Figure 1
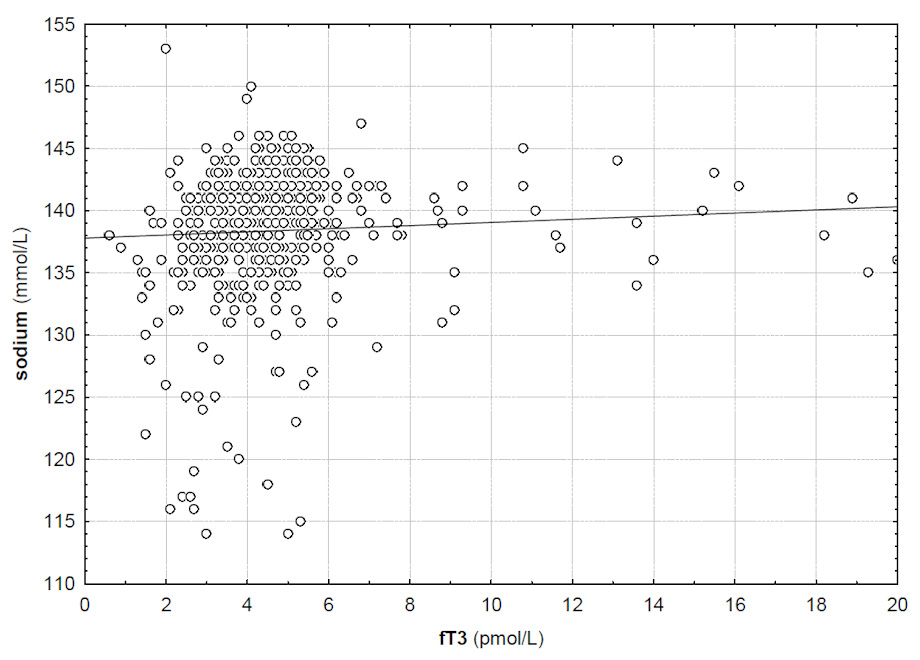
Correlation between serum fT3 and serum sodium (R = 0.11, p <0.05).
DOI: https://doi.org/10.4414/smw.2012.13669
Electrolyte disorders are common in hospitalised patients with dysnatraemias being the most common ones [1, 2]. In recent years research has focused on outcomes of patients with electrolyte disorders, mainly hypo- and hypernatraemia, which were found to be associated with increased mortality [3–6]. But also disorders of potassium, phosphate and magnesium showed to be predictors for increased mortality [7–9].
Thyroid hormone is a central regulator of body haemodynamics, thermoregulation and metabolism. It therefore has an influence on renal haemodynamics, glomerular filtration, as well as the renin angiotensin aldosterone system and renal electrolyte handling [10].
In many standard textbooks and reviews different electrolyte disorders were associated with thyroid dysfunction. In severe hypothyroidism and myxoedema hyponatraemia was described to be a consequence of enhanced renal water retention mediated by vasopressin [11]. On the other hand, hypokalaemia, hypomagnesaemia and hypercalcaemia were mentioned in patients with thyrotoxicosis [12–14].
Severe thyroid dysfunctions are rare and often additional factors contribute to the development of clinical signs as well as electrolyte disorders. Surprisingly, searching the literature by use of PubMed with “thyroid/thyroid hormones” and “electrolytes” as key words, no original investigations could be found on the prevalence of electrolyte disorders in patients with mild forms of hypo- or hyperthyroidism.
We therefore wanted to investigate the effects of thyroid stimulating hormone (TSH) on serum electrolytes in a broad spectrum of patients admitted to the emergency department of a large university hospital.
In this retrospective analysis, we screened the electronic database of the central laboratory for all thyroid stimulating hormone levels which were ordered by the Emergency Department of the Inselspital University Hospital Bern between 1 April 2008 and 31 March 2011.
