Improvement in function after spinal cord injury: the black-box entitled rehabilitation
DOI: https://doi.org/10.4414/smw.2012.13673
Summary
Rehabilitation can be referred to as a “black box” because little is known about what specific interventions comprise the rehabilitation process, including patients with spinal cord injury (SCI). Despite that rehabilitation professionals can “see” daily what rehabilitation looks like, the contribution of each intervention to the final outcome of rehabilitation remains unclear. Moreover, there is only limited evidence supporting the efficacy of those interventions. To determine the efficacy of these interventions with respect to the outcome of rehabilitation and general functional improvement, we need appropriate outcome measurements and we need to know how profiles of functional improvement look in patients with SCI. It is sometimes forgotten, but obviously profiles of recovery depend to a large extent on the assessment tool applied. International efforts have been made to select and recommend a number of assessments with good psychometric properties and some of these assessments have been applied to hundreds of patients with SCI providing us with patterns of functional improvement. Currently, information about the efficacy of specific interventions is still lacking. For the coming years, one focus of rehabilitation research should be to determine the contribution of each specific intervention to the overall outcome of the rehabilitation process. Only by applying well-controlled randomised trials or large-scale observational studies, the most effective interventions can be selected, improving the efficacy of rehabilitation and turning the black-box into a more translucent one.
Introduction
Abbreviations
10MWT 10 Meter Walk Test
ADL Activities of Daily Living
AIS ASIA Impairment Scale
ASIA American Spinal Injury Association
BWSTT Bodyweight supported treadmill training
CPG Central Pattern Generator
CUE Capabilities of Upper Extremity
EPT Electrical Perception Threshold
FES Functional Electrical Stimulation
GRASSP Graded and Redefined Assessment of Strength, Sensibility and Prehension
ICF International Classification of Functioning, Disability and Health
ISNCSCI International Standards for Neurological Classification of Spinal Cord Injury
MEP Motor Evoked Potential
NDT Neuro-Developmental Treatment
SCI Spinal Cord Injury
SCIM Spinal Cord Independence Measure
SD Standard Deviation
SSEP Somato-Sensory Evoked Potential
TMS Transcranial Magnetic Stimulation
WISCI II Revised Walking Index for Spinal Cord Injury
In science and engineering, a black box is a device, system or object which can be viewed solely in terms of its input, output and transfer characteristics without any knowledge of its internal workings. That is, its implementation is “opaque” (black; source: http://en.wikipedia.org/wiki/Black_box). Many things could be referred to as a black box: a transistor, the human mind, or even the rehabilitation of patients with a spinal cord injury (SCI). This might be somewhat of a surprise, because early during the rehabilitation process, rehabilitation professionals formulate the goals together with the patient and his/her relatives. Rehabilitation teams are multi-professional and include physicians, nurses, physical therapists, occupational therapists, psychologists, speech therapists, sports therapists, social workers, etc. Formulating the goals allows for careful planning of the rehabilitation programme customised for each individual patient. Interestingly, despite the fact that each rehabilitation specialist “knows” the patient’s rehabilitation plan, little is known about what specific interventions comprise the rehabilitation process. Furthermore, there is only limited evidence supporting the effectiveness of those interventions.
A clinical trial, where SCI patients who would normally undergo rehabilitation would not receive any rehabilitation at all, would be considered unethical. Nevertheless, rehabilitation should become more evidence-based. Rehabilitation research is difficult, as patients are often very heterogeneous, sample sizes are small, a large number of different interventions are provided simultaneously and many other confounding factors might influence the outcome. Rehabilitative interventional studies often “fail”, as the control treatment is often also an active intervention and not, like in most drug studies, a passive placebo controlled trial. Double-blinding is often difficult, and patients will know what therapy they are receiving, which could influence their subjective opinion about the treatment. This makes the quality of the largest number of trials relatively low and impedes meta-analyses, which would be one alternative to make decisions about treatment efficacy by summing up the evidence of several smaller trials.
Furthermore, a gold standard to assess the efficacy of the rehabilitation process in patients with SCI is lacking. The most important outcome of the rehabilitation process should be to achieve the highest level of independence in activities of daily living (ADL) for the patient, while taking into account the individual impairments and limitations of the patient. This is important, because high functional independence is a significant determinant of a positive course of life satisfaction after discharge [1]. Monitoring improvement in performing ADL during in-patient rehabilitation is therefore important. However, solely assessing changes in ADL performance might not be sensitive enough to pick up small, but perhaps already functionally relevant improvements, as multiple personal and environmental factors could influence ADL performance. Furthermore, as many professions contribute to the rehabilitation process (multi-professional approach), and each profession works on its own specific sub-goals, a test battery is required to cover improvement in these functions and activities.
The aim of this manuscript is to discuss the difficulties in determining the contribution of rehabilitative interventions of the observed improvements in outcome after SCI. It does not require much fantasy to translate several of the issues raised in this manuscript to other patient groups receiving rehabilitation. I intend to discuss three topics that I consider relevant: (1.) how to assess functional improvement after SCI, (2.) how do functional improvement patterns look in patients with SCI, and (3.) what rehabilitative interventions might contribute to the observed improvements?
Assessing functional improvement after SCI
In rehabilitation, the International Classification of Functioning, Disability and Health (ICF, see http://www.who.int/classifications/icf/en/) has proven to be valuable in classifying outcomes. The ICF considers the consequences of the health condition on several domains. Body-functions are the physiological functions of body systems (including psychological functions), while body-structures are anatomical parts of the body such as organs, limbs and their components. Impairments are problems in body-function or structure such as a significant deviation or loss. Activity is the execution of a task or action by an individual. With respect to outcome measures, we can distinguish between “capacity”, where the patient performs a task according to a strictly standardised protocol, and “performance”, where the actual performance of the task in daily life is assessed. Limitations are difficulties an individual may have in executing activities. Finally, participation is involvement in a life situation, and restrictions are problems an individual may experience in involvement in life situations. It might be difficult to estimate effects on participation in society by the rehabilitation team, as functional independence is an important, but not the only predictor for participating in the society after discharge [1]. Additional factors influencing the rehabilitation of a specific patient can be the personal factors (e.g., age, fitness), as well as environmental factors that make up the physical, social and attitudinal environment in which people live and conduct their lives.
