Figure 1
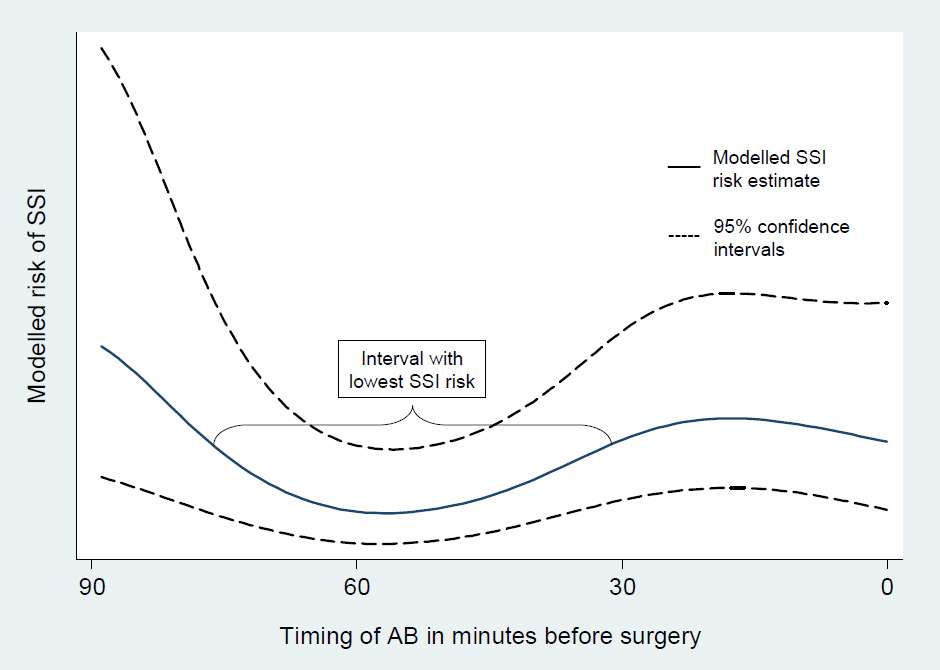
Risk of surgical site infection by timing of surgical antimicrobial prophylaxis. The lowest risk of surgical site infection was observed when the antibiotics were administered between 74 and 30 minutes before surgery.
DOI: https://doi.org/10.4414/smw.2012.13616
Surgical site infections (SSI) account for 14–16% of all nosocomial infections in inpatients and are considered the most common nosocomial infection among surgical patients [1]. The direct and indirect costs of treating SSI can be extremely high [2]. SSI are considered to reflect the quality of care, as they are potentially preventable complications directly linked to surgery.
The issue of risk factors and prevention measures for SSI has not been studied as thoroughly or as systematically as one would like, mostly for ethical or logistical reasons. Thus, many of the current recommendations of the Centres for Disease Control and Prevention (CDC) are based on a strong theoretical rationale or suggestive evidence in the absence of confirmatory scientific knowledge [1]. To address this problem, the Department of Surgery and the Division of Infectious Diseases and Hospital Epidemiology at the University Hospital of Basel followed a large prospective observational series of surgical patients closely for evidence of SSI, and then analysed the dataset for the influence of various risk factors.
It is hoped that this review of the Basel SSI Cohort Study further stimulates surgeons, operating room nurses, postoperative inpatient and clinic nurses, infection control professionals, anaesthesiologists and healthcare epidemiologists to engage actively in surgical research for the prevention of SSI.
The use of routine surgical antimicrobial prophylaxis (SAP) was a breakthrough in the prevention of SSI [4]. Today, single-shot administration of first- or second-generation cephalosporin is the state-of-the-art procedure in SAP in non-clean surgical interventions and implant surgery [5]. Because anaerobic activity is limited in most cephalosporins, treatment is supplemented with metronidazole in colorectal surgery.
In 1961, Burke [6] showed in animals that the timing of SAP was crucial. The [7] observational landmark paper of Classen et al. determined that in humans, the antimicrobial agent should be administered within 2 hours before surgery. The guidelines for prophylactic administration of cefuroxime (a second-generation cephalosporin), combined with metronidazole in colorectal surgery, were based on this time window at the University Hospital of Basel during the study period. Other authors [8, 9] have suggested that the optimal window for SAP is less than 60 minutes before skin incision, or have simply advised performing SAP immediately prior to the incision. Therefore, current guidelines recommend conducting SAP 60 minutes or less before surgery [4, 10].