Of all patients identified in the first step, we gathered information on age and sex. Data on serum sodium, potassium, chloride, calcium, phosphate, magnesium, creatinine, urea, and serum osmolality were collected. Serum levels of free T3 and T4 were obtained. Normal ranges for TSH, fT4 and fT3 were 0.35–4.5 mU/l, 9.5–25 pmol/l and 2.9–6.5 pmol/l, respectively. Hypothyroidism was defined as a fT4 concentration below the normal range value, hyperthyroidism as fT4 and/or fT3 concentration above the normal range.
TSH, fT3 and fT4 were measured by electrochemical luminescence immunoassays by use of Modular E170, Roche, Hoffmann-La Roche Ltd., Switzerland. Serum osmolality was measured by freezing point depression using the Advanced Osmometer, Advanced Instruments Inc., Norwood, MA. Sodium, potassium, chloride were measured by indirect potentiometry using the Modular ISE 900 by Roche, Hoffmann-La Roche Ltd., Switzerland. Magnesium was determined by use of a colour test, calcium by calcium o-kresolphtalein-komplexon test and phosphate by an ultraviolet test using Modular P800 by Roche, Hoffmann-La Roche Ltd., Switzerland. Creatinine was measured using an enzymatic photometric test using Modular P800 by Roche, Hoffmann-La Roche Ltd., Switzerland.
We defined electrolyte disorders according to the reference ranges for adults of the central laboratory of the Inselspital University Hospital Bern:
Hyponatraemia <135 mmol/l; hypernatraemia >145 mmol/l; hypokalaemia <3.5 mmol/l; hypochloraemia <97 mmol/l; hyperchloraemia >108 mmol/l; hyperkalaemia >4.7 mmol/l; hypocalcaemia <2.1 mmol/l; hypercalcaemia >2.55 mmol/l; hypophosphataemia <0.84 mmol/l; hyperphosphataemia >1.45 mmol/l; hypomagnesaemia <0.75 mmol/l; hypermagnesaemia >1.0 mmol/l.
Results are presented as median and first (Q1) and third (Q3) quartile as suggested by the performed Lilliefors test. Correlations, Mann-Whitney-U tests and chi-square tests were computed using STATISTICA 9.1, Statsoft Inc., Tulsa, Oklahoma.
The study was approved by the ethics committee of the Canton of Bern, Switzerland, which represents the local authority for ethics in medical science.
During the study period a total of 9,012 patients with available serum TSH levels ordered by the Department of Emergency Medicine could be identified. Median age of the patients was 61 years (Q1: 45, Q3: 74). 51% (N = 4,558) of patients were male.

Figure 1
Correlation between serum fT3 and serum sodium (R = 0.11, p <0.05).
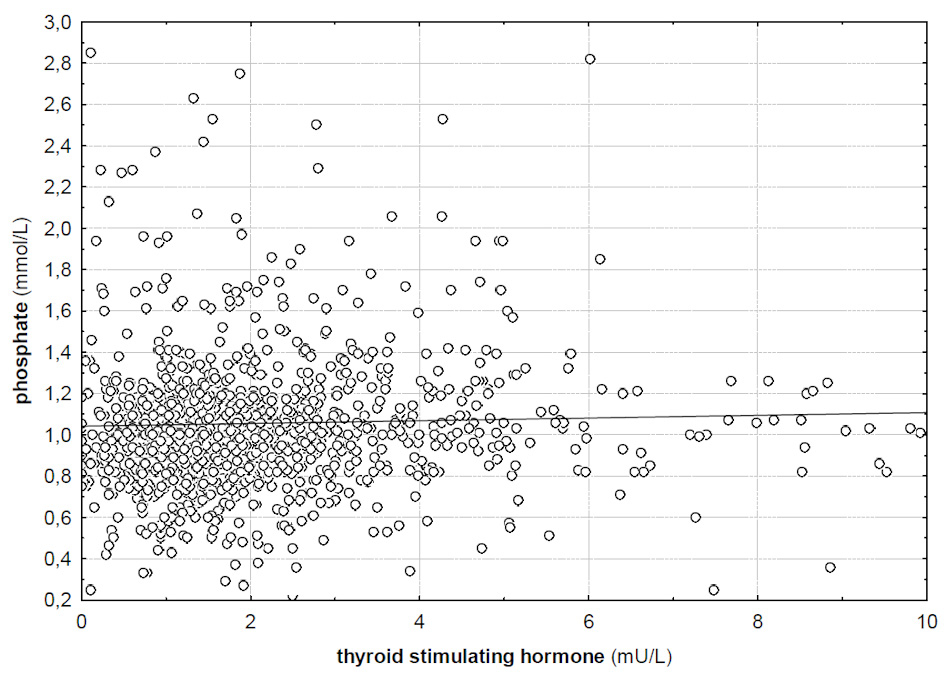
Figure 2
Correlation between TSH and serum phosphate (R = 0.08, p <0.05).
