
Figure 1
Age-specific cancer mortality from 1991 through 2008 in Swiss men ≥65 years old.
DOI: https://doi.org/10.4414/smw.2012.13637
Population aging is occurring worldwide but most rapidly in the developed countries. These societies will need to pay close attention to how they respond to the associated challenges and opportunities. Switzerland has one of the developed world’s most pronounced increases of life expectancy (women 84.4 years, men 79.8 years in 2009) [1]. Consequently, the population of Swiss older adults (≥65 years) which was nearly 17% in 2009 is projected to increase to 28% by 2050 [1]. For Switzerland and other developed nations cancer is a particular concern: cancer is a disease of aging and a leading cause of morbidity and mortality in older people. Cancer incidence and mortality rates rise steeply in both men and women after age 60 years. Similarly to other developed countries, 62% of all-cancer mortality in Switzerland occurs in persons ≥70 years old; reflected similarly for most cancer-specific rates [2, 3].

Figure 1
Age-specific cancer mortality from 1991 through 2008 in Swiss men ≥65 years old.
Analysis of mortality trends is an important tool for monitoring the burden of cancer and developing cancer control programmes. Trends in cancer mortality in Switzerland (from registration and mortality data) have been published 1980–2001 [4, 5], annually by federal organisations [3] and included in comprehensive overviews of cancer in Europe [6–8]. However, cancer mortality trends were examined without explicit consideration for older age groups (the most affected persons) or potentially important socio-demographic factors [3–6, 9].
It is crucial for aging societies to focus careful studies on cancer mortality trends in older adults because of their far-reaching implications for the individual, society, and healthcare policy. To address existing knowledge gaps, we conducted a comprehensive epidemiologic analysis of gender-age-specific trends of all-cancer and cancer-specific mortality for the most common cancers in older Swiss men and women over the last two decades [10]. Making use of the Swiss National Cohort (SNC) Study, a census-based national research platform, we considered socio-demographic and regional characteristics specifically focused on the cancer mortality experience of older adults in Switzerland [11].
The SNC based on the 1990 and 2000 national censuses is described elsewhere [11, 12]. In brief, census records were linked to federal death or emigration records by probabilistic record linkage using the Generalised Record Linkage System (GRLS) [13, 14]. Linkage was based on a set of key variables available in both datasets (e.g. sex, date of birth, place of residence, marital status, religion, nationality, profession, date of birth of partner, date of birth of children). Because participation in the Swiss census, residence registration, and standardised death certificate reporting are mandatory, enumeration is virtually complete; the 2000 census estimated coverage as 98.6% [15]. The current study population included all individuals recorded in the 1990 and/or 2000 census linked to mortality records to the end of 2008 (N = 8,527,638) providing maximally 18 years of follow-up (1 January 1991 to 31 December 2008). Because this was a priori analysis of the older Swiss population we restricted study entry age to those ≥65 years (N = 2,253,378).
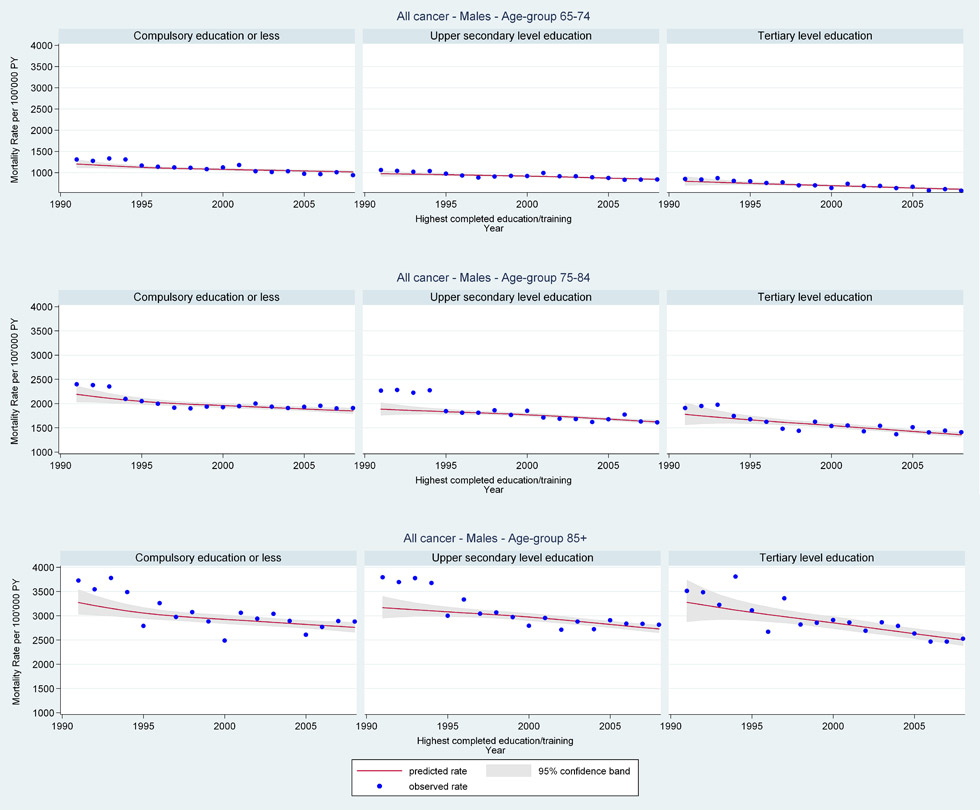
Web figure 1A
Age-specific all-cancer mortality rates from 1991 through 2008 for older Swiss men (≥65 years old) by education level.