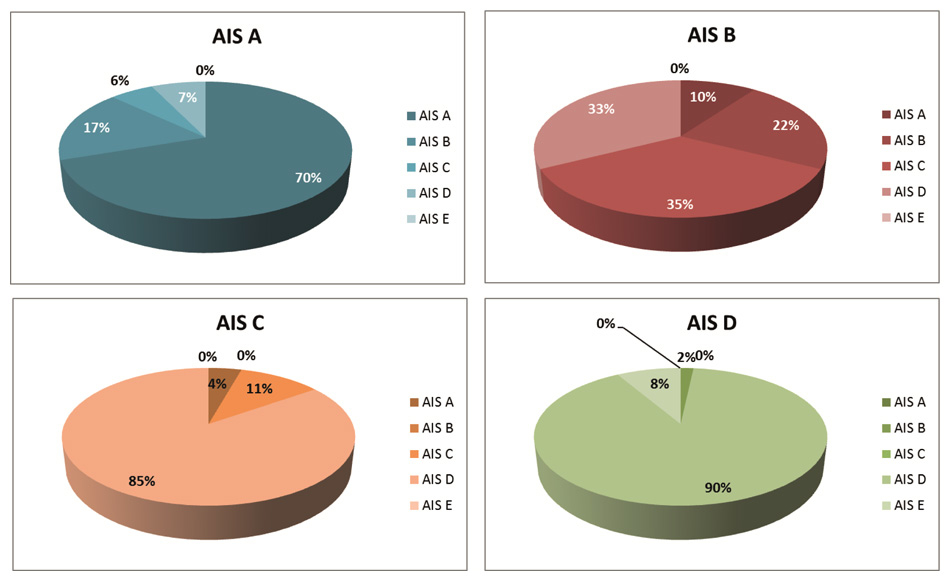
Figure 1
Conversion in neurological status after SCI. The percentage of patients that convert to another neurological category according to the International Standards for Neurological Classification of Spinal Cord Injury differs widely between the categories AIS A (sensory-motor complete), AIS B (motor complete, but sensory incomplete), AIS C and D, both sensory-motor incomplete, but AIS D patients have more than half of the muscles underneath the lesion graded as 3 or above. Conversion is largest in AIS B and C patients and smallest in AIS D patients. (Numbers are based on [12].)
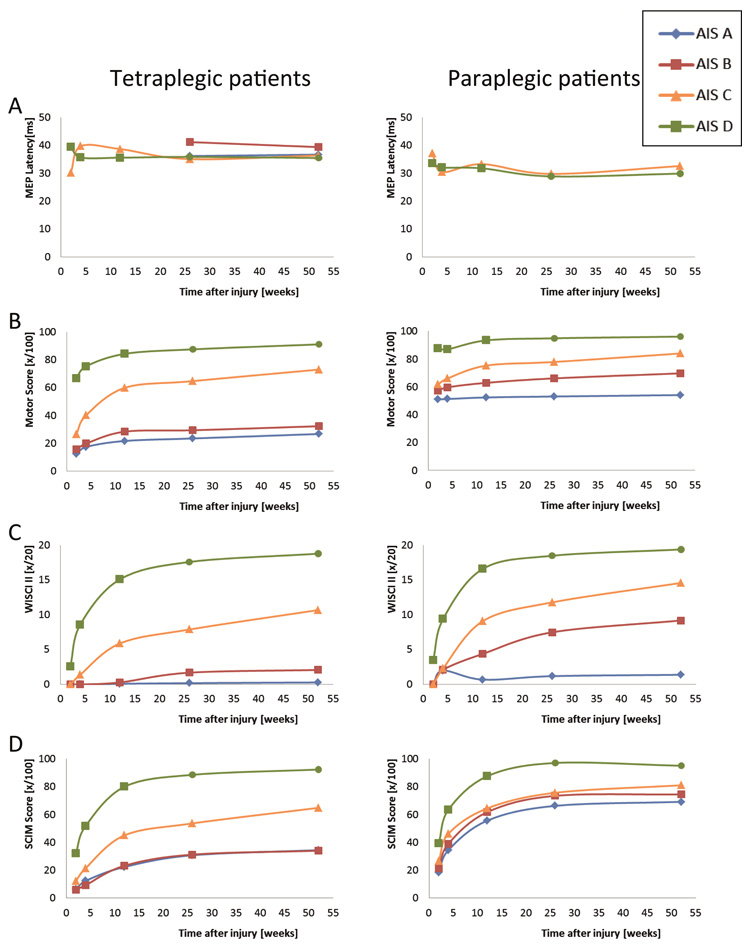
Figure 2
Improvement in motor functions and activities. Patterns of improvement in various motor functions and activities are shown for patient groups, which are categorised according to the level of lesion (tetraplegic versus paraplegic patients) and ASIA Impairment Scale (AIS). Patterns are shown of (A) the latency of motor evoked potentials (MEPs) evoked by transcranial magnetic stimulation, (B) the motor score of the key muscles according to the International Standards, (C) improvements in walking aids, braces and personal assistance as quantified with the revised Walking Index for Spinal Cord Injury (WISCI II) and (D) activities of daily living and independence as scored with the Spinal Cord Independence Measure (SCIM). (Numbers are based on [38].)
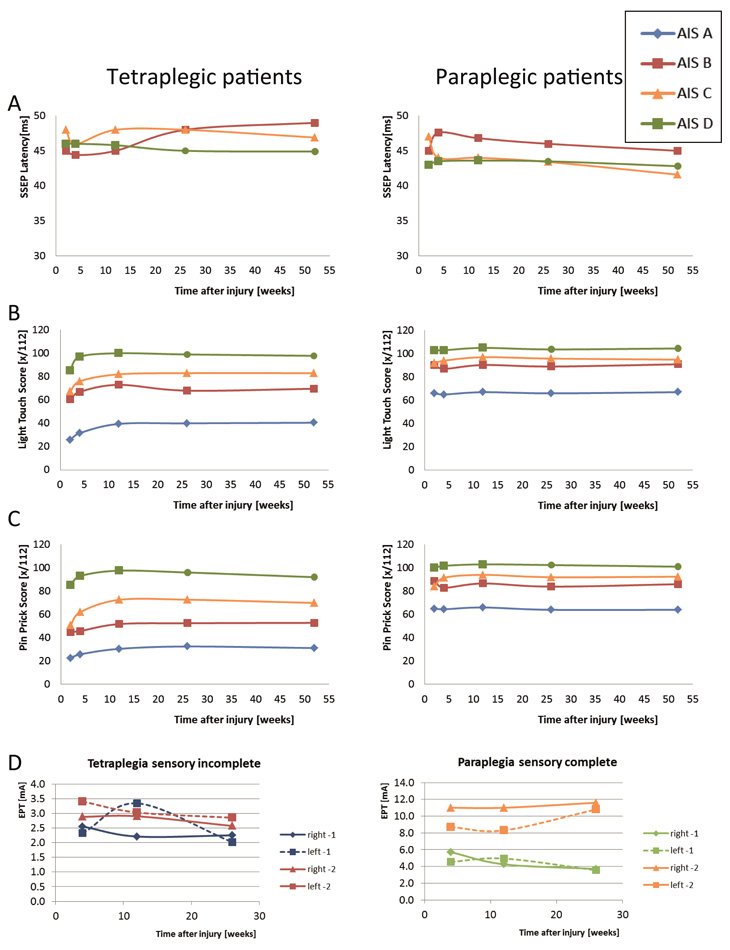
Figure 3
Improvement in sensory functions. Patterns of improvement in sensory functions are shown for patient groups, which are categorised according to the level of lesion (tetraplegic versus paraplegic patients) and ASIA Impairment Scale (AIS). Patterns are shown of (A) the latency of somato-sensory evoked potentials (SSEPs) elicited by electrical stimulation, (B) light touch and (C) pin prick sensation according to the International Standards. Numbers are based on [38]. (D) Changes in sensory perception as evaluated by the Electrical Perception Threshold (EPT) applied to the left and right dermatomes 1 and 2 segments below the neurological level of lesion (where most changes might be expected).
