight loss, especially in very obese patients at risk of gaining even more weight when starting insulin therapy.
Exenatide (Byetta®) is given by 5–10 μg sub-cutaneous injections twice a day and decreases glycosylated hemoglobin by approximately 0.7% to 1.5%, depending on baseline HbA1C.
Exenatide is primarily eliminated by glomerular filtration, followed by proteolytic breakdown. In patients with mild-to-moderate renal impairment, exenatide clearance is slightly reduced compared to subjects with no renal impairment (13% reduction in mild renal impairment and 36% reduction in moderate renal impairment). In patients with severe renal impairment or dialysis patients, clearance is reduced by 84%.
Exenatide is not recommended in patients with severe renal impairment (GFR <30 ml/min). In patients with a GFR of 30–60 ml/min, exenatide should only be used with great caution and at lower doses, as the incidence and intensity of gastrointestinal side effects are likely to increase. Clinical experience in these patients is very limited. The use of Bydureon® (extended release exenatide) is not recommended in CKD stage 3 and beyond.
GLP-1 receptors are expressed in renal tubules, and GLP-1 receptor agonists have been shown to have diuretic properties. As a result, the diuretic effect of such treatment may exacerbate renal impairment, especially in patients taking renin-angiotensin system inhibitors or diuretics. The extracelluar volume contraction can also be worsened by the gastro-intestinal side effects (vomiting) and precipitate acute renal failure. In addition, subjects with advanced kidney disease and diabetes frequently have autonomic neuropathy which exacerbate the gastric side effects of GLP-1 receptor agonists.
Liraglutide (Victoza®) is given by 0.6 to 1.8 mg subcutaneous injections once daily. Its efficacy with regard to HbA1c lowering is superior to the shorter acting analogs (Exenatide) due to the lowering of fasting glucose. The effect on weight is comparable [18].
Liraglutide is metabolised in a similar manner to large proteins, and no specific organ is responsible for its elimination. Intact liraglutide is recovered neither in the urine nor in the feces. Its elimination half-life is approximately 13 hours.
Unlike exenatide, liraglutide shows no increase in half-life and hence no reduced clearance in patients with mild, moderate, or even severe renal impairment. Therefore, the kidney is not a major site of liraglutide elimination or breakdown. Patients with mild renal impairment require no dose adjustment. Experience with liraglutide is however very limited in patients with moderate renal impairment and nonexistent in those with severe renal impairment. Therefore, this treatment is contraindicated in these settings.
|
Table 1: Clinical practice recommendations. |
|
Class
|
Drug
|
T1/2
|
Duration of action
|
Use in case of renal impairment
|
Dialysis
|
| |
| Sulfonylureas |
Glibenclamide |
6–10 h |
18–24 h |
GFR >60GFR <60 |
YesNo |
No |
| Glimepiride |
5–7 h |
>24 h |
| Gliclazide |
6–15 h |
18–24 h |
GFR >60GFR: 40–60GFR <40 |
YesWith cautionNo |
No |
| Glinides |
Repaglinide |
0.6–1.8 h |
4–6 h |
Yes |
Yes |
| Nateglinide |
1.2–1.8 h |
4 h |
With caution |
No |
| |
| Biguanide |
Metformin |
4–9 h |
|
GFR >60GFR: 45–60 |
YesWith caution, only in the absence of conditions that may increase the risk of lactic acidosis. |
No |
| |
| Glitazone |
Pioglitazone |
3–7 hActive and inactive metabolites: 16–24 h |
|
GFR >60GFR <60 |
YesWith caution, risk of water and sodium retention. |
Limited experience; great caution. |
| |
| Alpha-glucosidase inhibitors |
Acarbose |
|
|
GFR >60GFR <60 |
YesNo |
No |
| |
| GLP-1 receptor agonists |
Exenatide |
2.4 h |
|
GFR >60GFR: 30–60GFR <30 |
YesLimited experience; with caution.No |
No |
| Liraglutide |
13 h |
|
GFR >50GFR <50 |
YesNo |
No |
| |
| DPP-4 inhibitors |
Sitagliptin |
8–24 h |
|
GFR >50
GFR: 30–50
GFR <30 |
Yes
50 mg/day
25 mg/day, limited experience |
Limited experience, with caution. |
| Vildagliptin |
1.5–4.5 hrs |
|
GFR >50GFR <50 |
YesNo |
No |
| Saxagliptin |
2–4 hrs |
|
GFR >50GFR: 30–50GFR: 15–30 |
Yes2.5 mg/day2.5 mg/day; limited experience |
No (CH);limited experience, with caution (US) |
| Linagliptin |
10–40 hrs |
|
GFR >50GFR <50 |
YesYes; limited experience |
No experience, with caution. |
|
Table 2: Pharmacokinetic properties of incretins. |
|
|
Sitagliptin
(Januvia®; Xelevia®)
|
Vildagliptin
(Galvus®)
|
Saxagliptin
(Onglyza®)
|
Linagliptin (Trajenta®) |
Exenatide
(Byetta®)
|
Liraglutide
(Victoza®)
|
| Dose (mg/day) |
25–100 |
50–100 |
2.5–5
|
5 |
5–10 μg/day |
0.6–1.8 mg/day |
| Administration |
OD |
BID |
OD |
OD |
BID |
OD |
| Plasma protein binding |
Intermediate |
Low |
Very low |
High |
Low |
High |
| Renal excretion |
Predominant |
Intermediate |
Predominant |
Very low |
Predominant |
No |
DPP4 Inhibitors
Inhibitors of the DPP4 enzyme (gliptins) are not a homogeneous class of drugs; they have different chemical and pharmacokinetic properties (table 2), but share the same mechanism of action: inhibition of the DPP4 enzyme. Gliptins are less effective on average than GLP-1 receptor agonists as they achieve a reduction in glycosylated haemoglobin by approximately 0.6% to 1%. They are used either in combination with metformin or as add-on therapy to a combination of two antidiabetic drugs.