Administration of SAP less than 30 minutes before incision has been routine practice at the University Hospital of Basel. However, there is little evidence in the literature to show that tissue levels of cefuroxime can within just a few minutes reach the minimum inhibitory concentration at incision required to prevent SSI. Administration of SAP within the final half hour before surgery may be too late for optimal prevention of SSI.
Therefore, the Basel SSI Cohort Study was designed primarily with emphasis on the influence of the timing of SAP on the incidence of SSI [3].
Pathogens may be transferred both from the surgical team to patients [12, 13] and vice-versa [14]. Skin-borne pathogens on staff hands are particularly prone to transfer. Consequently, all staff members wear surgical gloves as a protective barrier to prevent hand-wound contamination during operations.
The risk of glove perforation increases with the duration of surgery [15, 16]. The factors causing glove perforation include puncture by needles and sharp surfaces on complex instruments [17, 18]. The frequency of glove perforation during surgery has been repeatedly studied and found to range from 8 to 50% [16, 19–24]. However, the impact of glove perforation on the risk of SSI has been unknown.
The second analysis of the Basel SSI Cohort Study was conducted to test the hypothesis that clinically visible surgical glove perforation is associated with an increased SSI risk.
The association of blood transfusion with the risk of SSI remains controversial. While many observational series suggested that blood transfusion was a risk factor for the development of SSI [26–33], others achieved contradictory results [34–36]. Several randomised controlled trials (RCT) investigated the relationship between the use of different types of blood transfusion and overall infectious complication rates among different surgical specialities [37–41]. However, the results of investigations into whether receiving versus not receiving blood transfusion is associated with infection after surgery were observational in nature and susceptible to confounding. Interpretation of previously published studies is difficult due to inconsistencies in the types of blood components and the timings of transfusions, and consideration of all possible confounding factors [1, 42, 43].
The primary objective of this analysis of the Basel SSI Cohort Study was to investigate the association of perioperative allogeneic blood transfusion (ABT) with the risk of SSI. Moreover, as perioperative ABT is mainly performed in patients with preoperative anaemia [34, 44, 45], we assessed whether preoperative anaemia was associated with the rate of SSI. Specifically, we hypothesised that perioperative ABT and preoperative anaemia increase the risk of SSI.
Surgical skills are acquired primarily in the operating room. According to William Halsted’s apprenticeship model (“see one, do one, teach one”), surgical training starts by observing and then continues by taking an increasingly active role in the procedure [47]. Recently, various alternative methods for teaching surgical techniques have been developed, such as box model or virtual reality simulation [48]. However, tutorial assistance during surgery continues to be crucial to acquire a full command of surgical skills. This training system can only be justified if it does not increase the complication rate.
The purpose of this analysis of the Basel SSI Cohort Study was to assess whether tutorial training in the operating room leads to a higher incidence of SSI compared with surgery performed autonomously by board-certified surgeons.
Many studies have demonstrated a direct economic impact of SSI on health systems and an indirect impact on patients [50–55]. However, the magnitude of the economic SSI-related burden differed across the studies, mainly because of differences in healthcare reimbursement systems, the methodology of the surveillance, and the heterogeneity of the complications [56]. The available information is difficult to apply to any specific setting such as the University Hospital of Basel. We therefore conducted a matched case-control study nested in the prospective observational Basel SSI Cohort Study to quantify the economic and medical burden of SSI at the University Hospital of Basel.
SSI surveillance involving a feedback from the infection control personnel to surgeons has been shown to reduce the incidence of SSI by >30% [58]. However, this sort of surveillance is time-consuming and expensive. Other methods of surveillance have been described that economise resources and optimise sensitivity [59, 60]. One possible approach is self-assessment by the surgical team before the patient is discharged. However, this simple and low cost approach has been questioned in terms of sensitivity and accuracy of data, due to the significant risk of underreporting [61, 62].
The aim of this analysis by the Basel SSI Cohort Study was to determine the quality of in-house SSI surveillance by surgeons during the study period compared with surveillance performed by an infection control team.
The type of SAP is determined by the spectrum and antimicrobial resistance of pathogens causing SSI. Continuous efforts to identify outbreaks of antimicrobial-resistant pathogens are therefore mandatory. The present study was conducted to describe the epidemiologic features of SSI at the University Hospital of Basel and to outline their microbiological patterns, including antimicrobial resistance.
The total study population consisted of 6,283 procedures in 4808 patients with full in-hospital data records. A long-term follow-up after discharge was achieved for 5,721 of these 6,283 procedures (91.1%).