Left: patients with a sensory incomplete tetraplegia; right: patients with a sensory-complete paraplegia. In both groups, there are no significant changes over time. (Numbers are based on [22].)
Large efforts have been made in the past years to develop appropriate outcome measures, determining the psychometric properties of these measures and making recommendations for their application, both for evaluating clinical rehabilitation as well as translational trials [2–5]. These developments were stimulated by the planning of translational trials, because outcome measures sensitive to change were missing. Apparently, many clinical trials in the past “failed” (i.e., no significant beneficial outcome for the interventional drug was found), because the primary outcome measure was insensitive to change [5]. Please note that this paper does not intend to provide a comprehensive overview of all outcome measures.
On the domain of body-functions and structures, recommended [2] are the neurological assessment according to the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) [6], as well as neurophysiological tests like transcranial magnetic stimulation (TMS), somato-sensory evoked potentials (SSEP), and the Electrical Perception Threshold (EPT) test [7]. Improvements in activities for example walking can be quantified by a combination of capacity measures like the 10 meter walk test (10MWT) [8] and the revised Walking Index for Spinal Cord Injury (WISCI II) [9], while the performance measure Spinal Cord Independence Measure (SCIM, currently version III) is recommended to quantify independence in ADL [10].
The ISNCSCI is the most widely applied protocol to assess the neurological damage and functional impairment in people with SCI. The ISNCSCI was developed out of the Frankel Scale [11] and consists of various parts. The motor part consists of testing 5 key muscles for the lower extremity and 5 key muscles for the upper extremity. Each muscle is graded from 0 (total paralysis) to 5 (active movement, full range of motion against gravity with sufficient resistance to be considered normal if identified inhibiting factors were not present). The maximal total sum score is 100. The sensory part consists of light touch testing (dorsal column function) and pin prick testing (spino-thalamic tract function) of all dermatomes from cervical 2 to sacral 4/5. Perception of each dermatome is scored as normal, impaired or absent. Very important is motor testing of deep anal contractions and sensory testing of deep anal sensations, as these factors decide to a large extent, whether a patient is classified as being sensory-motor complete or incomplete [12]. The ISNCSCI provides the neurological level of lesion, the completeness of the injury and the ASIA (American Spinal Injury Association) Impairment Scale (AIS). The AIS has 5 categories: A, sensory-motor complete; B, sensory incomplete, motor complete; C, sensory-motor incomplete, with more than half of the key muscles below the level of lesion having a grade less than 3; D, similar, but with half or more having a grade of at least 3 and E, normal testing of motor and sensory segments, while previously having deficits. The algorithms that are required to determine what SCI characteristics (AIS, level of lesion, etc.) are quite complex and computer algorithms were developed to improve classification [13]. Assessors have to be aware that the assessment and classification are two different skills. Both require considerable attention, as previous studies have shown poor reliability results (e.g., [14]). Moreover, in adults in the very acute stage the assessment is very difficult, while reliable assessments can only be performed after 48 hours [15]. With respect to children, the ISNCSCI cannot be performed reliably in children aged under 6 years [16], likely due to the extensive protocol and the high level of awareness that is required for responding to the sensory testing.
Neurophysiological testing can assess the integrity of motor and sensory pathways. TMS can be used in the early phase after the accident, and does not require cooperation from the patient [17]. Electrodes are placed on the muscle group of interest, while a magnetic coil is placed on the scalp and a pulse is released. Especially the latency has been used for clinical evaluations, as the amplitude and silent period are difficult to assess reliably in a clinical setting [18]. The latency represents the signal conduction of the fastest fibers and can be classified as normal, delayed (partly damaged pathway) or absent (completely disrupted cortico-spinal tracts).
SSEPs can be elicited by electrical stimulation of the peripheral nerve, while recording the signal on the scalp. SSEPs assess the integrity of dorsal column function (proprioceptive information). The latency, the amplitude and also the shape of the response can be of interest. SSEPs can improve the prediction of functional improvement, for example of walking [17]. Recent studies show that SSEPs can also be applied reliably to dermatomes, providing information about the segmental level of lesion [19–20]. While there is abundant literature on the application of neurophysiological testing in adult patients with SCI, there is not much known about these measures in children with SCI.
As the rough scaling of the ISNCSCI light touch testing could limit the sensitivity of finding increments in sensory perception over time, the EPT was introduced [7]. Electrodes are placed on the dermatome and a 3 Hz current is slowly increased until the patient perceives the stimulation. This threshold is then taken as a measure for perception. EPT should result in a higher resolution of increments in sensory perception compared to the ISNCSCI [21]. First results indicate that EPT assessments can be performed reliably, but reliability differs between dermatomes [22] and might be more sensitive to reveal lesions in segments surrounding the lesion site [21].
While the 10MWT records the time needed to walk 10 meters, preferably by measuring the intermediate 10 meters of a 14 meter walkway [8, 23], the WISCI II assesses the assistive devices, braces and personal assistance to walk 10 meters [9]. Both tests have been investigated extensively in hundreds of patients with SCI, and are considered valid and reliable. In patients with good walking ability, the 10MWT might be somewhat more responsive to change [24]. Clinically relevant threshold values of changes are known for the 10MWT and the WISCI II. For example, in chronic patients with SCI, a change of 1WISCI II category is considered clinically relevant [25]. The 10MWT has been applied to children and adolescents with neurological disorders, but there is no study specifically for children with SCI. The WISCI II has been applied to patients aged between 12 and 89 years [26] and there is a single case study in a 5 year old girl, where the WISCI II was applied [27].