Median serum levels for thyroid function parameters and electrolytes are given in table 1. 872 (10%) patients had hyponatraemia, 125 (1%) hypernatraemia, 1,009 (11%) had hypokalaemia and 434 (5%) had hyperkalaemia according to the reference range of the central laboratory. An overview of the prevalence rates of all electrolyte disturbances on admission to the emergency department is given in table 2.
7,759 (86%) patients had normal, 396 (4%) had suppressed and 857 (10%) had elevated TSH levels. 15 (2% of patients with elevated TSH) patients with elevated TSH levels had fT4 levels below normal range and 37 (4%) had fT3 levels below normal range. In patients with suppressed TSH, 61 (15% of patients with suppressed TSH) had elevated fT4 and 38 (10%) had elevated fT3.
Serum TSH and fT4 levels did not correlate with serum sodium levels (R = –0.02 and R = 0.022, p >0.05). However, there was a significant correlation between serum fT3 levels and serum sodium (R = 0.11, p <0.05). Figure 1 gives the correlation between fT3 and serum sodium. While there was no significant difference in serum sodium between patients with normal and low TSH levels (139 vs 139 mmol/l, p >0.05), serum sodium was significantly lower in patients with high TSH levels (138 vs 139 mmol/l, p <0.01). An overview of serum electrolyte levels stratified for TSH levels is given in table 3.
Serum TSH levels did not correlate significantly with potassium (R = 0.01, p >0.05), chloride (R = –0.003, p >0.05) and serum osmolality (R = –0.06, p >0.05).
There was a significant correlation between serum TSH levels and phosphate levels in serum (R = 0.08, p <0.05). Phosphate did not correlate with fT4 and fT3 levels in serum (R = –0.01 and R = –0.06, p >0.05). Phosphate levels were significantly higher in patients with elevated TSH than in patients with normal TSH values (1.19 vs 1.04 mmol/l, p <0.01). Additionally, serum calcium and magnesium levels correlated significantly with TSH levels (R = –0.03 and R = 0.1, p <0.05). Serum fT3 levels correlated significantly with serum calcium levels (R = 0.11, p <0.05) but serum fT4 did not (R = 0.01, p >0.05). A significant correlation could not be shown for magnesium and fT3 levels (R = –0.04, p >0.05) but fT4 correlated significantly (R = –0.19, p <0.05). Although marginal, serum calcium levels were significantly lower in patients with high TSH than with normal TSH (2.23 vs 2.25, p <0.01). There was no difference in chloride and magnesium levels between patients with normal and high TSH levels (p >0.05, respectively). Potassium was significantly higher in patients with low TSH levels than in patients with normal TSH (4.03 vs 3.9, p <0.01), while there was no difference for chloride, calcium, phosphate and magnesium.