A summary of the adjusted odds ratios from multivariable analyses of contracting surgical site infection by occurrence of the potential risk factors under study is given in table 1.
| Table 1: Adjusted odds ratio of contracting surgical site infection by occurrence of potential risk factors in multivariable analyses. | |||||
| Characteristics | Adjusted odds ratio | 95% CI | p-value | ||
| Timing of SAP* | –59 to –45 min before incision | 1 | Reference | ||
| –29 to –15 min before incision | 2.82 | 1.5–5.3 | 0.001 | ||
| –120 to –75 min before incision | 3.16 | 1.4–7.0 | 0.005 | ||
| Glove perforation | With SAP* | No | 1 | Reference | |
| Yes | 1.25 | 0.85–1.85 | 0.263 | ||
| Without SAP* | No | 1 | Reference | ||
| Yes | 4.24 | 1.7 to 10.8 | 0.003 | ||
| Allogeneic blood transfusion | No | 1 | Reference | ||
| Yes: 1–2 units | 1.25 | 0.8–1.9 | 0.310 | ||
| Yes: ≥3 units | 1.07 | 0.6–2.0 | 0.817 | ||
| Preoperative anaemia | No | 1 | Reference | ||
| Yes | 0.91 | 0.7–1.2 | 0.530 | ||
| Surgical training | No | 1 | Reference | ||
| Yes | 0.82 | 0.62–1.09 | 0.163 | ||
| *SAP = surgical antimicrobial prophylaxis. | |||||
SAP was applied in 4,265 of the 6,283 procedures and administered within 2 hours before incision in 3,836 procedures. These 3,836 procedures met the inclusion

Figure 1
Risk of surgical site infection by timing of surgical antimicrobial prophylaxis. The lowest risk of surgical site infection was observed when the antibiotics were administered between 74 and 30 minutes before surgery.
criteria for this analysis and were performed in 3,313 patients with a median hospital stay of 10 days (interquartile range 6–17 days). A total of 180 SSI were detected (4.7%), in 109 instances during inpatient and in 71 instances during outpatient follow-up.
SAP was applied in most patients between 44 and 0 minutes before surgical incision. The lowest risk of SSI, however, was recorded when the antibiotics were administered between 74 and 30 minutes before surgery (fig. 1). Univariable logistic regression analysis showed that when antibiotic prophylaxis was applied 29 to 15 (unadjusted OR = 2.96; 95% CI 1.6 to 5.5; p= 0.001) and 14 to 0 (unadjusted OR = 1.99; 95% CI 1.0 to 3.8; p= 0.041) or 120 to 75 minutes prior to surgery (unadjusted OR = 3.25; 95% CI 1.5 to 7.1; p= 0.003) , the likelihood of SSI was significantly higher than when the antibiotics were administered between 59 and 45 minutes prior to surgery. The heterogeneity of SSI risk with timing of SAP remained statistically significant after adjusting for twelve confounders in multivariable analyses (p-value from likelihood ratio test = 0.0002). In addition, these analyses confirmed various known SSI risk factors, such as ASA score, wound class, duration of surgery, smoking status, diabetes and intraoperative hypothermia.
For analysis of the impact of compromised asepsis on occurrence of SSI, 747 of the 6,283 procedures (12%) were excluded due to highly contaminated CDC wound class 4. In 1,389 cases (22%) the presence or absence of glove perforation during surgery was not recorded. The remaining 4,147 procedures, with a total of 188 SSI (4.5%), were accepted for this analysis.
Glove perforation was recorded in 677 interventions (16.3%). After these procedures, 51 SSI (7.5%) were recorded, compared to 137 SSI (3.9%) in 3,470 procedures where asepsis was not breached (crude OR = 1.98; 95% CI, 1.4–2.8; p <0.001). Multivariable logistic regression analyses showed that the increase of SSI likelihood with glove perforation differed between procedures with and without SAP (test for effect-modification: p = 0.005). SAP was given in 3,233 interventions, and glove perforations were detected in 605 of the 3,233 operations (18.7%). Multivariable logistic regression analysis showed that in procedures with SAP the odds of contracting SSI in the event of glove puncture were not significantly higher than when gloves remained intact (adjusted OR = 1.25; 95% CI 0.85 to 1.85; p = 0.263). In the absence of SAP (n = 914), however, glove leakage was associated with an SSI rate of 12.7%, as opposed to 2.9% where asepsis was not compromised. This difference was statistically significant in both univariable (OR = 4.9; 95% CI 2.2 to 11.0; p <0.001) and multivariable (OR = 4.24; 95% CI 1.7 to 10.8; p = 0.003) analysis.