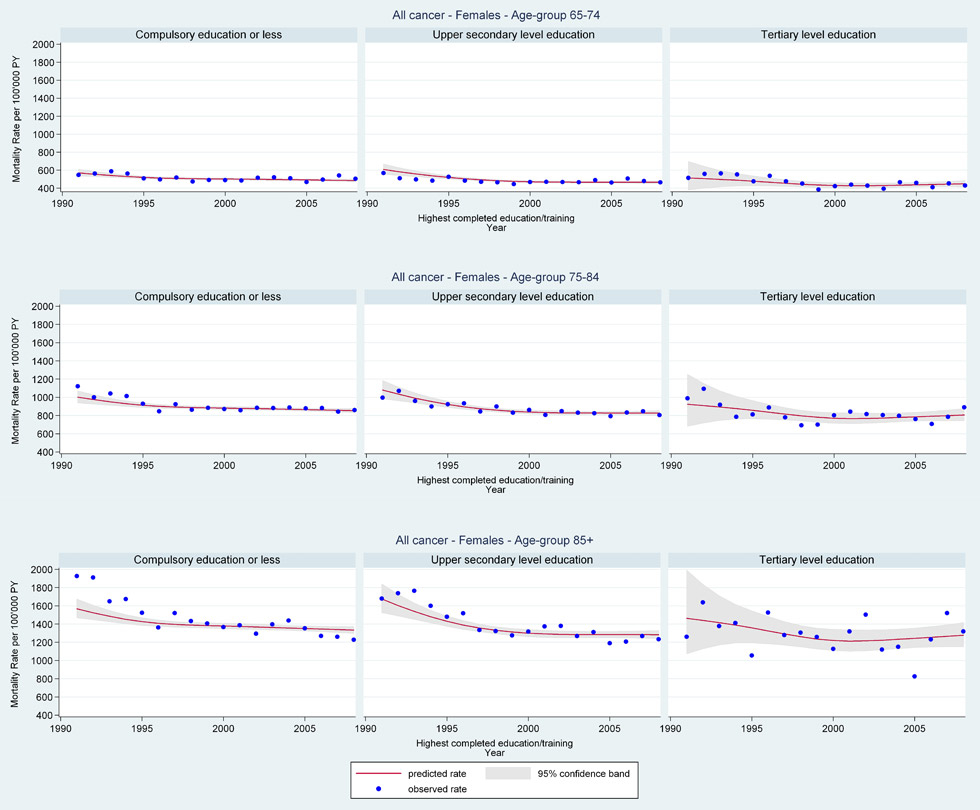
Web figure 1B
Age-specific all-cancer mortality rates from 1991 through 2008 for older Swiss women (≥65 years old) by education level.

Web figure 1C
Age-specific lung cancer mortality rates from 1991 through 2008 for older Swiss men (≥65 years old) by education level.
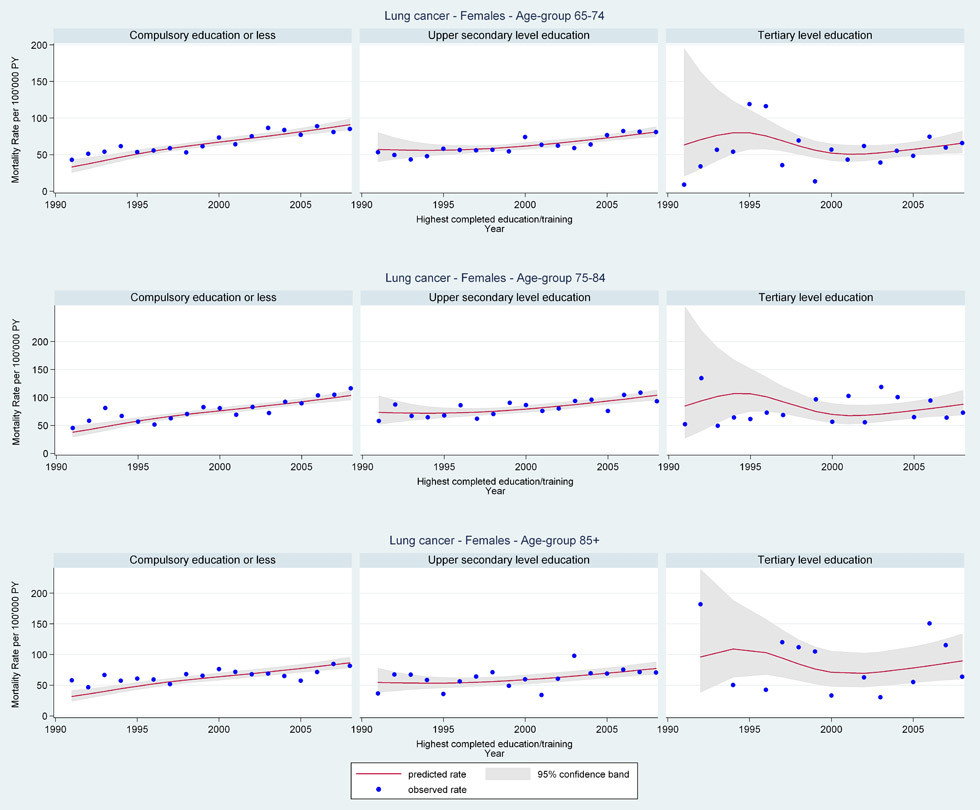
Web figure 1D
Age-specific lung cancer mortality rates from 1991 through 2008 for older Swiss women (≥65 years old) by education level.