Hyponatremia was present in 119 (14%) patients with high TSH and was significantly more common than in the group with normal TSH levels of which 719 (9%) had hyponatraemia (p <0.01). Only 34 (9%) patients with suppressed TSH levels had hyponatraemia on admission to the emergency department. Hyponatraemia was significantly more common in the high than in the low TSH group (14 vs 9%, p = 0.04). There was no difference in the prevalence rate of hypernatraemia between groups. Analogous to hyponatraemia, hypochloraemia was more common in patients with high TSH than in those with normal TSH (23 vs 13%, p = 0.03).
Hypokalaemia was significantly more common in the group with elevated TSH than in those with normal TSH levels (14 vs 11%, p = 0.016). Again there was no difference in the prevalence of hypokalaemia between patients with normal TSH levels and those who showed suppressed TSH. However, hyperkalaemia was more common in the group with high TSH levels (7%) than in those with normal TSH (7 vs 4%, p <0.01). There was no significant difference seen for hypokalaemia between patients with high TSH and those with low TSH (14 vs 11%, p = 0.07). Also, there was no significant difference for hyperkalaemia between patients with suppressed and those with high TSH (8 vs 7%, p = 0.43).
Hyperphosphataemia and hypermagnesaemia were more common in patients with high TSH levels than in those with normal TSH (14 vs 7%, p = 0.01 and 7 vs 4%, p = 0.02).
Hypochloraemia was more common in patients with high TSH than in those with normal TSH (23 vs 13%, p = 0.03).
Serum creatinine levels were significantly higher in patients with low TSH levels than in those who had normal TSH (99 vs 82 μmol/l, p <0.01). In the group with high TSH levels creatinine was highest (105 μmol/l, p <0.01).
In the group of patients with highest TSH levels (≥40 mU/l, 16 patients) only 2 patients had hyponatraemia, 2 had hypernatraemia, 1 had hypophosphataemia and hyperphosphataemia, 2 had hypocalcaemia, 1 had hypermagnesaemia and 1 had hyperchloraemia.
| Table 1: Thyroid function parameters and electrolytes for all patients. | ||||
| N (%) | Median | Q1 | Q3 | |
| TSH | 9,012 (100) | 1.7 | 1.06 | 2.75 |
| fT3 | 777 (8.6) | 4.3 | 3.5 | 5.1 |
| fT4 | 813 (9) | 16.5 | 13.9 | 19.7 |
| Na+ | 9,012 (100) | 140 | 138 | 141 |
| K+ | 8,995 (99.8) | 3.9 | 3.7 | 4.2 |
| Cl- | 851 (9) | 104 | 100 | 106 |
| Ph-- | 1,096 (12) | 1.01 | 0.84 | 1.18 |
| Ca++ | 4,315 (48) | 2.26 | 2.17 | 2.33 |
| Mg++ | 1,548 (17) | 0.81 | 0.74 | 0.87 |
| Creatinine | 4,526 (50) | 71 | 59 | 87 |
| Urea | 5,021 (56) | 5.8 | 4.5 | 7.8 |
| Osmolality | 778 (8.6) | 290 | 283 | 302 |
| TSH = thyroid stimulating hormone; fT3 = triiodothyronine; fT4 = thyroxine; Na+ = sodium; K+ = potassium; Cl- = chloride; Ph-- = phosphate; Ca++ = calcium; Mg++ = magnesium. TSH in mU/l, fT3 and fT4 in pmol/ml, creatinine in μmol/l, all other values in mmol/l. | ||||
| Table 2: Prevalence of electrolyte disorders on admission to the emergency department. | |||||
| N (%) | Median* | Quartile 1* | Quartile 3* | Low/High* | |
| Hyponatraemia | 872 (9.7) | 128 | 124 | 130 | 103 |
| Hypernatraemia | 125 (1.4) | 143 | 143 | 144 | 163 |
| Hypokalaemia | 1,009 (11.2) | 3.3 | 3.1 | 3.4 | 1.5 |
| Hyperkalaemia | 434 (4.8) | 5.0 | 4.9 | 5.3 | 9.6 |
| Hypochloraemia | 121 (14.2) | 93 | 88 | 95 | 71 |
| Hyperchloraemia | 106 (12.4) | 111 | 110 | 113 | 126 |
| Hypocalcaemia | 489 (11.3) | 2.03 | 1.95 | 2.06 | 1.09 |
| Hypercalcaemia | 55 (1.2) | 2.6 | 2.57 | 2.72 | 3.32 |
| Hypomagnesaemia | 396 (26%) | 0.69 | 0.62 | 0.72 | 0.2 |