This analysis included 5,873 procedures with 284 SSI (4.8%). Univariable analysis showed significant associations between anaemia or blood transfusion and risk of SSI. After including duration of surgery as confounder in multivariate analysis, these associations disappeared (anaemia: OR, 0.91; 95% CI 0.7 to 1.2; p = 0.53 / transfusion: OR, 1.07; 95% CI 0.6 to 2.0; p= 0.817).
This analysis included 6,103 procedures and 290 SSI (4.8%). Surgery was performed with tutorial assistance in 39% (2,388/6,103) and autonomously in 61% (n = 3,715/6,103).Surprisingly, univariable analysis showed a significant increase in the rate of SSI for autonomously performed procedures compared to those under supervision (5.4% vs 3.8%; OR = 0.70; 95% CI 0.543–0.902; p = 0.006). However, this association did not remain significant after adjusting for confounders in multivariable analysis (OR = 0.82; 95% CI 0.62–1.09; p = 0.163).
For this matched case-control study, 183 case patients with in-hospital SSI were primarily considered and 168 (92%) were successfully matched to a suitable control patient.
In the event of SSI, the mean additional hospital cost was CHF 19,638 (95% CI CHF 8,492–30,784). The mean postoperative length of hospitalisation for case patients was more than double that for control patients (29.0 vs 12.3 days; p= .001), resulting in a mean additional postoperative hospital stay of 16.8 days (95% CI, 13–20.6 days). The mean duration of additional in-hospital antibiotic therapy was 7.4 days (95% CI 5.1–9.6). Logistic regression analyses showed significantly higher odds of antibiotic therapy for patients with SSI (OR = 3.23; 95% CI 2.0– 5.2; p= .001). The overall mean increase in SSI-related hospital costs was 60.6%
This analysis included 6,283 procedures and 187 in-hospital SSI. The surgical staff documented only 91/187 (48.7%) of in-hospital SSI, while the ICT registered the remaining 96/187 (51.3%). By division, the visceral surgeons documented 59/105 (56.2%), the vascular surgeons 14/37 (37.8%) and the trauma surgeons 18/45 (40.0%) in-hospital SSI.
Microbiological evaluation has not been performed or was not conclusive in 164 of 293 SSI (56%). The germ spectrum in the remaining 129 SSI (44%) identified not a single case of methicillin-resistant Staphylococcus aureus (MRSA) or other bacteria with increased antimicrobial resistance. Staphylococcus aureus was the most common SSI-causing pathogen in trauma and vascular surgery, whereas Escherichia coli was more frequently found in SSI after visceral surgery. Overall, Staphylococcus aureus was the most common SSI-causing pathogen (29% of all SSI with documented microbiology).
The prospective observational Basel SSI Cohort Study of 6,283 procedures performed on 4,808 patients allowed us to investigate several a priori and post hoc hypotheses. Administration of SAP within the final half hour versus administration at earlier points in time before surgery and glove perforation in the absence of SAP have been identified as significant independent risk factors for SSI. Anaemia, transfusion and tutorial assistance did not increase the risk of SSI. The substantial economic burden of in-hospital SSI has been confirmed. SSI surveillance by the surgical staff detected only half of all in-hospital SSI. Due to the absence of multiresistant SSI-causing pathogens, the continuous use of single-shot single-drug SAP with cefuroxime (plus metronidazole in colorectal surgery) has been validated.
The a priori hypothesis proved to be true inasmuch as administration of SAP within the final half hour before surgery may be too late for optimal prevention of SSI. Importantly, healthcare providers in the United States and in Europe often fail to meet the present broad recommendation to get drugs started during the 60-minute window before surgery [4, 5, 64]. Further constricting that time window and requiring that the infusion should be completed 30 minutes before incision may make this target even more difficult. Nonetheless, SAP has been delivered during the hour before incision >90% of the time during the study period, and the goal should be to apply SAP at the optimal time, despite all difficulties. Hence the guidelines in place at the University Hospital of Basel now demand that SAP be completed 30 minutes before the start of surgery.
However, two large prospective studies observed the lowest risk of SSI when SAP was given within 30 minutes prior to incision [65, 66]. Therefore, current international guidelines for the correct timing of SAP are based on observational studies, which have recently achieved discordant results. A well conducted RCT seems warranted to obtain a clear answer on the optimal timing. We plan to conduct a bicentre prospective RCT at two tertiary referral centers in Switzerland, the University Hospital of Basel and the Cantonal Hospital of Aarau. The study is supported by the Swiss National Science Foundation. This RCT will compare two different delivery modi for SAP, which will result in different average administration times: SAP delivery in the anaesthesia room (more than 30 minutes before incision) vs SAP delivery in the operating room (less than 30 minutes before incision). We hypothesise that the rate of SSI is significantly lower with administration of SAP more than 30 minutes before the scheduled incision as compared with less than 30 minutes before the scheduled incision. We plan to include 5,000 patients undergoing visceral, vascular and trauma procedures – 2,500 per treatment arm – and assess the occurrence of SSI during a 30 day follow-up period (1 year if an implant is in place). We expect the study to be completed within 3 years.