Sitagliptin (Januvia®, Xelevia®) shows high plasma protein binding. Sitagliptin is eliminated predominantly unchanged, via the urine (87%) or via the feces (13%).
In patients with mild, moderate or severe renal impairment, or dialysis patients, the increase in sitagliptin plasma levels is respectively 1.7, 2.3, 3.8 or 4.5 fold compared to controls. Therefore, the half-life of sitagliptin in patients with renal impairment is increased and correlated with the degree of renal impairment.
No dose adjustment is necessary in patients with mild renal impairment (GFR >50 ml/min). Patients with moderate renal impairment (GFR 30–50 ml/min) need to have their dose reduced by half to 50 mg/day. Patients with GFR <30 ml/min or requiring dialysis may be treated with 25 mg/day, but clinical experience is very limited in these patients. When sitagliptin is combined with metformin, the contraindications to metformin should obviously be considered, especially if GFR is <45 ml/min. This applies to all gliptins used in combination with metformin.
Around 80% of a Vildagliptin (Galvus®) dose is metabolised into non-active metabolites by various mechanisms that do not involve cytochrome P450 enzymes; 20% of a dose is thus not metabolised. Approximately 85% of a vildagliptin dose is eliminated in the urine, and 15% is recovered in the feces.
Exposure to vildagliptin in patients with moderate-to-severe renal impairment is increased compared to controls. However, the degree of exposure to vildagliptin does not correspond to the severity of renal impairment. Therefore, vildagliptin can be used without dose adjustment in patients whose creatinine clearance is >50 ml/min. While vildagliptin is, in principle, contraindicated in patients with moderate-to-severe renal impairment, a recent 24-week study suggests that vildagliptin 50 mg is effective and well tolerated in this setting [19].
Saxagliptin (Onglyza®) is excreted primarily by the kidneys, with approximately 20% of a dose being recovered unchanged in the urine and 20–50% as metabolites. Saxagliptin metabolism is essentially mediated by cytochrome 3A4/5 enzymes, thus the co-administration of saxagliptin with cytochrome 3A4/5 inhibitors requires a dose reduction by half. Saxagliptin metabolites still have 50% of the activity of the parent compound.
Saxagliptin is thus eliminated both by biotransformation and by renal excretion. In patients with mild renal impairment, patient exposure to saxagliptin and to its major metabolites was respectively 1.2 and 1.7 fold greater compared to controls. Values for patients with moderate renal impairment were 1.4 and 2.9 fold higher and those for patients with severe renal impairment were 2.1 and 4.5 fold higher. Therefore, no dose adjustment is required for patients with mild renal impairment, whereas dose reduction by half is necessary for patients with moderate renal impairment. The use of saxagliptin is not recommended for patients with a more severe degree of renal impairment although recent data suggest that saxagliptin is effective and well tolerated in this setting [20].
Linagliptin (Trajenta®) was accepted in Switzerland in March 2012 for the treatment of type 2 diabetes. Linagliptin shows very high plasma protein binding and is subject to little biotransformation. Its elimination is essentially via the feces. As the elimination of linagliptin via the kidneys is extremely low (approximately 1%), no dose adjustment is required for any degree of renal impairment [21, 22].
Recapitulation
In summary (table 1 and 2), there are significant differences between the various gliptins. Sitagliptin has a relatively long half-life, no active metabolites, and significant renal excretion; it is therefore necessary to adjust the sitagliptin dose as a function of renal impairment. Vildagliptin has a much shorter half-life, with only a quarter of the active metabolite eliminated by the kidney. No dose adjustment is necessary in patients with mild renal impairment, but is required in more advanced stages of renal impairment. Saxagliptin also has a short half-life, but has active metabolites. Both saxagliptin and its main metabolite are excreted via the kidneys; therefore, dose adjustment is necessary in case of renal impairment. Linagliptin is subject to high plasma protein binding, has a relatively long half-life, and is subject to virtually no renal excretion and, therefore, requires no dose adjustment in patients with kidney disease.