| Hypermagnesaemia | 63 (4.1) | 1.1 | 1.05 | 1.16 | 1.35 |
| Hypophosphataemia | 269 (24.5) | 0.74 | 0.61 | 0.79 | 0.14 |
| Hyperphosphataemia | 91 (8.3) | 1.74 | 1.62 | 2.27 | 4.51 |
| *All values are given in mmol/l. Low/high stands for the lowest and highest measured value, respectively. | |||||
| Table 3: Serum electrolytes (values are medians) on admission to the emergency department stratified for TSH. | ||||
| Normal TSH* | High TSH* | Low TSH* | Significance** | |
| Sodium | 139 | 138 | 139 | p <0.01 |
| Potassium | 3.94 | 3.96 | 4.03 | p <0.01 |
| Chloride | 103 | 102 | 103 | ns |
| Calcium | 2.25 | 2.23 | 2.24 | p <0.01 |
| Magnesium | 0.81 | 0.82 | 0.81 | ns |
| Phosphate | 1.04 | 1.19 | 1.1 | p <0.01 |
| *Serum TSH is given in mU/l. ** p-values refer to low versus high TSH groups. ns = non-significant. | ||||
It was the aim of the study to investigate the effects of thyroid function on serum electrolytes. According to different case reports in the literature someone could expect electrolyte disturbances in any sort of thyroid dysfunction, but those reports only include patients with severe forms of hypo- or hyperthyroidism. In the present study, only mild forms of hypo- and hyperthyroidism, based on elevated or low TSH levels respectively were available and most of the patients showed normal TSH levels.
Of 9,012 patients admitted to the emergency department of our university hospital, 9.7% showed hyponatraemia. Hyponatraemia was significantly more common in patients with elevated serum TSH levels. Although serum sodium did not correlate with TSH levels, a significant correlation could be shown between fT3 levels and serum sodium. While no correlation could be found between TSH levels and serum potassium and chloride levels, serum phosphate, calcium and magnesium correlated significantly with TSH.
Hypothyroidism is mentioned as a cause of hyponatraemia in a lot of specialty textbooks as well as in reviews published in highly ranked journals [2, 15–18]. However, to our knowledge, the present study is the first original contribution studying the association of thyroid hormone on serum sodium and other electrolytes in a large collection of patients. In contrast to a letter presenting original data, which did not find an association between high serum TSH levels and hyponatraemia, we found that hyponatraemia was more common in patients with elevated TSH compared to those with normal TSH [19, 20]. Although serum TSH levels per se did not correlate with serum sodium, the biologically most active fT3 correlated significantly with serum sodium concentrations. However, it should be stated that the effects shown in this study are minute and the higher prevalence rate for hyponatraemia in the group with high TSH levels was only slight. The theoretical mechanisms explaining an association between thyroid function and serum sodium were reviewed recently [10]. An impaired urinary dilution capacity due to non-osmotic release of anti-diuretic hormone, as well as increased urine sodium loss was the major mechanism for hypothyroid induced hyponatraemia in rats [21].
Prospective studies with long term follow up in patients with newly diagnosed hypothyroidism and hyponatraemia could help to determine whether the electrolyte disorder really resolves itself after starting hormone substitution. Hyponatraemia was recently shown to be associated with an increased risk of falls and fractures, making the subject more relevant for patients prognosis [22, 23], especially the elderly. This would justify the efforts of a prospective observational study.