All-cancer and cancer-specific deaths recorded on official Swiss death certificates between 1 January 1990 and 31 December 2008 were included in these analyses. Death certificates have been shown to be an accurate source of assigning cancer deaths [16, 17]. All-cancer deaths (N = 208,239) were coded as underlying cause of death by International Classification of Diseases (ICD) version 8 (before 1995 codes 140–209) and ICD-10 (after 1995 codes C00–C97). Cancer-specific deaths included: breast cancer (N = 16,733) codes 174–175 and C50; colorectal cancer (N = 24,582) codes 153–154 and C18–C21; lung cancer (N = 33,454) codes 162 and C33–C34; prostate cancer (N = 22,740) codes 185 and C61.
All socio-demographic and regional characteristics were measured using variables from the census. Questionnaires (German, French, Italian, English) and variable lists (German, French) are available from the SNC website at http://www.swissnationalcohort.ch/index.php?id=2991 [12]. Specifically included in these analyses were: sex; age at census (<65, 65-74, 75-84, 85+ years); nationality (Swiss, non-Swiss); educational attainment (compulsory schooling of up to 9 years’ education or less; secondary education, tertiary education, not known); marital status (single, married, widowed, divorced); household type (lives alone, two or more persons, institution); religion (Catholic, Protestant, no denomination, other/undeclared); urbanisation (urban, suburban, rural, for additional detail in German/French see http://www.bfs.admin.ch/bfs/portal/de/index/infothek/nomenklaturen/blank/blank/raum_glied/01.html ); language region (German, French, Italian). For additional regional analyses we also considered canton and the seven greater geographical regions in Switzerland (Central, Eastern, Zurich, Espace Mittelland, Lake Geneva, Northwestern, and Ticino, for additional detail in German/French see www.bfs.admin.ch/bfs/portal/).
Cancer mortality rates were calculated using person years (PY) at risk. At risk time began on 1 January 1991 or 5 December 2000 (census date) if ≥65 years at census date, otherwise on date of 65th birthday. It ended on (first occurrence of) date of death, date of emigration from Switzerland, 4 December 2000 for persons identified only in 1990 census, or 31 December 2008. At risk time accrued based on age; so the day someone reached an upper age-group limit their PY were thereafter assigned to the next older age group. Total at risk person-time (person years [PY] age 65–74, 75–84, 85+ years respectively) was 4,747,989PY; 2,640,244PY; 715,836PY for men and 5,826,815PY; 4,211,652PY; 1,769,929PY for women. All unlinked death records were assigned an appropriately chosen (matched for sex, age within 1 year) census record to avoid underestimation of absolute mortality rates [18]. For several cancer sites, a previously described sharp drop in mortality rates 1994–1995 (most pronounced in cancers with longer survival and older age groups) was the byproduct of Swiss Federal Statistical Office coding changes (revised official cause of death priority policy and switched ICD-8 to ICD-10) [19]. Consequently, we adjusted mortality rates before 1995 to compensate for this overestimation by including an indicator variable for calendar years after 1994 [18].
Gender-age-specific all-cancer and cancer-specific absolute rates per calendar year 1991–2008 were calculated and stratified by socio-demographic and regional variables described above. Observed rates were calculated as events (count of all-cancer or cancer-specific deaths) over PY. Poisson regression models estimated rates over time with the natural logarithm of the number of events as the outcome including the natural logarithm of PY as fixed offset [19]. Restricted cubic splines were included to flexibly model time trends. Models estimated cancer mortality rates with 95% confidence intervals (CI). Reported herein observed rates, estimated (EST) rates, and EST rate ratios (RR) comparing individual yearly rates (e.g. 1991or 2008) with 2000 yearly rate (approximated mid-follow-up point) as reference (e.g. RR >1.0 1991/2008 comparison yearly rate higher than 2000; RR <1.0 1991/2008 comparison yearly rate lower than 2000) [18]. All analyses were done using Stata version 11.1 [20].
Due to the comprehensive nature of these analyses (number of cancer sites and independent variables) we include herein only the most important results. Additional selected results are available as web figures online and full results are available upon request from the authors.

Figure 2
Age-specific cancer mortality from 1991 through 2008 in Swiss women ≥65 years old.

Web figure 2A
Age-specific lung cancer mortality rates from 1991 through 2008 for older Swiss men (≥65 years old) by household type.
