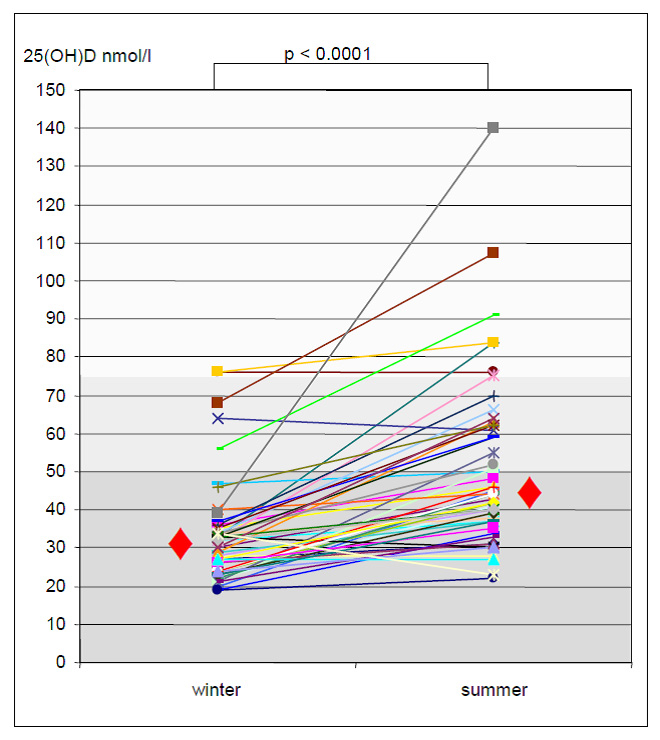
Figure 1
Change of 25(OH)D from winter to summer.
DOI: https://doi.org/10.4414/smw.2012.13672
Vitamin D is essential for calcium, phosphorus and bone metabolism. In addition there are links between vitamin D deficiency and infectious diseases, cardiovascular diseases and cancer [1].
Vitamin D deficiency is very common in the general population especially during winter with an even higher prevalence in patients with chronic kidney diseases and kidney transplant patients [2–7]. The major source of vitamin D is the cutaneous production by exposure to solar ultraviolet B (UVB). Only very few foods contain vitamin D. Inadequate exposure to UVB is the major cause of vitamin D deficiency [8]. Factors that influence the cutaneous vitamin D production are time of day, season of the year, latitude, age, skin pigmentation and sunscreen use [8–14]. The high prevalence of vitamin D deficiency in kidney transplant patients is thought to be due the general recommendation of sun protection and sun avoidance in these patients because of their high risk of skin cancer [15]. Furthermore there are some animal data suggesting that glucocorticoids, commonly used for immune-suppression, can increase vitamin D catabolism and additionally lower vitamin D levels [16]. Whether sun protection alone can decrease cutaneous vitamin D production reversing circannual rhythm of vitamin D in these patients is not known. We therefore measured vitamin D during winter and summer in kidney transplant patients with steroid free immunosuppressive maintenance therapy.
In this single centre prospective observational study all kidney transplanted patients visiting our outpatient clinic in January and February 2011 were screened for eligibility to participate in the study. Exclusion criterion were: any vitamin D, calcium or cinacalcet substitution during the last 6 months, any treatment for osteoporosis, severe hypocalcaemia, uncontrolled hyper-parathyreoidism, treatment with steroids and travels in the southern hemisphere within the previous 3 months.

Figure 1
Change of 25(OH)D from winter to summer.