In addition, a correlation of TSH levels with serum phosphate could be found. Animal studies propose thyroid hormones as long term regulators for phosphate metabolism. fT3 elevates renal phosphate reabsorption and elevates serum phosphate levels in rats [24]. Although no significant correlation between serum phosphate and fT3 could be shown, elevated TSH levels were related to higher serum-phosphate levels in our study. The higher prevalence of hyperphosphataemia in the group with high TSH levels in our study is controversial to the current basic and clinical research data, and may potentially be explained be a different underlying pathology in these patients [25].
The correlation of thyroid function (TSH and fT3) with serum-calcium levels in our study fits with clinical and animal studies. Hypercalcaemia was described in patients with hyperthyroidism due to an enhanced bone turn over [26]. Additionally, renal calcium excretion is influenced by thyroid hormones, as in rats FE(Ca) was decreased in hyperthyroidism and increased in hypothyroidism [27].
Serum-potassium levels were normal in myxoedema, but often decreased in thyrotoxicosis. A potassium shift in the cell as well as an enhanced renal potassium excretion where the reasons for hypokalaemia in hyperthyroidism [28]. In our study no conclusive statement for thyroid function and serum-potassium could be given, because elevated TSH levels were correlated with hyper- as well as hypokalaemia.
Hypomagnesaemia was described in thyrotoxicosis, but not found in our study. On the other hand we could show elevated TSH levels in patients with hypermagnesaemia. The mechanisms of changes in serum magnesium levels due to thyroid dysfunctions were not described in the literature.
Our study is limited by the retrospective design. Additionally, the list of potential confounders for electrolyte disorders is long. An unknown proportion of patients presented in this study suffered from acute illness influencing both electrolyte as well as thyroid hormone homeostasis. However, many if not most of our patients present with minor problems such as simple viral infections of the upper respiratory tract or small traumas. A detailed case by case review would be necessary in order to rule out all potential other causes for changes of serum electrolyte levels. However, this would not be possible due to the large number of patients included in the analysis. Additionally, free thyroid hormones were only available for a small proportion of our patients limiting our findings on this issue.
In conclusion, we observed marginal and not clinical relevant changes in serum electrolytes in patients with thyroid dysfunction. It is necessary to note, that clinically relevant electrolyte disorders most probably are only found in severe thyroid dysfunctions like thyrotoxicosis or myxoedema.
1 Adrogue HJ, Madias NE. Hypernatremia. N Engl J Med. 2000;342(20):1493–9.
2 Adrogue HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581–9.
3 Funk GC, Lindner G, Druml W, Metnitz B, Schwarz C, Bauer P, et al. Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med. 2010;36(2):304–11.
4 Darmon M, Timsit JF, Francais A, Nguile-Makao M, Adrie C, Cohen Y, et al. Association between hypernatraemia acquired in the ICU and mortality: a cohort study. Nephrol Dial Transplant. 2010;25(8):2510–5.
5 Lindner G, Funk GC, Lassnigg A, Mouhieddine M, Ahmad SA, Schwarz C, et al. Intensive care-acquired hypernatremia after major cardiothoracic surgery is associated with increased mortality. Intensive Care Med. 2010;36(10):1718–23.
6 Lindner G, Funk GC, Schwarz C, Kneidinger N, Kaider A, Schneeweiss B, et al. Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis. 2007;50(6):952–7.
7 Hwang JC, Wang CT, Chen CA, Chen HC. Hypokalemia is associated with increased mortality rate in chronic hemodialysis patients. Blood Purif. 2011;32(4):254–61.
8 Soliman HM, Mercan D, Lobo SS, Melot C, Vincent JL. Development of ionized hypomagnesemia is associated with higher mortality rates. Crit Care Med. 2003;31(4):1082–7.
9 Geerse DA, Bindels AJ, Kuiper MA, Roos AN, Spronk PE, Schultz MJ. Treatment of hypophosphatemia in the intensive care unit: a review. Crit Care. 2010;14(4):R147.