Lacking, is a widely accepted test for upper extremity function. Several tests have been developed, but none of them have gained international acceptance. Currently several groups are working on improved outcome measures for the upper extremity. An international panel has developed the Graded and Redefined Assessment of Strength, Sensibility and Prehension (GRASSP) and showed acceptable validity and reliability in patients aged 16 years and above [28]. Another group developed the Capabilities of Upper Extremity test (CUE) and showed recently good validity [29]. Yet, it has to be determined whether the GRASSP or CUE will become generally accepted.
As previously stated, the rehabilitation process aims to achieve the highest level of independence in ADL. Therefore, a performance measure should be used to evaluate the “overall efficacy” of the rehabilitation process. Two performance measures are widely applied: the Functional Independence Measure (FIM) [30] and the newer SCIM [31]. The FIM was originally developed to assess the burden of care in a broad range of patient groups. It consists of 13 motor and 5 cognitive items. Patients with SCI often show ceiling effects for the cognitive items, as cognition is less affected by SCI compared to for example stroke. FIM scores can vary between 18 (poorest performance, indicating complete dependency requiring total assistance) to 126 (best performance). The SCIM covers the categories self-care, respiration and bladder/bowel management and mobility and is currently in its third version [10]. In contrast to FIM, it has been developed specifically for patients with SCI. The scoring of the items and categories is weighted according to what is considered important by the patients [31]. SCIM scores can vary between 0 (poorest performance) and 100. Already the first version proved to be more sensitive than the FIM in detecting improvements in performance of ADL over time [31]. Furthermore, the SCIM appears valid and reliable and detects changes in ADL, even in patients with motor complete lesions [32]. Children and juveniles aged 12 and above have been evaluated with the SCIM [26, 33]. Currently, in my opinion, the SCIM might be considered one of the most favourable outcome measures to document the “overall efficacy” of rehabilitation after SCI.
Recovery versus compensation
The terminology can be confusing when we discuss “recovery” or “improvement” in patients with SCI. In general, improvement can be caused by recovery or compensation. Levin proposed definitions (for patients with stroke) on three ICF domains [34]. On the domain health condition, recovery can be seen as restoration of function in tissue that was initially lost after injury, while compensation can be described as that neural tissue which acquires a function that it did not have prior to injury. Recovery here implies a certain structural redundancy around the affected lesion [35]. At the domain of body function, recovery refers to restoring the ability to perform a movement in the same manner as it was performed before injury, while performing an old movement in a new manner would be compensation. Finally, at the domain of activities, successful task accomplishment using limbs or end effectors typically used by non-disabled individuals would refer to recovery, while successful task accomplishment using alternate limbs or end effectors would be compensation [34]. Compensatory strategies could for example be performing a task with two hands (e.g., holding a cup and drinking) instead of just a single hand, but also using assistive devices (e.g., crutches or a walker for walking, adapted grips of forks, knives and spoons) or adapted environments (e.g., removing curbs at home, an elevator instead of stairs) allowing the patient to achieve a higher level of independence without actually influencing his neurological status. It is clear that many clinical assessments applied nowadays, cannot distinguish between recovery and compensation.
Currently the trend in rehabilitation is to focus more on recovery and exploit restorative mechanisms, as this might improve performance across a range of tasks, whereas compensation appears to be more limited to the task that is specifically trained [36]. If we understand better how a specific therapy could exploit these mechanisms, we might be able to improve the rehabilitation process. Improving our assessments to enable us to distinguish between recovery and compensation might provide us with clues for what mechanism contributed to the observed changes in functions and activities.
Improvement in functions and activities after spinal cord injury
In persons with spinal cord injury (SCI), “spontaneous recovery” is sometimes used by scientists to describe the improvements in functions and activities in patients who have not undergone any experimental regenerative or reparative interventions. The wording is somewhat misleading, as spontaneous might imply that even in the case of a completely passive behavior of the patient during rehabilitation, improvement occurs. This does not reflect the physical and psychological efforts of the patient throughout and after the rehabilitation process.
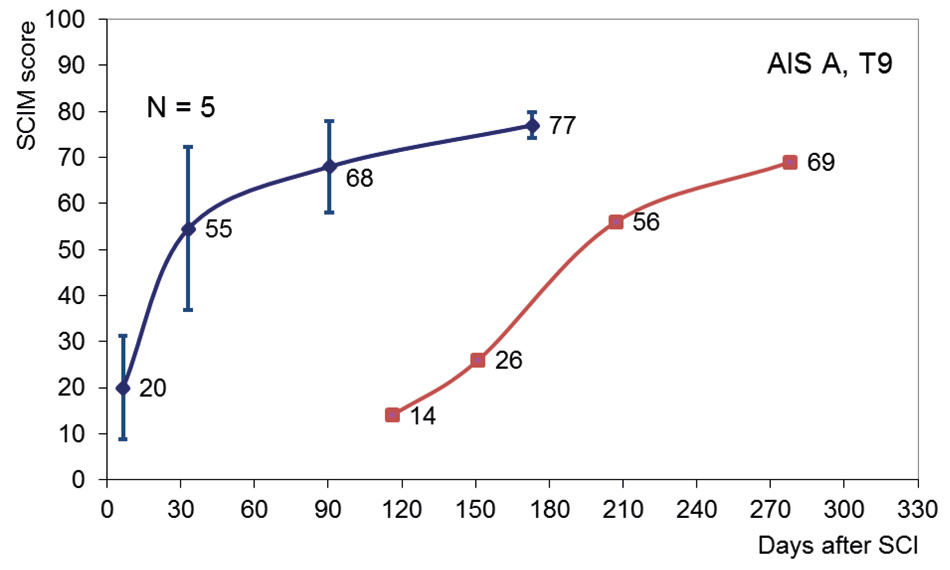
Figure 4
Delayed onset of rehabilitation. Improvement in Spinal Cord Independence Measure (SCIM) scores from a patient with a sensory motor complete (AIS A) lesion at thoracic 9 (T9), who entered rehabilitation with an almost 4 month delay in onset (red line). Averaged improvements in SCIM score (with SD) of 5 patients matched for severity and level of lesion (blue line). The results indicate that without rehabilitation, spontaneous improvement in activities of daily life is limited.
A typical example of spontaneous recovery could be the changes in reflex activity that can be observed within the first couple of days marking the end of the spinal shock [37]. The continuation of this increased reflex activity up to a level considered as pathological (the developing spastic syndrome due to the upper motor neuron lesion) is another indicator that spontaneous neurological changes occur after a SCI.