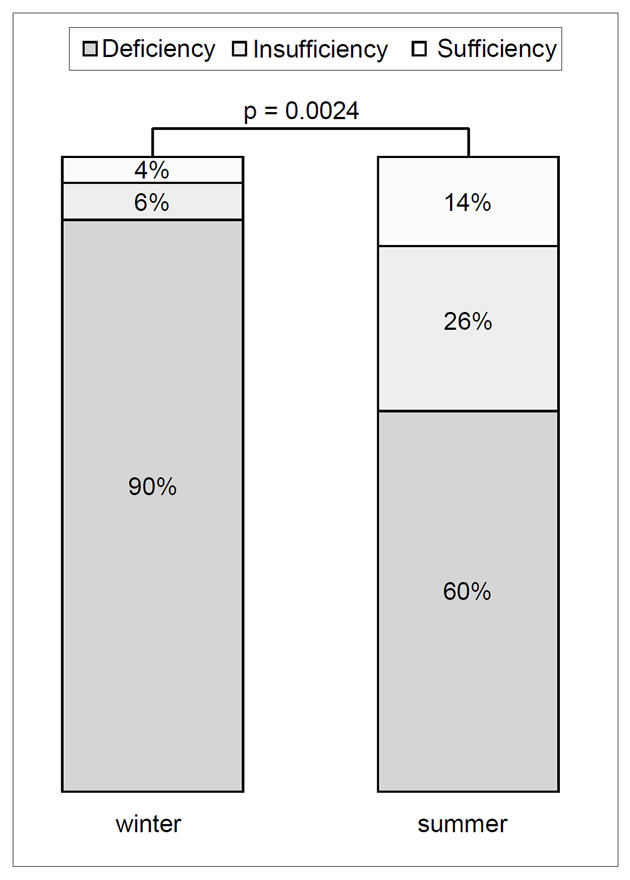
Figure 2
Seasonal prevalence of 25(OH)D deficiency (<50 nmol/l), insufficiency (50–74 nmol/l) and sufficiency (>74 nmol/l).
In total 50 patients were eligible and were included in the study after giving written informed consent. Demographics data are shown in table 1. This prospective single centre study was performed after approval by the local ethics committee of the University of Basel.
Non-fasting blood samples were drawn during winter (January/February) and during summer (July/August). 25-hydroxvitamin D (25[OH]D, 1-25-hydroxvitamin D (1-25[OH]D, intact parathormone (iPTH), creatinine, phosphate and calcium were measured.
25(OH)D was measured with an enzyme-linked immunosorbent assay (ELISA) (Immuno-Diagnostic-System DSX, Germany) in the clinical biochemical laboratory at University Hospital Basel. We defined deficiency when 25(OH)D was below 50 nmol/l, insufficiency by a level of 50–74 nmol/l and sufficiency by a level above 74 nmol/l according to the guideline of the endocrine society of clinical practice [17]. 1-25(OH)D concentrations (pmol/l) were assessed by ELISA (Immuno-Diagnostic-System Victor, Germany) in the clinical biochemical laboratory at University Hospital Berne and intact PTH by a solid phase chemiluminescence (Immulite 2000, Siemens Germany) in the clinical biochemical laboratory at University Hospital Basel.
Kidney function was assessed from the estimated glomerular filtration rate (GFR) according to the equation from the Modification of Diet in Renal Diseases Study [18].
The patients were asked about dietary supplement (e.g., multivitamins, calcium or vitamin D intake) and about their travels during the previous 3 months before study entry.
All the patients were meticulously instructed about sun protection after transplantation and education was repeated annually. They were recommended to avoid sun exposure from 11 a.m. until 3 p.m., to wear long sleeves, long trousers and a hat or cap. They were instructed to apply the sunscreen to the head and neck, forearms, dorsum of each hands and other sun exposed skin areas, and to use sunscreen with sun protector factor 50 at least once daily. In addition they were given written instructions on how to apply the sunscreen.
Sun avoidance was assessed by a questionnaire during summer visit. The patients were asked the following questions: “Do you have direct sun exposure during the spring and summer months between 11.00 a.m. and 3.00 p.m.?”, “Do you use sunscreen every day during summer?”, “How many times per day do you apply sunscreen?”, “When in the sun, do you always wear: a hat or cap, long sleeves, long trousers?”
We used JMP software version 8.0 (SAS Institute Inc., Cary, NC) for statistical analysis. Descriptive statistical values are presented as median (range). For categorical data, Fisher’s exact test or Pearson’s chi-square test were used. Continuous variables were compared with Wilcoxon rank-sum tests. P values were two-sided and a value of less than 0.05 was considered statistically significant.
A total of 50 patients were included. The median time of transplantation was 11.1 years (range 0.8–33.6) and all patients had stable allograft function during the study period (median GFR at study entry 63.3 ml/min [range 28.7–109.7] and 59.1 ml/min [range 27.1–136.0]) at the follow up visit. We found vitamin D deficiency (25[OH]D <50 nmol/l) in 90% (45/50) of the study population during winter. The median 25(OH)D level of the study population during winter was 31 nmol/l (range 21–76; fig. 1). There was a significant rise of 25(OH)D in 94% (47/50) patients from winter to summer (p <0.0001) leading to a decline of patients with 25(OH)D deficiency from 90 to 60% (30/50). 25(OH)D insufficiency increased from 6 to 26% and 25(OH)D values normalised from 4 to 14% (p = 0.0024; fig. 2). The median 25(OH)D level of the study population during summer was 44 nmol/l (range 23–140 nmol/l). There was a rise of 1-25(OH)D from a median of 54 pmol/l (range 21–153) during winter up to 72.5 pmol/l (range 21–127) during summer (p = 0.003). We did not find a significant decline of iPTH (p = 0.917) from winter (median 83.7pg/ml; range 24.5–1075) to summer (median 80.9 pg/ml; range 14–896). Patients with normal 25(OH)D values during summer were younger (median 51.6 vs 63.8 years), but the difference was not statistically significant (p = 0.44). Correlation analyses of 25(OH)D changes with 1-25(OH)D and iPTH changes respectively showed poor correlations (r2 <0.03566).