While various studies have assessed the frequency of glove perforation, the relevant consequences in terms of SSI risk have been largely neglected. The second analysis showed that in the absence of SAP glove perforation increased the risk of SSI. Efforts to decrease the frequency of glove perforation in procedures without SAP, such as double gloving or routinely changing gloves in longer surgical procedures, are therefore encouraged as the first line of SSI prevention. These measures are effective and safe. However, implementing them in clinical practice can be difficult. Alternatively, the indication for SAP may be broadened to all surgical procedures. SAP has been shown to prevent SSI after clean surgery in several RCT [67–69], but there is no current consensus regarding its use in this area. The present results theoretically support an extended indication of SAP to all clean procedures when no strict precautions are taken to prevent glove perforation. However, the advantages of generalised SAP administration must be balanced against the adverse effects of the prophylactic antibiotics, such as increased costs, drug reactions and, most importantly, bacterial resistance.
The results of the third analysis strengthen existing doubts on the role of transfusion of leukocyte-depleted packed red cells during surgery and preoperative anaemia as risk factors for SSI. No significant relationship could be found after including duration of surgery in the multivariable model, which was found to be the most important confounder, presumably as a surrogate for the complexity of the patients and procedures. This underlines the role of covariate selection in the statistical analyses conducted on this topic, which may help to explain the disagreement among the published observational studies [42, 43].
Surgical training did not result in higher infection rates in this study. Therefore, while other forms of training are useful, tutorial assistance in the operating room continues to be the mainstay of surgical education at our hospitals.
The matched case-control study confirmed the substantial economic impact of in-hospital SSI and provided an estimate of the resources that may be saved by reducing the SSI-related heavy burden on patients and healthcare providers. Hence, future research in this field has the potential to become a matter of significant interest in terms of national and international healthcare economics.
The SSI surveillance system used by the surgical staff during the study period detected only half of all in-hospital SSI. This documented poor performance called for a major revision of the procedures to ascertain in-hospital SSI. The main procedural problem identified was that the resident had to complete a paper form to register SSI when he prepared all the discharge documents, a time of considerable time pressure, which resulted in many missed (i.e. non-registered) infections mostly in patients on a longer hospital stay. Therefore, substantial efforts have been undertaken to develop an electronic SSI surveillance system, which has recently been introduced at the University Hospital of Basel and the Cantonal Hospital of Aarau. It allows residents to prospectively register detailed information on SSI in much less time compared with the old system. Furthermore, surveillance is now a continuous procedure, allowing registration of events immediately after diagnosis, for example after the resident’s round with an attending surgeon. When the patient is discharged, the resident reviews all the information on patients’ hospital course and then presses the save button on the electronic surveillance form. This creates an E-mail to the attending surgeon in charge of the patient asking him to confirm the findings. The electronic SSI surveillance system is much more user-friendly compared with the former version. The system generates a weekly reminder E-mail to the attending surgeons including a list of their patients with missing information on wound surveillance after discharge. In addition, it allows the attending surgeons to continuously control surveillance by residents with a detailed list on the intranet.
The spectrum of SSI-causing pathogens identified and the very low incidence of antimicrobial resistance at the University Hospital Basel validate the continuous use of single shot single-drug SAP with cefuroxime (plus metronidazole in colorectal surgery).
By way of summary, current guidelines for SSI prevention have been evaluated in various ways, and future research projects in this field have been defined. We hope that the combined efforts of the University Hospital of Basel and the Cantonal Hospital of Aarau will translate in a significant reduction of SSI rates, corresponding costs and provision of specialised care.
1 Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20(4):250–78.
2 Urban JA. Cost analysis of surgical site infections. Surg Infect. (Larchmt ) 2006;7(Suppl 1):S19–22.
3 Weber WP, Marti WR, Zwahlen M, Misteli H, Rosenthal R, Reck S, et al. The timing of surgical antimicrobial prophylaxis. Ann Surg. 2008;247(6):918–26.
4 Dellinger EP, Gross PA, Barrett TL, Krause PJ, Martone WJ, McGowan JE, Jr. et al. Quality standard for antimicrobial prophylaxis in surgical procedures. Infectious Diseases Society of America. Clin Infect Dis. 1994;18(3):422–7.