The course of improvement depends strongly on what outcome measurement is applied and what function, structure or activity is evaluated. Figures 1–3 display the improvement of several of the previously mentioned outcome measurements in patients with SCI and are based on papers investigating functional improvement during the first year after traumatic SCI [12, 22, 38]. Conversion in neurological status (AIS, see fig. 1) is relatively poor in AIS D patients (likely because of ceiling effects) and in AIS A patients (likely due to extensive damage of the spinal cord), while AIS B and C patients show greater conversion rates [12]. With the exception of paraplegic AIS A and B patients, most other patient categories show improvement in motor score (fig. 2A–B), while no neurophysiological change in tibialis anterior motor evoked potentials can be observed [38]. Improvements in walking capacity as quantified with the WISCI II vary considerably between patient groups, but are high in patients with motor incomplete lesions (fig. 2C). Interestingly, all patients appear to improve in ADL (fig. 2D), even those patients with sensory-motor complete paraplegia, who show in the other measures no large recovery [38]. This could indicate that currently compensatory mechanisms are especially exploited to improve ADL for these patients.
Improvements in sensory function are limited, as changes in SSEPs (tibialis posterior nerve, fig. 3A), as well as light touch and pin prick (fig. 3B–C) [38] and even in EPT values around the level of lesion (fig. 3D) are small, even in patients with incomplete lesions [22].
Factors that could influence recovery after SCI could be manifold, however, there are not many studies addressing this topic. Some factors are clear predictors of recovery, like the initial localisation and severity of the lesion (fig. 1). Poly-traumatic SCI lengthens the duration of rehabilitation compared to mono-traumatic paraplegic patients, but neurological and functional outcome appears similar [39]. Older age at injury appears to negatively influence the transfer of improvements of functions in daily life, despite similar changes in the neurological status of younger and elderly patients [40–41]. The influence of initial care on recovery is difficult to determine (e.g., surgery, conservative treatment, time between the accident and first care), due to the occurrence of other confounding factors following initial treatment. A recent trial suggested that early decompression resulted in improved neurological outcome compared to delayed decompression [42]. Nevertheless, rehabilitation is a prerequisite to ensure the success (i.e. best possible outcome) of acutely applied care and interventions. The occurrence of complications (decubitus, bladder infections, contractures) might slow down the rehabilitation programme, as the intensity of active interventions needs to be temporarily decreased.
The black-box entitled neuro-rehabilitation
A large part of the improvement in functions and activities will depend on active rehabilitation interventions, despite the occurrence of some spontaneous neurological recovery. One clue indicating that improvement in ADL does not occur spontaneously, but is induced by active rehabilitation programmes, comes from patients with a delayed onset of rehabilitation. Figure 4 shows the improvement in SCIM scores of a patient with a four months delay in rehabilitation onset (AIS A, neurological level thoracic 9) compared with data from 5 patients matched for AIS and neurological level. These patient-data were derived from the European Multicenter Study about Spinal Cord Injury (EMSCI) database ( http://www.emsci.org ). At rehabilitation onset, the SCIM score is clearly below the four months SCIM level of the EMSCI patients. During rehabilitation, SCIM performance increases, although the course appears somewhat different. While the end level appears similar, an Italian study showed that a delayed onset of rehabilitation actually results in a poorer outcome [43].
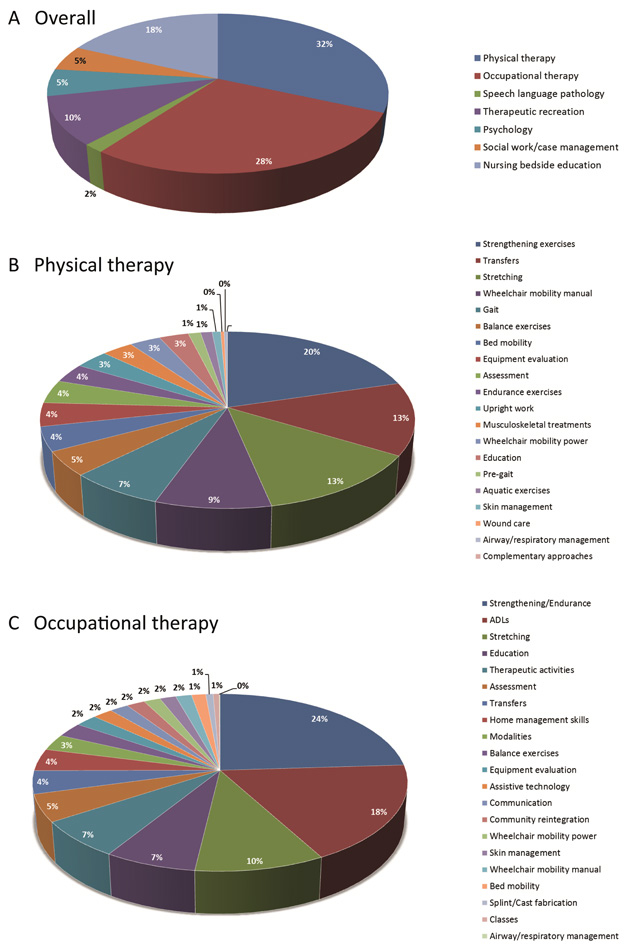
Figure 5
Percentages of time spent during rehabilitation. Numbers from the SCIRehab project showing the percentage of time of 600 patients (132 high cervical AIS A, B and C; 151 low cervical AIS A, B and C; 223 paraplegic AIS A, B and C and 94 AIS D patients) spent during rehabilitation. (A), overall distribution of the various disciplines, (B), percentages of time spent in physical therapy activities and (C) occupational therapy activities. Please note that large differences between the patient groups exist. For more details, see the original publications from the SCIRehab project [44, 66–67].
As current rehabilitation programmes are multi-disciplinary, it remains difficult to determine the contribution of each therapy to the overall observed change in outcome during rehabilitation, making rehabilitation a sort of a black-box. Besides the influence of acute care, surgery, medication and psychological support, professions that provide active training interventions have a slightly different focus (e.g., physical therapists focus often more (but not exclusively) on improving body functions and capacities, while occupational therapists aim to improve ADL performance and independence), and many different active interventions exist. Furthermore, it is currently unclear how much time is spent in each intervention, confounding the relationship between therapy efficacy and provided duration.
First insight into the duration of therapy-specific time spent during rehabilitation is now provided by the SCIRehab project (http://scirehab.net). Six centres in the USA collected information about the nature and duration of each therapy. A recent report including data of 600 SCI patients revealed extensive variation in the amount of treatment received between and within neurologic groups [44]. On average, patients received 180 hours (SD = 106 hours) of treatment, which is about 24 hours per week; 31.7% during physical therapy and 28.4% during occupational therapy [44] (fig. 5A). A taxonomy system was developed to classify and categorise interventions, e.g., treatment activities performed during physical therapy [45] or occupational therapy [46] (see also fig. 5B–C). Correlating such numbers with functional outcome at the end of rehabilitation having taken into account the severity of the lesion and other personal characteristics might identify the elements of the rehabilitation programme that contribute to a successful outcome.