Questionnaires were obtained from 78% of patients (39/50). Result of these questionnaires revealed consequent application of sunscreen (at least once daily) in all but one patient. Sun protection by clothes was lower. A total of 62% (24/39) wore a hat or cap, 64% (25/39) long trousers and only 36% (14/39) long sleeves. Consequent avoidance of direct sun exposure during daytime (between 11.00 am and 3.00 pm) was performed by 89% (34/39) of patients. In total, 9 patients adhered strictly to all parts of the recommendations. Only one of these nine patients showed no rise of 25(OH)D from winter to summer. He had a 25(OH)D of 64 nmol/l in the winter and 61 nmol/l during summer (range of insufficiency). We found no differences in the rise of 25(OH)D levels when comparing these 9 patients with the 21 patients with the poorest adherence (median rise 13 nmol/l vs 15 nmol/l, p = 0.91).
| Table 1: Demographics of study population at study start. | |
| Age | Median 61.2 (range 28–81) |
| Gender (f/m) | 17/33 |
| Years since transplantation | 11.1 years (range 0.8–33.6) |
| GFR by MDRD | Median 63.3 ml/min (range 30–106) |
| iPTH | Median 83.7 pg/ml (range 24–1075) |
| Calcium | Median 2.32 mmol/l (range 2.07–2.52) |
| Phosphorus | Median 0.98 mmol/l (range 0.37–1.6) |
Sun protection is the main reason for vitamin D deficiency in kidney transplant patients [15]. However, vitamin D deficiency is also common during winter in the whole central-European general population [7, 19]. Hintzpeter found a high prevalence of vitamin D deficiency in the German population, (latitude 47°16`N to 55°04`N) in 68% of men and in 61% of women during winter with a decline to 45% in men, and to 55% in women during summer [20].
The current study from Switzerland (latitude of 47°33`N) revealed that 90% of kidney transplant patients are vitamin D deficient during winter with a significant decrease to 60% during summer, despite very efficient sun protection in our study population. Ewers also found a 81% prevalence of hypovitaminosis D during winter in kidney transplant patients in Denmark where 30% had 25(OH)D deficiency and 51% had insufficiency [2]. This slightly lower prevalence of any hypovitaminosis and especially the less severe degree of hypovitaminosis is probably due to the fact that the majority of these patients (61% of the women and 51% of the men) took vitamin D supplements. In 94% of our patients we found a significant circannual rise of 25(OH) Vitamin D. A probable reason for preserved circannual rhythm of 25(OH)D is that even just 20–30 minutes of sun exposure during the daytime, three times a week, are sufficient for cutaneous vitamin D3 production during seasons with high UVB radiation. So, even very good sun protection will not totally prevent any cutaneous vitamin D3 production. This would also imply that our above mentioned sun protection recommendations for kidney transplant patients were not sufficient enough to inhibit any cutaneous vitamin D production. Marks and colleagues made a similar observation in patients with skin cancer who were recommended strict sun avoidance in a randomised double-blind placebo control trial showing an equal rise of 25(OH) during the study period from winter to summer in both groups [21]. Another reason for preserved circannual rhythm could be that non-compliance with the sun protection recommendations is common in kidney transplant patients despite the answers of high adherence in the questionnaire. Only three patients (6%) in the study population showed absolute no rise of 25(OH)D. One patient was hospitalised for several months during the study period. The second one adhered very strictly to the sun protection recommendations. He remained stable with his 25(OH)D level in the range of 64–61 nmol/l and so was only mildly vitamin D insufficient. No obvious reason was found in the third patient.