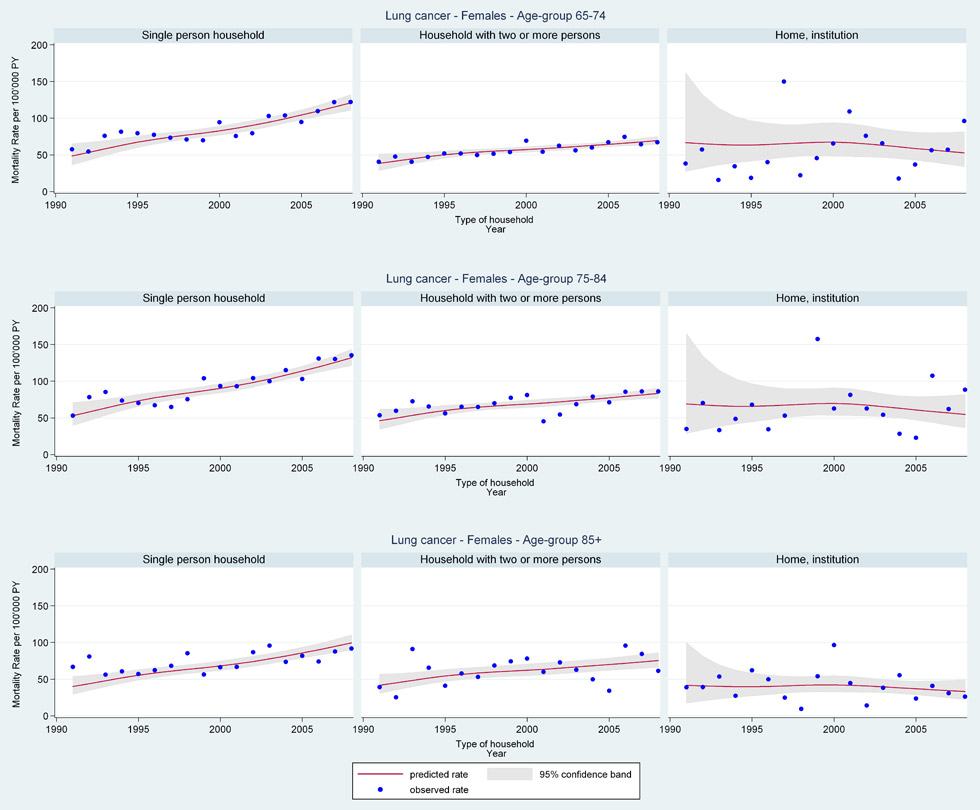
Web figure 2B
Age-specific lung cancer mortality rates from 1991 through 2008 for older Swiss women (≥65 years old) by household type.
The characteristics of the study population are listed in table 1. Consistent with older adult populations, more women than men were included. Approximately one-third were age 65–74 years at census and nearly 40% turned 65 after census date. The population was overwhelmingly Swiss (>90%), well educated (majority secondary or tertiary education), married (>60%), and lived in multi-person households (>65%). The population reflected expected Swiss language distributions, while only one-quarter lived in rural communities. The population was similar across gender, except (characteristically of older populations) men were younger, more educated, and more often married living in multi-person households.
Gender-age-specific cancer mortality trends of the population are shown in figures 1 and 2. Cancer mortality, except for lung cancer, increased with advancing age. Older men in all age-groups had overall higher all-cancer and cancer-specific mortality rates than older women. Older men in all age groups also showed a consistent decline in all-cancer mortality 1991–2008 (fig. 1 Panel A: age 65–74 years EST 1991 RR = 1.13 [95%CI 1.08, 1.19]; 2008 RR = 0.88 [95%CI 0.86, 0.90]). This was reflected in overall declining cancer-specific rates in older men.
In contrast, older women in all age-groups showed early declines with a levelling-off of all-cancer mortality beginning in 2000 (fig. 2 Panel A: age 65–74 years EST 1991 RR = 1.20 [95%CI 1.14, 1.27]; 2008 RR = 0.96 [95%CI 0.93, 0.98]). Increasing lung cancer mortality rates in older women (fig. 2 Panel D: age 65–74 years EST 1991 RR = 0.65 [95%CI 0.53, 0.79]; 2008 RR = 1.30 [95%CI 1.21, 1.40]) were in part offset by decreases in rates for other common cancers (e.g. colorectal and breast cancer) across age-groups over the period.
We analysed all-cancer and cancer-specific mortality rates from 1991 to 2008 for older men and women by all socio-demographic variables listed in table 1. For older men there appeared to be an education effect on trends for all-cancer and lung cancer mortality with the highest rates among older men with compulsory education (web fig. 1A: men all-cancer age 65–74 years EST 1991 1205/100,000PY [95%CI 1118, 1299] compulsory, 978/100,000PY [95%CI 913, 1046] secondary, 797/100,000PY [95%CI 703, 904] tertiary and EST 2008 1016/100,000PY [95%CI 982, 1052] compulsory, 842/100,000PY [95%CI 822, 863] secondary, 608/100,000PY [95%CI 585, 632] tertiary; web fig. 1C: men lung cancer age 65–74 years EST 1991 370/100,000PY [95%CI 318, 430] compulsory, 310/100,000PY [95%CI 268, 358] secondary, 175/100,000PY [95%CI 129, 236] tertiary and EST 2008 337/100,000PY [95%CI 315, 360] compulsory, 242/100,000PY [95%CI 230, 254] secondary, 140/100,000PY [95%CI 128, 153]) tertiary). Other socio-demographic variables showed little effect on mortality rates.
In general, for older women mortality rates remained comparatively constant regardless of education level, marital status, religion, or type of household. Surprisingly, however, young older women (≤74 years) living in single person households had the most sharply increasing lung cancer mortality rates (web fig. 2B: age 65–74 years EST 1991 RR = 0.59 [95%CI 0.44, 0.79], 2008 RR = 1.46 [95%CI 1.31, 1.63] single; 1991 RR = 0.67 [95%CI 0.50, 0.90], 2008 RR = 1.21 [95%CI 1.09, 1.34] multi-person; 1991 RR = 0.99 [95%CI 0.41, 2.40], 2008 RR = 0.78 [95%CI 0.48, 1.27] institution).