5 Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;38(12):1706–15.
6 Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 196;50:161–8.
7 Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326(5):281–6.
8 Geroulanos S, Marathias K, Kriaras J, Kadas B. Cephalosporins in surgical prophylaxis. J Chemother. 2001;13 Spec No 1(1):23–6.
9 van Kasteren ME, Kullberg BJ, de Boer AS, Mintjes-de GJ, Gyssens IC. Adherence to local hospital guidelines for surgical antimicrobial prophylaxis: a multicentre audit in Dutch hospitals. J Antimicrob Chemother. 2003;51(6):1389–96.
10 Bratzler DW, Hunt DR. The surgical infection prevention and surgical care improvement projects: national initiatives to improve outcomes for patients having surgery. Clin Infect Dis. 2006;43(3):322–30.
11 Misteli H, Weber WP, Reck S, Rosenthal R, Zwahlen M, Fueglistaler P, et al. Surgical glove perforation and the risk of surgical site infection. Arch Surg. 2009;144(6):553–8.
12 Esteban JI, Gomez J, Martell M, Cabot B, Quer J, Camps J, et al. Transmission of hepatitis C virus by a cardiac surgeon. N Engl J Med. 1996;334(9):555–60.
13 Harpaz R, Von SL, Averhoff FM, Tormey MP, Sinha SD, Kotsopoulou K, et al. Transmission of hepatitis B virus to multiple patients from a surgeon without evidence of inadequate infection control. N Engl J Med. 1996;334(9):549–54.
14 Kiyosawa K, Sodeyama T, Tanaka E, Nakano Y, Furuta S, Nishioka K, et al. Hepatitis C in hospital employees with needlestick injuries. Ann Intern Med. 1991;115(5):367–9.
15 Greco RJ, Wheatley M, McKenna P. Risk of blood contact through surgical gloves in aesthetic procedures. Aesthetic Plast Surg. 1993;17(2):167–8.
16 Kojima Y, Ohashi M. Unnoticed glove perforation during thoracoscopic and open thoracic surgery. Ann Thorac Surg. 2005;80(3):1078–80.
17 Maffulli N, Capasso G, Testa V. Glove perforation in elective orthopedic surgery. Acta Orthop Scand. 1989;60(5):565–6.
18 Yinusa W, Li YH, Chow W, Ho WY, Leong JC. Glove punctures in orthopaedic surgery. Int Orthop. 2004;28(1):36–9.
19 Dodds RD, Guy PJ, Peacock AM, Duffy SR, Barker SG, Thomas MH. Surgical glove perforation. Br J Surg. 1988;75(10):966–8.
20 Gunasekera PC, Fernando RJ, de Silva KK. Glove failure: an occupational hazard of surgeons in a developing country. J R Coll Surg Edinb. 1997;42(2):95–7.
21 Laine T, Aarnio P. How often does glove perforation occur in surgery? Comparison between single gloves and a double-gloving system. Am J Surg. 2001;181(6):564–6.
22 Nicolai P, Aldam CH, Allen PW. Increased awareness of glove perforation in major joint replacement. A prospective, randomised study of Regent Biogel Reveal gloves. J Bone Joint Surg Br. 1997;79(3):371–3.
23 Punyatanasakchai P, Chittacharoen A, Ayudhya NI. Randomized controlled trial of glove perforation in single- and double-gloving in episiotomy repair after vaginal delivery. J Obstet Gynaecol Res. 2004;30(5):354–7.
24 Quebbeman EJ, Telford GL, Wadsworth K, Hubbard S, Goodman H, Gottlieb MS. Double gloving. Protecting surgeons from blood contamination in the operating room. Arch Surg. 1992;127(2):213–6.
25 Weber WP, Zwahlen M, Reck S, Misteli H, Rosenthal R, Buser AS, et al. The association of preoperative anemia and perioperative allogeneic blood transfusion with the risk of surgical site infection. Transfusion. 2009;49(9):1964–70.
26 Blumetti J, Luu M, Sarosi G, Hartless K, McFarlin J, Parker B, et al. Surgical site infections after colorectal surgery: do risk factors vary depending on the type of infection considered? Surgery. 2007;142(5):704–11.
27 Ford CD, VanMoorleghem G, Menlove RL. Blood transfusions and postoperative wound infection. Surgery. 1993;113(6):603–7.
28 Friedman ND, Bull AL, Russo PL, Leder K, Reid C, Billah B, et al. An alternative scoring system to predict risk for surgical site infection complicating coronary artery bypass graft surgery. Infect Control Hosp Epidemiol. 2007;28(10):1162–8.