Nevertheless, it remains questionable how well the SCIRehab results can be generalised to other regions, as there are considerable differences in rehabilitation programmes between centres and regions, for example due to different health-care systems, the relative “importance” of each profession, etc. One relatively simple indicator of differences in treatment regimes is the length of stay. In the SCIRehab consortium, the average length of stay was 55 days (SD = 37 days). In the EMSCI Consortium, which included German, Dutch, Spanish and Swiss centres, unpublished data from 1918 patients showed that the mean length of stay was 126 days (SD = 84 days), making comparisons about outcome difficult.
Therefore, well designed randomised controlled trials are still necessary to determine the efficacy of rehabilitative interventions. In one review investigating the effectiveness of physical interventions, 31 randomised controlled trials were identified, but only 6 trials reported a between-group mean difference with an important treatment effect on at least one outcome measure [47]. These trials supported the use of fitness, strength and gait training as well as acupuncture. A tremendous effort was made by a Canadian research collaboration, the Spinal Cord Injury Rehabilitation Evidence (SCIRE), to collect a comprehensive overview about rehabilitation evidence in SCI (see http://www.scireproject.com ). The following paragraphs shortly summarise the evidence obtained for a handful of treatment interventions.
Bodyweight Supported Treadmill Training is based on a large number of experimental animal studies on the ability of the spinal cord to generate stepping movements [48]. It can be considered a safe intervention that allows performing a high number of step repetitions, while the bodyweight support and additional assistance of therapists assures a physiological walking pattern, also in patients with impaired voluntary control of their legs. The current level of evidence indicates that BWSTT is good, but not better than other approaches aimed at improving gait [49–50].
Robot-assisted therapy appears to exploit restorative rather than compensational mechanisms. For the lower extremity, it is increasingly popular due to extensive engineering efforts made during the last couple of years. These devices have certain advantages when it comes to documenting the amount and intensity of training (number of repetitions, actual support provided by the device, etc.), providing instant augmented feedback using virtual reality scenarios and offering a playful environment improving motivation, especially for young patients [51]. Nevertheless, there is currently no evidence showing superior efficacy of robot-supported treadmill training compared to conventional interventions [52]. The currently observed trend to substitute rather than to complement conventional therapy by robot-supported systems is therefore undesirable [53–54].
Almost no information about robot- and computer- assisted therapy for improving upper extremity function in patients with SCI is available, as the first study that applied a gravity-supporting device to improve arm and hand function has just been published [55].
Electrical stimulation can be used for “simply” producing muscle contractions to increase muscle force of weakened muscles or in assisting learning and performing purposeful movements such as walking (functional electrical stimulation or FES). There is evidence that these applications improve muscle strength [56] and reduce atrophy (e.g., [57]). Regular use of FES in ADL including walking can lead to improvements even when the stimulator is turned off [58]. However, these applications have failed in becoming widely applied during and after rehabilitation.
Physical exercise trainingis assumed to have an impact on all four components of physical fitness: physical capacity, muscular strength and endurance, body composition and functional performance. Several reviews were performed, all with the same unfortunate conclusion, namely that the overall quality of the studies was poor [59–61]. Nevertheless, physical exercise programmes could reduce pain, while aspects of subjective well-being, namely depression and quality of life, improved [62]. Evidence on the impact on performing ADL is still inconclusive.
In my opinion, the importance of strength training during rehabilitation might be somewhat underestimated, likely because of the difficulties in differentiating between limitations in performance due to reduced strength or impaired voluntary control. Patients with an incomplete SCI showed especially deficits in muscle strength, while the timing and fine-grading of muscle strength remained unaffected [63]. In patients with stroke, however, fine muscle coordination was impaired, even in the “unaffected” leg. While these observations were made in a single-joint task, recently we could show that a four week strength training programme in chronic patients with an incomplete SCI, improved walking capacity more compared to a similar amount of robot-assisted gait training [64].
Bracingappears beneficial for ambulation in patients with incomplete lesions, but as none of the studies investigating bracing were randomised or blinded, there is limited evidence that bracing alone can induce relevant improvements in ambulation in patients with complete SCI.
The Bobath conceptwas developed around 1943 by Bertha Bobath (1907–1991) and Karl Bobath (1906–1991). It is nowadays also known as Neuro-Developmental treatment (NDT). It tries to regulate muscle tonus and facilitate physiological movement patterns. As it is widely applied in German and Swiss rehabilitation centres, especially in neurorehabilitation of children and adults with brain injury, there is hardly any information about treatment efficacy in adults with SCI. In PubMed, only 1 study was found describing a cell therapy approach for chronic SCI mentioning that the two patients received a rehabilitation programme consisting of Vojta and Bobath therapy [65]. A PEDRO Database search revealed no studies. In short, evidence for the efficacy of Bobath in patients with SCI is lacking.
Vojta therapy was developed by Prof. Vaclav Vojta (1917–2000) to extend the therapeutic possibilities for children with cerebral palsy. “Reflex locomotions” become activated by therapeutically applied external stimuli. Goal-directed pressures are administered to defined areas of the patient, who is in a prone, supine or lying position and result in identical automatical simultaneous movement responses. Some therapists apply Vojta therapy to adult patients with SCI. The therapy appears passive as no active goal-directed training takes place and patients remain in passive positions throughout therapy. A PEDRO database search revealed 9 publications on Vojta therapy, all performed in children, none in adults and none in SCI. In short, evidence for the efficacy of Vojta in patients with SCI does not exist.
Conclusion
In conclusion, impressive efforts have been made in the last decade to establish new outcome measurements to document improvement in functions and activities in patients with SCI. A large part of these improvements can be attributed to the rehabilitation programme, but it is still unclear which pieces out of this complex rehabilitation jigsaw puzzle deliver the largest contribution to the improvement. Elucidating the contribution of each intervention to the observed improvement, as well as determining whether exploiting restorative approaches would indeed be more successful than compensatory strategies, must be important rehabilitative research goals for the near future to optimise the rehabilitation process.
Acknowledgement:I would like to acknowledge the Neuroscience Center Zurich (ZNZ), the Zurich Center for Integrative Human Physiology (ZIHP), as well as the Children’s Research Center (CRC) from the University Children’s Hospital Zurich. Finally, I would like to express my thanks to the colleagues of the EMSCI Network, especially Prof. Armin Curt.