Mortality rates by language region, canton, and greater regions showed little variation in gender-age-specific all-cancer or cancer-specific trends over the period. However, stratification by urbanisation showed older women in suburban and rural areas had the most steeply increasing rates of lung cancer mortality (web fig. 3B: lung cancer women age 65–74 years EST 1991 RR = 0.85 [95%CI 0.63, 1.14], 2008 RR = 1.22 [95%CI 1.08, 1.37] urban; 1991 RR = 0.48 [95%CI 0.34, 0.67], 2008 RR = 1.33 [95%CI 1.19, 1.50] suburban; 1991 RR = 0.53 [95%CI 0.32, 0.88], 2008 RR = 1.45 [95%CI 1.23, 1.71] rural). Although most often older urban women had the overall highest rates and 2008 female lung cancer mortality rates were still well below male rates. Older men living in suburban areas had the lowest lung cancer mortality rates.
| Table 1: Characteristics of the analytical study population (older Swiss adults ≥65 years old), 1991–2008. | ||
| Males | Females | |
| Total | 986,634 (43.8%) | 1,266,744 (56.2%) |
| Age at 1990 census <65 years § 65–74 years 75–84 years 85+ years | 300,592 (30.5%) 231,609 (23.5%) 130,688 (13.2%) 28,774 (2.9%) | 345,094 (27.2%) 296,476 (23.4%) 221,246 (17.5%) 74,971 (5.9%) |
| Age at 2000 census <65 years § 65–74 years 75–84 years 85+ years | 281,521 (28.5%) 263,056 (26.7%) 146,993 (14.9%) 41,109 (4.2%) | 302,211 (23.9%) 324,726 (25.6%) 234,578 (18.5%) 103,008 (8.1%) |
| Nationality Swiss Non-Swiss | 869,840 (88.2%) 116,794 (11.8%) | 1,169,358 (92.3%) 97,386 (7.7%) |
| Educational attainment Compulsory schooling or less Secondary education Tertiary education Not known | 272,837 (27.7%) 483,990 (49.0%) 211,761 (21.5%) 18,046 (1.8%) | 662,961 (52.3%) 501,777 (39.6%) 69,617 (5.5%) 32,389 (2.6%) |
| Marital status Single Married Widowed Divorced | 76,507 (7.8%) 778,765 (78.9%) 73,345 (7.4%) 58,017 (5.9%) | 136,867 (10.8%) 675,448 (53.3%) 361,231 (28.5%) 93,198 (7.4%) |
| Household type Single person Multi-person Institution | 135,712 (13.8%) 818,364 (82.9%) 32,558 (3.3%) | 396,031 (31.3%) 790,671 (62.4%) 80,042 (6.3%) |
| Religion Protestant Roman Catholic No denomination Other / unknown | 456,793 (46.3%) 412,358 (41.8%) 79,404 (8.0%) 38,079 (3.9%) | 617,559 (48.8%) 539,006 (42.5%) 66,342 (5.2%) 43,837 (3.5%) |
| Language region German French Italian | 712,950 (72.3%) 226,029 (22.9%) 47,655 (4.8%) | 907,334 (71.6%) 294,272 (23.2%) 65,138 (5.2%) |
| Urbanisation Urban Surburban Rural | 305,133 (30.9%) 419,611 (42.5%) 261,890 (26.6%) | 443,788 (35.0%) 509,519 (40.2%) 313,437 (24.8%) |
| § Study entry after census date on 65th birthday. | ||
This comprehensive epidemiological analysis of cancer mortality trends in older adults provides further evidence that in Switzerland (like other developed countries) cancer is a disease of aging. But it also elucidates gender-age-specific cancer mortality variation in older age-groups by socio-demographic and regional factors. Overall, trends in cancer mortality were higher in men than women, increased by age, and except for lung cancer decreased over the past two decades. Of particular interest were findings of attenuated male cancer mortality rates by educational attainment and pronounced lung cancer mortality rates in younger older women living alone and/or in non-urban settings.
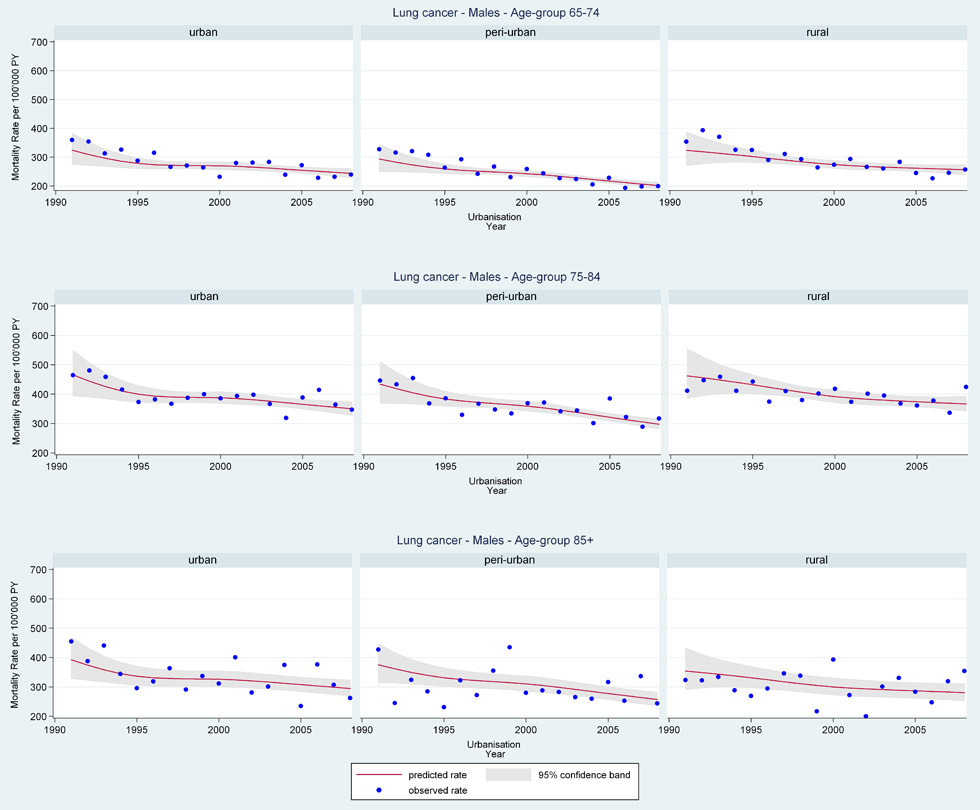
Web figure 3A
Age-specific lung cancer mortality rates from 1991 through 2008 for older Swiss men (≥65 years old) by urbanisation of community of residence.