29 Morris CD, Sepkowitz K, Fonshell C, Margetson N, Eagan J, Miransky J, et al. Prospective identification of risk factors for wound infection after lower extremity oncologic surgery. Ann Surg Oncol. 2003;10(7):778–82.
30 Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116(22):2544–52.
31 Talbot TR, D’Agata EM, Brinsko V, Lee B, Speroff T, Schaffner W. Perioperative blood transfusion is predictive of poststernotomy surgical site infection: marker for morbidity or true immunosuppressant? Clin Infect Dis. 2004;38(10):1378–82.
32 Tang R, Chen HH, Wang YL, Changchien CR, Chen JS, Hsu KC, et al. Risk factors for surgical site infection after elective resection of the colon and rectum: a single-center prospective study of 2,809 consecutive patients. Ann Surg. 2001;234(2):181–9.
33 Walz JM, Paterson CA, Seligowski JM, Heard SO. Surgical site infection following bowel surgery: a retrospective analysis of 1446 patients. Arch Surg. 2006;141(10):1014–8.
34 Ali ZA, Lim E, Motalleb-Zadeh R, Ali AA, Callaghan CJ, Gerrard C, et al. Allogenic blood transfusion does not predispose to infection after cardiac surgery. Ann Thorac Surg. 2004;78(5):1542–6.
35 Johnston P, Wynn-Jones H, Chakravarty D, Boyle A, Parker MJ. Is perioperative blood transfusion a risk factor for mortality or infection after hip fracture? J Orthop Trauma. 2006;20(10):675–9.
36 Koval KJ, Rosenberg AD, Zuckerman JD, Aharonoff GB, Skovron ML, Bernstein RL, et al. Does blood transfusion increase the risk of infection after hip fracture? J Orthop Trauma. 1997;11(4):260–5.
37 Bilgin YM, van de Watering LM, Eijsman L, Versteegh MI, Brand R, van Oers MH, et al. Double-blind, randomized controlled trial on the effect of leukocyte-depleted erythrocyte transfusions in cardiac valve surgery. Circulation. 2004;109(22):2755–60.
38 Busch OR, Hop WC, Hoynck van Papendrecht MA, Marquet RL, Jeekel J. Blood transfusions and prognosis in colorectal cancer. N Engl J Med. 1993;328(19):1372–6.
39 Heiss MM, Mempel W, Jauch KW, Delanoff C, Mayer G, Mempel M, et al. Beneficial effect of autologous blood transfusion on infectious complications after colorectal cancer surgery. Lancet. 1993;342(8883):1328–33.
40 Houbiers JG, Brand A, van de Watering LM, Hermans J, Verwey PJ, Bijnen AB, et al. Randomised controlled trial comparing transfusion of leucocyte-depleted or buffy-coat-depleted blood in surgery for colorectal cancer. Lancet. 1994;344(8922):573–8.
41 Jensen LS, Kissmeyer-Nielsen P, Wolff B, Qvist N. Randomised comparison of leucocyte-depleted versus buffy-coat-poor blood transfusion and complications after colorectal surgery. Lancet 1996;348(9031):841–5.
42 Vamvakas EC, Carven JH, Hibberd PL. Blood transfusion and infection after colorectal cancer surgery. Transfusion. 1996;36(11-12):1000–8.
43 Vamvakas EC, Carven JH. Transfusion of white-cell containing allogeneic blood components and postoperative wound infection: effect of confounding factors. Transfus Med. 1998;8(1):29–36.
44 Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM. Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res. 2002;102(2):237–44.
45 Shander A, Knight K, Thurer R, Adamson J, Spence R. Prevalence and outcomes of anemia in surgery: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):58S–69S.
46 Rosenthal R, Weber WP, Zwahlen M, Misteli H, Reck S, Oertli D, et al. Impact of surgical training on incidence of surgical site infection. World J Surg. 2009;33(6):1165–73.
47 Halsted WS. The training of the surgeon. Bull Johns Hop Hosp. 15, 267–275. Ref Type: Generic
48 Sutherland LM, Middleton PF, Anthony A, Hamdorf J, Cregan P, Scott D, et al. Surgical simulation: a systematic review. Ann Surg. 2006;243(3):291–300.
49 Weber WP, Zwahlen M, Reck S, Feder-Mengus C, Misteli H, Rosenthal R, et al. Economic burden of surgical site infections at a European university hospital. Infect Control Hosp Epidemiol. 2008;29(7):623–9.