10 Mariani LH, Berns JS. The renal manifestations of thyroid disease. J Am Soc Nephrol. 2012;23(1):22–6.
11 Iwasaki Y, Oiso Y, Yamauchi K, Takatsuki K, Kondo K, Hasegawa H, et al. Osmoregulation of plasma vasopressin in myxedema. J Clin Endocrinol Metab. 1990;70(2):534–9.
12 Farnsworth AE, Dobyns BM. Hypercalcaemia and thyrotoxicosis. Med J Aust. 1974;2(21):782–4.
13 Wuttke H, Kessler FJ. Clinical significance of serum magnesium concentration in thyrotoxicosis (author’s transl). Med Klin. 1976;71(6):235–8.
14 Manoukian MA, Foote JA, Crapo LM. Clinical and metabolic features of thyrotoxic periodic paralysis in 24 episodes. Arch Intern Med. 1999;159(6):601–6.
15 Gross P, Benzing T, Hensen J, Monig H. Practical approach to hyponatremia. Dtsch Med Wochenschr. 2011;136(34-35):1728–32.
16 Rose BD. Clinical physiology of acid-base and electrolyte disorders. 5 ed: Mcgraw-Hill, 2001.
17 Halperin ML, Kamel KS, Goldstein MB. Fluid, electrolyte, and acid-base physiology: a problem based approach. Philadelphia: Saunders ElSevier; 2009;4th Edition.
18 Greenberg A, Adrogue HJ, Aggarwal N, Allon M, Anderson S, Andreoli SP. A primer on kidney diseases. ElSevier Health Science 2010;5th Edition.
19 Croal BL, Blake AM, Johnston J, Glen AC, O’Reilly DS. Absence of relation between hyponatraemia and hypothyroidism. Lancet. 1997;350(9088):1402.
20 Hanna FW, Scanlon MF. Hyponatraemia, hypothyroidism, and role of arginine-vasopressin. Lancet. 1997;350(9080):755–6.
21 Schmitt R, Klussmann E, Kahl T, Ellison DH, Bachmann S. Renal expression of sodium transporters and aquaporin-2 in hypothyroid rats. Am J Physiol Renal Physiol. 2003;284(5):F1097–104.
22 Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71 e1–8.
23 Kinsella S, Moran S, Sullivan MO, Molloy MG, Eustace JA. Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin J Am Soc Nephrol. 2010;5(2):275–80.
24 Alcalde AI, Sarasa M, Raldua D, Aramayona J, Morales R, Biber J, et al. Role of thyroid hormone in regulation of renal phosphate transport in young and aged rats. Endocrinology. 1999;140(4):1544–51.
25 Yamashita H, Yamazaki Y, Hasegawa H, Yamashita T, Fukumoto S, Shigematsu T, et al. Fibroblast growth factor-23 in patients with Graves’ disease before and after antithyroid therapy: its important role in serum phosphate regulation. J Clin Endocrinol Metab. 2005;90(7):4211–5.
26 Iqbal AA, Burgess EH, Gallina DL, Nanes MS, Cook CB. Hypercalcemia in hyperthyroidism: patterns of serum calcium, parathyroid hormone, and 1,25-dihydroxyvitamin D3 levels during management of thyrotoxicosis. Endocr Pract. 2003;9(6):517–21.
27 Kumar V, Prasad R. Molecular basis of renal handling of calcium in response to thyroid hormone status of rat. Biochim Biophys Acta. 2002;1586(3):331–43.
28 Park CW, Shin YS, Ahn SJ, Kim SY, Choi EJ, Chang YS, et al. Thyroxine treatment induces upregulation of renin-angiotensin-aldosterone system due to decreasing effective plasma volume in patients with primary myxoedema. Nephrol Dial Transplant. 2001;16(9):1799–806.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.
Authors’ contribution: C. Schwarz and A. B. Leichtle contributed equally.