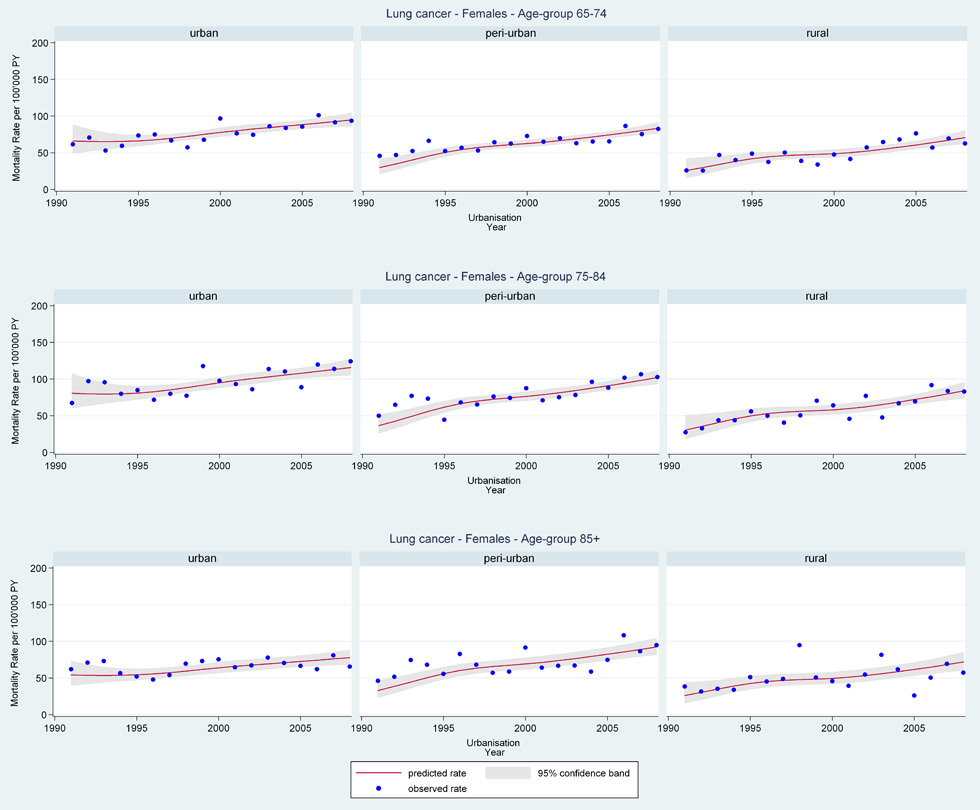
Web figure 3B
Age-specific lung cancer mortality rates from 1991 through 2008 for older Swiss women (≥65 years old) by urbanisation of community of residence.
Although cancer mortality rates declined in older women there was a distinct levelling-off of the decline after 2000 (earlier sharper declines similar within European Union) [21]. The lack of appreciable decline for nearly 10 years suggests that older women in Switzerland may receive less benefit from cancer screening and improvements in treatments than older men and younger adults. Findings may also be influenced by gender-specific changes in smoking patterns. By international comparison population-based data from other European countries (e.g. Austria, France, Germany) and the United States show decreases in all-cancer mortality for older women within similar periods [22, 23]. A recent study from Japan also showed mortality rates declining in both older men and women over the past 10 years [24]. The potential healthcare inequalities underlying these minimal improvements in cancer mortality rates for older Swiss women should be further evaluated. They are perhaps an important indicator of a major public health challenge arising from an increasing cancer burden with inadequate prevention and treatment in the growing number of older women.
Societal effects of smoking are reflected in these gender-age-specific lung cancer mortality trends. Reported trends correlate with smoking prevalence (considered the most important lung cancer risk factor) which are reported as declining and/or levelling-off in European men over the past five decades, while simultaneously trending upward in women until only much more recently [7, 25–27]. Previous studies of smoking prevalence and lung cancer in Switzerland indicate that from 1980–1984 approximately 90% of lung cancer deaths in men and 40% in women could be attributed to smoking (overall proportion 85%) [28]. Given the long latency period of lung cancer and the lag in smoking prevalence in Swiss women (women were not allowed to smoke in Switzerland before 1918) the increasing lung cancer mortality trends were as expected and similar to other reports for European women [29–31]. But what is interesting and, to the best of the authors’ knowledge as yet unnoted, is that these effects are seemingly restricted to younger old women living alone and/or in non-urban areas. These effects were seen even though no colinearity between the variables existed. Also important is that the highest lung cancer mortality rates were seen in older men with lower educational attainment. These specific variations present a unique public health opportunity for targeting tobacco cessation programmes (shown to improve prognosis in older adults) and heightened awareness of lung cancer monitoring in preventing the later stage diagnosis common in older adults [32–34]. For further discussion of educational effects see our companion paper [35].