50 Coello R, Charlett A, Wilson J, Ward V, Pearson A, Borriello P. Adverse impact of surgical site infections in English hospitals. J Hosp Infect. 2005;60(2):93–103.
51 Engemann JJ, Carmeli Y, Cosgrove SE, Fowler VG, Bronstein MZ, Trivette SL, et al. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin Infect Dis. 2003;36(5):592–8.
52 Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20(11):725–30.
53 Poulsen KB, Bremmelgaard A, Sorensen AI, Raahave D, Petersen JV. Estimated costs of postoperative wound infections. A case-control study of marginal hospital and social security costs. Epidemiol Infect. 1994;113(2):283–95.
54 Vegas AA, Jodra VM, Garcia ML. Nosocomial infection in surgery wards: a controlled study of increased duration of hospital stays and direct cost of hospitalization. Eur J Epidemiol. 1993;9(5):504–10.
55 Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23(4):183–9.
56 Fry DE. The economic costs of surgical site infection. Surg Infect. (Larchmt ) 2002;3 (Suppl 1):S37–S43.
57 Rosenthal R, Weber WP, Marti WR, Misteli H, Reck S, Dangel M, et al. Surveillance of surgical site infections by surgeons: biased underreporting or useful epidemiological data? J Hosp Infect. 2010 March 12.
58 Haley RW, Morgan WM, Culver DH, White JW, Emori TG, Mosser J, et al. Update from the SENIC project. Hospital infection control: recent progress and opportunities under prospective payment. Am J Infect Control. 1985;13(3):97–108.
59 Glenister HM, Taylor LJ, Bartlett CL, Cooke EM, Mulhall AB. Introduction of laboratory based ward liaison surveillance of hospital infection into six district general hospitals. J Hosp Infect. 1993;25(3):161–72.
60 Glenister HM. How do we collect data for surveillance of wound infection? J Hosp Infect. 1993;24(4):283–9.
61 Broderick A, Mori M, Nettleman MD, Streed SA, Wenzel RP. Nosocomial infections: validation of surveillance and computer modeling to identify patients at risk. Am J Epidemiol. 1990;131(4):734–42.
62 Poulsen KB, Meyer M. Infection registration underestimates the risk of surgical wound infections. J Hosp Infect. 1996;33(3):207–15.
63 Misteli H, Widmer AF, Rosenthal R, Oertli D, Marti WR, Weber WP. Spectrum of pathogens in surgical site infections at a Swiss university hospital. Swiss Med Wkly. 2011;140:w13146.
64 Bratzler DW, Houck PM, Richards C, Steele L, Dellinger EP, Fry DE et al. Use of antimicrobial prophylaxis for major surgery: baseline results from the National Surgical Infection Prevention Project. Arch Surg. 2005;140(2):174–82.
65 Steinberg JP, Braun BI, Hellinger WC, Kusek L, Bozikis MR, Bush AJ, et al. Timing of antimicrobial prophylaxis and the risk of surgical site infections: results from the Trial to Reduce Antimicrobial Prophylaxis Errors. Ann Surg. 2009;250(1):10–6.
66 van Kasteren ME, Mannien J, Ott A, Kullberg BJ, de Boer AS, Gyssens IC. Antibiotic prophylaxis and the risk of surgical site infections following total hip arthroplasty: timely administration is the most important factor. Clin Infect Dis. 2007;44(7):921–7.
67 Platt R, Zaleznik DF, Hopkins CC, Dellinger EP, Karchmer AW, Bryan CS, et al. Perioperative antibiotic prophylaxis for herniorrhaphy and breast surgery. N Engl J Med. 1990;322(3):153–60.
68 Yerdel MA, Akin EB, Dolalan S, Turkcapar AG, Pehlivan M, Gecim IE, et al. Effect of single-dose prophylactic ampicillin and sulbactam on wound infection after tension-free inguinal hernia repair with polypropylene mesh: the randomized, double-blind, prospective trial. Ann Surg. 2001;233(1):26–33.
69 Lewis RT, Weigand FM, Mamazza J, Lloyd-Smith W, Tataryn D. Should antibiotic prophylaxis be used routinely in clean surgical procedures: a tentative yes. Surgery 1995;118(4):742–6.
Authors’ contribution:The first two authors contributed equally to this work.
Funding / potential competing interests: The authors declare that they have no conflicts of interest relevant to the manuscript submitted to Swiss Medical Weekly. This research was funded by the Department of General Surgery, University Hospital of Basel, and the Freiwillige Akademische Gesellschaft Basel. The study sponsors had no role in the design or conduct of the study, collection, management, analysis, or interpretation of the data, nor in the preparation, review, or approval of the manuscript.