References
1 Van Leeuwen CM, Post MW, van Asbeck FW, Bongers-Janssen HM, van der Woude LH, de Groot S, et al. Life satisfaction in people with spinal cord injury during the first five years after discharge from inpatient rehabilitation. Disabil Rehabil. 2012;34:76–83.
2 Alexander MS, Anderson KD, Biering-Sorensen F, Blight AR, Brannon R, Bryce TN, et al. Outcome measures in spinal cord injury: recent assessments and recommendations for future directions. Spinal Cord. 2009;47:582–91.
3 Ellaway PH, Kuppuswamy A, Balasubramaniam AV, Maksimovic R, Gall A, Craggs MD, et al. Development of quantitative and sensitive assessments of physiological and functional outcome during recovery from spinal cord injury: a clinical initiative. Brain Res Bull. 2011;84:343–57.
4 Labruyere R, Agarwala A, Curt A. Rehabilitation in spine and spinal cord trauma. Spine (Phila Pa 1976). 2010;35:S259–62.
5 Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF, et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord. 2007;45:206–21.
6 ASIA. International standards for neurological classification of spinal cord injury, revised 2002. Chicago, IL: American Spinal Injury Association 2002.
7 Davey NJ, Nowicky AV, Zaman R. Somatopy of perceptual threshold to cutaneous electrical stimulation in man. Experimental physiology. 2001;86:127–30.
8 van Hedel HJ, Wirz M, Dietz V. Assessing walking ability in subjects with spinal cord injury: validity and reliability of 3 walking tests. Arch Phys Med Rehabil. 2005;86:190–6.
9 Ditunno PL, Ditunno JF, Jr. Walking index for spinal cord injury (WISCI II): scale revision. Spinal Cord. 2001;39:654–6.
10 Catz A, Itzkovich M, Tesio L, Biering-Sorensen F, Weeks C, Laramee MT, et al. A multicenter international study on the Spinal Cord Independence Measure, version III: Rasch psychometric validation. Spinal Cord. 2007;45:275–91.
11 Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7:179–92.
12 Spiess MR, Muller RM, Rupp R, Schuld C, van Hedel HJ. Conversion in ASIA impairment scale during the first year after traumatic spinal cord injury. J Neurotrauma. 2009;26:2027–36.
13 Schuld C, Wiese J, Hug A, Putz C, Hedel HJ, Spiess MR, et al. Computer implementation of the international standards for neurological classification of spinal cord injury for consistent and efficient derivation of its subscores including handling of data from not testable segments. J Neurotrauma. 2012;29:453–61.
14 Jonsson M, Tollback A, Gonzales H, Borg J. Inter-rater reliability of the 1992 international standards for neurological and functional classification of incomplete spinal cord injury. Spinal Cord. 2000;38:675–9.
15 Burns AS, Lee BS, Ditunno JF, Jr., Tessler A. Patient selection for clinical trials: the reliability of the early spinal cord injury examination. J Neurotrauma. 2003;20:477–82.
16 Mulcahey MJ, Gaughan JP, Chafetz RS, Vogel LC, Samdani AF, Betz RR. Interrater reliability of the international standards for neurological classification of spinal cord injury in youths with chronic spinal cord injury. Arch Phys Med Rehabil. 2011;92:1264–9.
17 Curt A, Dietz V. Electrophysiological recordings in patients with spinal cord injury: significance for predicting outcome. Spinal Cord. 1999;37:157–65.
18 van Hedel HJ, Murer C, Dietz V, Curt A. The amplitude of lower leg motor evoked potentials is a reliable measure when controlled for torque and motor task. J Neurol. 2007;254:1089–98.
19 Kramer JK, Taylor P, Steeves JD, Curt A. Dermatomal somatosensory evoked potentials and electrical perception thresholds during recovery from cervical spinal cord injury. Neurorehabil Neural Repair. 2010;24:309–17.
20 Kramer JL, Moss AJ, Taylor P, Curt A. Assessment of posterior spinal cord function with electrical perception threshold in spinal cord injury. J Neurotrauma. 2008;25:1019–26.
21 Savic G, Bergstrom EM, Frankel HL, Jamous MA, Ellaway PH, Davey NJ. Perceptual threshold to cutaneous electrical stimulation in patients with spinal cord injury. Spinal Cord. 2006;44:560–6.
22 Van Hedel HJ, Kumru H, Rohrich F, Galen S. Changes in electrical perception threshold within the first 6 months after traumatic spinal cord injury: a multicenter responsiveness study. Neurorehabil Neural Repair. 2012;26:497–506.
23 van Hedel HJ, Wirz M, Dietz V. Standardized assessment of walking capacity after spinal cord injury: the European network approach. Neurol Res. 2008;30:61–73.
24 van Hedel HJ, Wirz M, Curt A. Improving walking assessment in subjects with an incomplete spinal cord injury: responsiveness. Spinal Cord. 2006;44:352–6.
25 Burns AS, Delparte JJ, Patrick M, Marino RJ, Ditunno JF. The reproducibility and convergent validity of the walking index for spinal cord injury (WISCI) in chronic spinal cord injury. Neurorehabil Neural Repair. 2011;25:149–57.
26 Morganti B, Scivoletto G, Ditunno P, Ditunno JF, Molinari M. Walking index for spinal cord injury (WISCI): criterion validation. Spinal Cord. 2005;43:27–33.
27 Prosser LA. Locomotor training within an inpatient rehabilitation program after pediatric incomplete spinal cord injury. Phys Ther. 2007;87:1224–32.
28 Kalsi-Ryan S, Beaton D, Curt A, Duff S, Popovic MR, Rudhe C, et al. The Graded Redefined Assessment of Strength Sensibility and Prehension: reliability and validity. J Neurotrauma. 2012;29:905–14.
29 Marino RJ, Patrick M, Albright W, Leiby BE, Mulcahey M, Schmidt-Read M, et al. Development of an objective test of upper-limb function in tetraplegia: the capabilities of upper extremity test. Am J Phys Med Rehabil. 2012;91:478–86.
30 Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. 1987;1:6–18.
31 Catz A, Itzkovich M, Agranov E, Ring H, Tamir A. SCIM – spinal cord independence measure: a new disability scale for patients with spinal cord lesions. Spinal Cord. 1997;35:850–6.
32 Wirth B, van Hedel HJ, Kometer B, Dietz V, Curt A. Changes in activity after a complete spinal cord injury as measured by the Spinal Cord Independence Measure II (SCIM II). Neurorehabil Neural Repair. 2008;22:145–53.
33 Ackerman P, Morrison SA, McDowell S, Vazquez L. Using the Spinal Cord Independence Measure III to measure functional recovery in a post-acute spinal cord injury program. Spinal Cord. 2010;48:380–7.
34 Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair. 2009;23:313–9.