In particular, the observed regionally similar reduction in breast cancer mortality before the year 2000 in all age groups of older Swiss women is noteworthy. Previous publications on breast cancer mortality and survival in Switzerland have shown regional (by language) differences, as does our companion paper examining relative risks [35–37]. Further, Ess et al. found considerable geographical variation in early detection and breast cancer care in Switzerland, although the findings did not explore older-age specific differences [38]. Others have proposed that differences are related to mammography screening programmes that are generally operational in French-speaking areas of Switzerland, only beginning in Eastern Switzerland, and opportunistic in other regions, may be influential. But if one considers the pattern of trends reported herein it is plausible that improvement in treatment before 2000 (e.g. widespread use of hormonal therapy) could have been the primary reason for the earlier declines in breast cancer mortality. If treatment changes were more consistently introduced across Switzerland and in older age groups (unlike mammography) they would explain why trends in absolute rates were level from 2000 onward despite increased mammography in French-speaking areas. However, if differences in breast cancer mortality in women are in fact related to mammography screening (supporting evidence mixed), then older Swiss women are not being screened (despite age-specific supporting evidence) and represent a sizable, growing, targetable at risk population and public health opportunity [39–46]. Additional research is needed and caution is warranted in comparing results across studies. Previous studies used data from only a subset of regional cancer registries (≤47% of population) and several cited papers were age truncated (restricted younger age-group).
Several strengths and weaknesses of this study are to be considered. First, it included a very large representative sample of older Swiss adults, allowing it to be generalised to the older population in Switzerland with statistical precision. In addition, it is one of the few investigations of cancer mortality trends that is specific to older adults, includes exact PY with a long follow-up, and explores gender-age-specific variations across a broad set of socio-demographic and regional characteristics. On the other hand, our age-group categories, although commonplace, created smaller distributions in the oldest old (85+ years) group leading to less stability of stratified results than in younger old age-groups. Additionally, our study population was based on probabilistic linkage and therefore subject to associated limitations [47]. Although previous evaluations of the SNC have shown no indication of selection bias and that linkage is least successful primarily in younger adults (a highly mobile group) that by design were excluded from these analyses [12]. It should also be mentioned that unfortunately our nearly complete Swiss mortality data did not include disease information (i.e. stage by age at diagnosis, treatment characteristics, etc.), health, lifestyle, and/or fully comprehensive socio-demographic characteristics. Additional analyses are planned to include cancer incidence as well as detailed lifestyle and health behaviour data linked to a representative subset of the current population. These future analyses will greatly expand our current findings.
In conclusion and despite the lack of more detailed information, our findings underscore that cancer mortality in older adults represents a major public health challenge where trends seemingly vary less regionally in older populations than previously suggested in all-age analyses [3, 48]. This first Swiss older age-specific evidence on cancer mortality trends can help inform public health decision-making in the fight against cancer. With older adults becoming an ever-increasing proportion of the population, Switzerland like other developed countries, will need to accommodate inevitable increases in cancer-related healthcare expenditures and focus on effective preventive and curative treatment for them. Efforts should be informed by continually updated age-specific epidemiologic investigations aimed towards reducing the physical and psychological burden of cancer, knowing that cancer is a disease of aging.
Acknowledgement: This manuscript contains original material not previously published and made possible with support from the members of the SNC Study Group: Felix Gutzwiller (Chairman of the Executive Board), Matthias Bopp and David Faeh (Zurich, Switzerland); Matthias Egger (Chairman of the Scientific Board), Kerri Clough-Gorr, Kurt Schmidlin, Adrian Spoerri and Marcel Zwahlen (Bern, Switzerland); Nino Kuenzli (Basel, Switzerland); Fred Paccaud (Lausanne, Switzerland); and Michel Oris (Geneva, Switzerland). We also thank the Swiss Federal Statistical Office, whose support made the SNC and these analyses possible.
1 The population of Switzerland 2009. In: Neuchâtel, Switzerland: Federal Department of Home Affairs, Swiss Federal Statistical Office 2010.
2 Switzerland statistics of cancer incidence1984–2008. In: Zurich, Switzerland: National Institute for Cancer Epidemiology and Registration 2010.
3 Switzerland statistics of cancer mortality 1984–2008. In: Zurich & Neuchâtel, Switzerland: National Institute for Cancer Epidemiology and Registration & Swiss Federal Statistics Office 2010.
4 Levi F, La Vecchia C, Randimbison L. Cancer mortality in Switzerland, 1990–1994. Soz Praventivmed. 1997;42:37–54.
5 Levi F, Lucchini F, La Vecchia C. Trends in cancer mortality in Switzerland, 1980–2001. Eur J Cancer Prev. 2006;15:1–9.
6 Levi F, Lucchini F, Negri E, La Vecchia C. Trends in mortality from major cancers in the European Union, including acceding countries, in 2004. Cancer. 2004;101:2843–50.
7 Karim-Kos HE, de Vries E, Soerjomataram I, Lemmens V, Siesling S, Coebergh JW. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. 2008;44:1345–89.