Beside reduced exposure to UVB radiation as the main factor for hypovitaminosis D in kidney transplant patients there may be other contributing factors leading to this very high incidence of hypovitaminosis D compared to the general population during all the seasons. It is known that glucocorticoid use is associated with low 25-hydroxyvitamin D levels [22] probably due to enhanced 24-hydroxylase activity with glucocorticoid use and thereby decreasing 25(OH)D levels [16, 23]. However our patients were all on a steroid-free low dose dual maintenance therapy with a calcineurin-inhibitor (cyclosporine or tacrolimus) and mycophenolate mofetil or azathioprine, so this factor would have had no impact on the vitamin D metabolism in our patients.
Cutaneously produced vitamin D3 is converted to 25(OH)D in the liver by the hepatic vitamin D 25-hydroxylase. It is known that the cytochrome P450 enzyme CYP3A4 in the human liver has some 25-hydroxylase activity [24], which could be inhibited by cyclosporine or tacrolimus [25] decreasing hepatic 25(OH)D hydroxylation and finally lowering 25(OH) levels in these patients. However this hypothesis is purely speculative.
We also observed a significant rise of the 1-25(OH)D (p = 0.003) during the study period, which is probably a consequence of the rise of 25(OH)D as GFR remained stable. Ewers also found a positive association between 25(OH)D and 1-25(OH)D levels in kidney transplant patients which was independent of eGFR [2]. This significant rise of 1-25(OH)D is probably also promoted by the up-regulated vitamin D hydroxylation in kidney transplant patients which was shown by Mazzaferro [26]. However the higher level of 1-25(OH)D during summer did not lead to a significant fall in iPTH. Kanter also reported a lack of iPTH fall after oral supplementation with calcidiol 8,000 U per week over 6 months despite higher 25(OH)D levels [27]. In contrast Courbebaisse reported a significant fall of iPTH (76 to 63 pg/ml, p <0.05) in renal transplant patients after high-dose parenteral substitution with 100,000 U cholecalciferol every 2 weeks over 3 months with an increase of 25(OH)D levels from 14 nmol/l to 107 nmol/l [28]. As parenteral substitution is not convenient in daily practice it remains unclear whether higher doses of oral substitution would have the same effect on iPTH as parenteral substitution.
In the non-transplant population 25(OH)D deficiency is strongly associated with osteomalacia, osteoporosis, reduced muscle strength and increased risks of fractures with a less strong association with cancers, autoimmune and cardiovascular diseases [1]. Whether this is also the case in renal transplant patients is not known. In addition there is not a defined target level of 25(OH)D in kidney transplant patient so far. KDIGO guidelines recommend assessment of vitamin D status and vitamin D supplementation in cases of vitamin D deficiency in CKD stage 3 to 5 [29].
There are some limitations with the current study. No current data of the Swiss population in the same area are available. Therefore the results were compared with data from the German population with a slightly higher latitude. Only 76% of the patients returned the questionnaire. Therefore no conclusive data about the adherence to sun protection are available. The questionnaire might have mainly been filled out by compliant patients. However, even when excluding the patients who did not return the questionnaire, there were no substantial differences in the results showing a persistent circannual rhythm of 25(OH)D (data not shown).
We conclude that vitamin D deficiency in winter and in summer is common in this sample of kidney transplant patients on a corticosteroid free immunosuppressive maintenance therapy. However circannual rhythm of vitamin D was preserved in a substantial number despite avoidance of exposure to UVB. Due to the high prevalence of vitamin D deficiency in these patients, we recommend routine assessment of vitamin D status and vitamin D supplementation in cases of 25(OH)D levels below 75 nmol/l.
1 Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81.
2 Ewers B, Gasbjerg A, Moelgaard C, Frederiksen AM, Marckmann P. Vitamin D status in kidney transplant patients: need for intensified routine supplementation. Am J Clin Nutr. 2008;87:431–7.
3 Thuesen B, Husemoen L, Fenger M, Jakobsen J, Schwarz P, Toft U, et al. Determinants of vitamin D status in a general population of Danish adults. Bone. 2012;50(3):605–10.
4 Ishimura E, Nishizawa Y, Inaba M, Matsumoto N, Emoto M, Kawagishi T, et al. Serum levels of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure. Kidney Int. 1999;55:1019–27.
5 Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75:88–95.
6 Mehrotra R, Kermah D, Budoff M, Salusky IB, Mao SS, Gao YL, et al. Hypovitaminosis D in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1144–51.
7 Reusch J, Ackermann H, Badenhoop K. Cyclic changes of vitamin D and PTH are primarily regulated by solar radiation: 5-year analysis of a German (50 degrees N) population. Horm Metab Res. 2009;41:402–7.