35 Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–7.
36 Huang VS, Krakauer JW. Robotic neurorehabilitation: a computational motor learning perspective. J Neuroeng Rehabil. 2009;6:5.
37 Ditunno JF, Little JW, Tessler A, Burns AS. Spinal shock revisited: a four-phase model. Spinal Cord. 2004;42:383–95.
38 Curt A, Van Hedel HJ, Klaus D, Dietz V. Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J Neurotrauma. 2008;25:677–85.
39 Putz C, Schuld C, Gantz S, Grieser T, Akbar M, Moradi B, et al. The effect of polytrauma as a possible confounder in the outcome of monotraumatic vs polytraumatic paraplegic patients: a clinical cohort study. Spinal Cord. 2011;49:721–7.
40 Furlan JC, Fehlings MG. The impact of age on mortality, impairment, and disability among adults with acute traumatic spinal cord injury. J Neurotrauma. 2009;26:1707–17.
41 Jakob W, Wirz M, van Hedel HJ, Dietz V. Difficulty of elderly SCI subjects to translate motor recovery – “body function” – into daily living activities. J Neurotrauma. 2009;26:2037–44.
42 Fehlings MG, Vaccaro A, Wilson JR, Singh A, D WC, Harrop JS, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS One. 2012;7:e32037.
43 Scivoletto G, Morganti B, Molinari M. Early versus delayed inpatient spinal cord injury rehabilitation: an Italian study. Arch Phys Med Rehabil. 2005;86:512–6.
44 Whiteneck G, Gassaway J, Dijkers M, Backus D, Charlifue S, Chen D, et al. The SCIRehab project: treatment time spent in SCI rehabilitation. Inpatient treatment time across disciplines in spinal cord injury rehabilitation. J Spinal Cord Med. 2011;34:133–48.
45 Natale A, Taylor S, LaBarbera J, Bensimon L, McDowell S, Mumma SL, et al. SCIRehab Project series: the physical therapy taxonomy. J Spinal Cord Med. 2009;32:270–82.
46 Ozelie R, Sipple C, Foy T, Cantoni K, Kellogg K, Lookingbill J, et al. SCIRehab Project series: the occupational therapy taxonomy. J Spinal Cord Med. 2009;32:283–97.
47 Harvey LA, Lin CW, Glinsky JV, De Wolf A. The effectiveness of physical interventions for people with spinal cord injuries: a systematic review. Spinal Cord. 2009;47:184–95.
48 Duysens J, Van de Crommert HW. Neural control of locomotion; the central pattern generator from cats to humans. Gait Posture. 1998;7:131–41.
49 Dobkin B, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006;66:484–93.
50 Mehrholz J, Kugler J, Pohl M. Locomotor training for walking after spinal cord injury. Cochrane Database Syst Rev. 2008:CD006676.
51 Brutsch K, Koenig A, Zimmerli L, Merillat-Koeneke S, Riener R, Jancke L, et al. Virtual reality for enhancement of robot-assisted gait training in children with central gait disorders. J Rehabil Med. 2011;43:493–9.
52 Swinnen E, Duerinck S, Baeyens JP, Meeusen R, Kerckhofs E. Effectiveness of robot-assisted gait training in persons with spinal cord injury: a systematic review. J Rehabil Med. 2010;42:520–6.
53 Dobkin BH, Duncan PW. Should body weight-supported treadmill training and robotic-assistive steppers for locomotor training trot back to the starting gate? Neurorehabil Neural Repair. 2012;26:308–17.
54 Harvey L, Wyndaele JJ. Are we jumping too early with locomotor training programs? Spinal Cord. 2011;49:947.
55 Zariffa J, Kapadia N, Kramer JL, Taylor P, Alizadeh-Meghrazi M, Zivanovic V, et al. Feasibility and efficacy of upper limb robotic rehabilitation in a subacute cervical spinal cord injury population. Spinal Cord. 2012;50:220–6.
56 Shields RK, Dudley-Javoroski S. Musculoskeletal plasticity after acute spinal cord injury: effects of long-term neuromuscular electrical stimulation training. J Neurophysiol. 2006;95:2380–90.
57 Baldi JC, Jackson RD, Moraille R, Mysiw WJ. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord. 1998;36:463–9.
58 Wieler M, Stein RB, Ladouceur M, Whittaker M, Smith AW, Naaman S, et al. Multicenter evaluation of electrical stimulation systems for walking. Arch Phys Med Rehabil. 1999;80:495–500.
59 Hicks AL, Martin Ginis KA, Pelletier CA, Ditor DS, Foulon B, Wolfe DL. The effects of exercise training on physical capacity, strength, body composition and functional performance among adults with spinal cord injury: a systematic review. Spinal Cord. 2011;49:1103–27.
60 Rimaud D, Calmels P, Devillard X. [Training programs in spinal cord injury]. Ann Readapt Med Phys. 2005;48:259–69.
61 Valent L, Dallmeijer A, Houdijk H, Talsma E, van der Woude L. The effects of upper body exercise on the physical capacity of people with a spinal cord injury: a systematic review. Clin Rehabil. 2007;21:315–30.
62 Hicks AL, Martin KA, Ditor DS, Latimer AE, Craven C, Bugaresti J, et al. Long-term exercise training in persons with spinal cord injury: effects on strength, arm ergometry performance and psychological well-being. Spinal Cord. 2003;41:34–43.
63 van Hedel HJ, Wirth B, Curt A. Ankle motor skill is intact in spinal cord injury, unlike stroke: implications for rehabilitation. Neurology. 2010;74:1271–8.
64 Labruyère R, van Hedel HJ. Strength training versus robot-assisted gait training after incomplete spinal cord injury: a randomized pilot study in patients dependent on walking asistance. Neurorehabil Neural Repair. Submitted for publication.
65 Moviglia GA, Fernandez Vina R, Brizuela JA, Saslavsky J, Vrsalovic F, Varela G, et al. Combined protocol of cell therapy for chronic spinal cord injury. Report on the electrical and functional recovery of two patients. Cytotherapy. 2006;8:202–9.
66 Foy T, Perritt G, Thimmaiah D, Heisler L, Offutt JL, Cantoni K, et al. The SCIRehab project: treatment time spent in SCI rehabilitation. Occupational therapy treatment time during inpatient spinal cord injury rehabilitation. J Spinal Cord Med. 2011;34:162–75.
67 Taylor-Schroeder S, LaBarbera J, McDowell S, Zanca JM, Natale A, Mumma S, et al. The SCIRehab project: treatment time spent in SCI rehabilitation. Physical therapy treatment time during inpatient spinal cord injury rehabilitation. J Spinal Cord Med. 2011;34:149–61.