In addition to the degradation of GLP-1, the DPP-IV enzyme is involved in the catabolism of other important proteins such as substance P. We should remain cautious about the use of DPP-IV inhibitors in metabolically complex situations like severe chronic kidney disease. Because the increase in substance P has been linked to ACE inhibitor (ACEI) induced angiooedema, there has been some concern that the association of an ACEI with a DPP-IV inhibitor could exacerbate the risk of angiooedema [23]. A meta-analysis of phase 3 trials showed that although DPP-IV inhibitor therapy alone did not increase the risk of angiooedema, there were clearly more cases when associated with ACEI, but not with angiotensin receptor blockers (ARB) [24]. These findings need confirmation but could be an argument to favour the association of DPP-IV with ARB’s rather than with ACEIs.
Conclusion
With the increasing incidence of type 2 diabetic subjects with renal impairment, regular kidney function monitoring and adjustment of antidiabetic drug therapy according to GFR and pharmacokinetic data are of major importance. Because large studies of the safety of oral hypoglycaemic agents in renal failure are lacking, these recommendations will need to be regularly updated following the results of larger randomised trials with longer follow-ups.
References
1 Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–50,
2 Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–41.
3 Meyers JL, Candrilli SD, Kovacs B. Type 2 diabetes mellitus and renal impairment in a large outpatient electronic medical records database: rates of diagnosis and antihyperglycemic medication dose adjustment. Postgrad Med. 2011:123:133–43.
4 Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–12.
5 Stamm C, Burnier M, Zanchi A. Diabetes and end stage renal disease. Eight year progression in the Canton de Vaud, Switzerland. Rev Med Suisse. 2011;7:495–9.
6 Firmann M, Mayor V, Vidal PM, Bochud M, Pecoud A, Hayoz D, et al. The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc Disord. 2008;8:6.
7 Jerums G, Panagiotopoulos S, Premaratne E, MacIsaac RJ. Integrating albuminuria and GFR in the assessment of diabetic nephropathy. Nat Rev Nephrol. 2009;5:397–406.
8 de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–9.
9 Snyder RW, Berns JS. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial. 2004;17:365–70.
10 Reilly JB, Berns JS. Selection and dosing of medications for management of diabetes in patients with advanced kidney disease. Semin Dial. 2010;23:163–8.
11 Bodmer M, Meier C, Krahenbuhl S, Jick SS, Meier CR. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: a nested case-control analysis. Diabetes Care. 2008;31:2086–91.
12 Lehman R, Fischer-Taescler D, Iselin HU, Pavan M, Pralong F, Seeger R, et al. Directives conernant l’aptitude à conduire lors de diabète sucré. Forum Med Suisse. 2011;11:273–5.
13 Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43.
14 Nye HJ, Herrington WG. Metformin: the safest hypoglycaemic agent in chronic kidney disease? Nephron Clin Pract. 2011;118:c380–3.
15 Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care. 2011;34:1431–7.
16 von Mach MA, Gauer M, Meyer S, Omogbehin B, Schinzel H, Kann PH, et al. Antidiabetic medications in overdose: a comparison of the inquiries made to a regional poisons unit regarding original sulfonylureas, biguanides and insulin. Int J Clin Pharmacol Ther. 2006;44:51–6.
17 Padwal RS, Gabr RQ, Sharma AM, Langkaas LA, Birch DW, Karmali S, et al. Effect of gastric bypass surgery on the absorption and bioavailability of metformin. Diabetes Care. 2011;34:1295–300.
18 Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–56.
19 Lukashevich V, Schweizer A, Shao Q, Groop PH, Kothny W. Safety and efficacy of vildagliptin versus placebo in patients with type 2 diabetes and moderate or severe renal impairment: a prospective 24-week randomized placebo-controlled trial. Diabetes Obes Metab. 2011;13:947–54.
20 Nowicki M, Rychlik I, Haller H, Warren M, Suchower L, Gause-Nilsson I, et al. Long-term treatment with the dipeptidyl peptidase-4 inhibitor saxagliptin in patients with type 2 diabetes mellitus and renal impairment: a randomised controlled 52-week efficacy and safety study. Int J Clin Pract. 2011;65:1230–9.
21 Graefe-Mody U, Friedrich C, Port A, Ring A, Retlich S, Heise T, et al. Effect of renal impairment on the pharmacokinetics of the dipeptidyl peptidase-4 inhibitor linagliptin. Diabetes Obes Metab. 2011;13:939–46.
22 Barnett AH. Linagliptin: a novel dipeptidyl peptidase 4 inhibitor with a unique place in therapy. Adv Ther. 2011;28:447–59.
23 Grouzmann E, Buclin T, Biollaz J. Gliptins. Lancet. 2007;369(9558):269.
24 Brown NJ, Byiers S, Carr D, Maldonado M, Warner BA. Dipeptidyl peptidase-IV inhibitor use associated with increased risk of ACE inhibitor-associated angioedema. Hypertension. 2009;54:516–23.
25 Schernthaner G, Ritz E, Schernthaner GH. Strict glycaemic control in diabetic patients with CKD or ESRD: beneficial or deadly? Nephrol Dial Transplant. 2010;25:2044–7.