8 Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Thomsen J, et al. Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. J Intern Med. 2000;247:260–8.
9 Woitge HW, Knothe A, Witte K, Schmidt-Gayk H, Ziegler R, Lemmer B, et al. Circaannual rhythms and interactions of vitamin D metabolites, parathyroid hormone, and biochemical markers of skeletal homeostasis: a prospective study. J Bone Miner Res. 2000;15:2443–50.
10 Engelsen O, Brustad M, Aksnes L, Lund E. Daily duration of vitamin D synthesis in human skin with relation to latitude, total ozone, altitude, ground cover, aerosols and cloud thickness. Photochem Photobiol. 2005;81:1287–90.
11 Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501.
12 Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–92.
13 Holick MF, Matsuoka LY, Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet. 1989;2:1104–5.
14 Matsuoka LY, Wortsman J, Hanifan N, Holick MF. Chronic sunscreen use decreases circulating concentrations of 25-hydroxyvitamin D. A preliminary study. Arch Dermatol. 1988;124:1802–4.
15 Querings K, Girndt M, Geisel J, Georg T, Tilgen W, Reichrath J. 25-hydroxyvitamin D deficiency in renal transplant recipients. J Clin Endocrinol Metab. 2006;91:526–9.
16 Akeno N, Matsunuma A, Maeda T, Kawane T, Horiuchi N. Regulation of vitamin D-1alpha-hydroxylase and -24-hydroxylase expression by dexamethasone in mouse kidney. J Endocrinol. 2000;164:339–48.
17 Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30.
18 Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70.
19 Mosekilde L, Nielsen LR, Larsen ER, Moosgaard B, Heickendorff L. Vitamin D deficiency. Definition and prevalence in Denmark. Ugeskr Laeger. 2005;167:29–33.
20 Hintzpeter B, Mensink GB, Thierfelder W, Muller MJ, Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2008;62:1079–89.
21 Marks R, Foley PA, Jolley D, Knight KR, Harrison J, Thompson SC. The effect of regular sunscreen use on vitamin D levels in an Australian population. Results of a randomized controlled trial. Arch Dermatol. 1995;131:415–21.
22 Skversky AL, Kumar J, Abramowitz MK, Kaskel FJ, Melamed ML. Association of glucocorticoid use and low 25-hydroxyvitamin D levels: results from the National Health and Nutrition Examination Survey (NHANES): 2001–2006. J Clin Endocrinol Metab. 2011;96:3838–45.
23 Dhawan P, Christakos S. Novel regulation of 25-hydroxyvitamin D3 24-hydroxylase (24(OH)ase) transcription by glucocorticoids: cooperative effects of the glucocorticoid receptor, C/EBP beta, and the Vitamin D receptor in 24(OH)ase transcription. J Cell Biochem. 2010;110:1314–23.
24 Gupta RP, Hollis BW, Patel SB, Patrick KS, Bell NH. CYP3A4 is a human microsomal vitamin D 25-hydroxylase. J Bone Miner Res. 2004;19:680–8.
25 Niwa T, Yamamoto S, Saito M, Shiraga T, Takagi A. Effect of cyclosporine and tacrolimus on cytochrome p450 activities in human liver microsomes. Yakugaku Zasshi. 2007;127:209–16.
26 Mazzaferro S, Pasquali M, Pugliese F, Citterio F, Gargiulo A, Rotondi S, et al. Distinct impact of vitamin D insufficiency on calcitriol levels in chronic renal failure and renal transplant patients: a role of FGF23. J Nephrol. 2012 Feb 28:0.doi:10.5301/jn.5000102. [Epub ahead of print]
27 Kanter Berga J, Crespo Albiach J, Beltran Catalan S, Gavela Martinez E, Sancho Calabuig A, Avila Bernabeu A, et al. Vitamin D deficiency in a renal transplant population: safe repletion with moderate doses of calcidiol. Transplant Proc. 2010;42:2917–20.
28 Courbebaisse M, Thervet E, Souberbielle JC, Zuber J, Eladari D, Martinez F, et al. Effects of vitamin D supplementation on the calcium-phosphate balance in renal transplant patients. Kidney Int. 2009;75:646–51.
29 KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009;(113):S1–130.
Authors’ contribution: F. Burkhalter participated in research design; F. Burkhalter and M. Dickenmann participated in the writing of the paper; F. Burkhalter participated in the performance of the research; F. Burkhalter and S. Schaub participated in data analysis.
Funding / potential competing interests: No financial support and no other potential conflict of interest relevant to this article were reported.