8 Bray F, Sankila R, Ferlay J, Parkin DM. Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer. 2002;38:99–166.
9 Jatoi I, Chen BE, Anderson WF, Rosenberg PS. Breast cancer mortality trends in the United States according to estrogen receptor status and age at diagnosis. J Clin Oncol. 2007;25:1683–90.
10 Hansen J. Common cancers in the elderly. Drugs Aging. 1998;13:467–78.
11 Spoerri A, Zwahlen M, Egger M, Bopp M. The Swiss National Cohort: a unique database for national and international researchers. Int J Public Health. 2010;55:239–42.
12 Bopp M, Spoerri A, Zwahlen M, Gutzwiller F, Paccaud F, Braun-Fahrländer C, et al. Cohort profile: the Swiss National Cohort – a longitudinal study of 6.8 million people. Int J Epidemiol. 2009;38:379–84.
13 Fair M. Generalized record linkage system – Statistics Canada’s record linkage software. Austrian J Stat. 2004;33:37–53.
14 Fellegi IP, Sunter AB. A theory of record linkage. Journal of the American Statistical Association. 1969;64:1183–210.
15 Renaud A. Methodology report – coverage estimation for the Swiss population census 2000. In. Neuchâtel, Switzerland: Swiss Federal Statistical Office 2004.
16 Doria-Rose VP, Marcus PM. Death certificates provide an adequate source of cause of death information when evaluating lung cancer mortality: an example from the Mayo Lung Project. Lung Cancer. 2009;63:295–300.
17 Doria-Rose VP, Marcus PM, Miller AB, Bergstralh EJ, Mandel JS, Tockman MS, et al. Does the source of death information affect cancer screening efficacy results? A study of the use of mortality review versus death certificates in four randomized trials. Clin Trials. 2010;7:69–77.
18 Schmidlin K, Clough-Gorr KM, Spoerri A, Eggers M, Zwahlen M. Impact of unlinked deaths and coding changes on mortality estimates in the Swiss National Cohort. BMC Med Inform Decis. Submitted.
19 Lutz JM, Pury P, Fioretta G, Raymond L. The impact of coding process on observed cancer mortality trends in Switzerland. Eur J Cancer Prev. 2004;13:77–81.
20 Data Analysis and Statistical Software (STATA). In 11.1 Edition. College Station, TX: StataCorp LP 2009.
21 Levi F, Lucchini F, Negri E, Boyle P, La Vecchia C. Changed trends of cancer mortality in the elderly. Ann Oncol. 2001;12:1467–77.
22 Chiolero A, Gervasoni JP, Rwebogora A, Mkamba M, Waeber B, Paccaud F, et al. Discordant prevalence of hypertension using two different automated blood pressure measurement devices: a population-based study in Dar es Salaam (Tanzania). Blood Press Monit. 2004;9:59–64.
23 World Health Organization (WHO) cancer mortality database. In: World Health Organization 2011.
24 Yang L, Fujimoto J, Qiu D, Sakamoto N. Trends in cancer mortality in the elderly in Japan, 1970–2007. Ann Oncol. 21:389–96.
25 Bray F, Tyczynski JE, Parkin DM. Going up or coming down? The changing phases of the lung cancer epidemic from 1967 to 1999 in the 15 European Union countries. Eur J Cancer. 2004;40:96–125.
26 Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P, et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122:155–64.
27 Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M; Comparative Risk Assessment collaborating group (Cancers). Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784–93.
28 La Vecchia C, Levi F, Decarli A, Wietlisbach V, Negri E, Gutzwiller F. Trends in smoking and lung cancer mortality in Switzerland. Prev Med. 1988;17:712–24.
29 Bosetti C, Levi F, Lucchini F, Negri E, La Vecchia C. Lung cancer mortality in European women: recent trends and perspectives. Ann Oncol. 2005;16:1597–604.
30 Forey B, Hamling J, Haming J, Lee P. International smoking statistics: A collection of worldwide historical data – Switzerland. In: International Smoking Statistics, 2nd Edition. Sutten, U.K.: Wolfson Institute of Preventive Medicine 2007.
31 Borras JM, Fernandez E, Gonzalez JR, Negri E, Lucchini F, La Vecchia C, Levi F. Lung cancer mortality in European regions (1955–1997). Ann Oncol. 2003;14:159–61.
32 Owonikoko TK, Ragin CC, Belani CP, Oton AB, Gooding WE, Taioli E, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol. 2007;25:5570–7.
33 Towe TP, O’Toole E, Engelhardt K, Nagaiah G, Berger NA. Changing healthcare practice for older adults with late-stage non-small cell lung cancer: practical considerations of targeted therapy in primary care and oncology. J Am Geriatr Soc. 2009;57(Suppl 2):S253–8.
34 Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569.
35 Schmidlin K, Spoerri A, Egger M, Zwahlen M, Stuck A, Clough-Gorr KM, et al. Cancer a disease of aging - part 2 - Risk factors for older adult cancer mortality in Switzerland 1991–2008. Swiss Med Wkly. 2012;142:w13637.
36 Fisch T, Pury P, Probst N, Bordoni A, Bouchardy C, Frick H, et al. Variation in survival after diagnosis of breast cancer in Switzerland. Ann Oncol. 2005;16:1882–8.
37 Bulliard JL, La Vecchia C, Levi F. Diverging trends in breast cancer mortality within Switzerland. Ann Oncol. 2006;17:57–9.
38 Ess S, Savidan A, Frick H, Rageth Ch, Vlastos G, Lütolf U, et al. Geographic variation in breast cancer care in Switzerland. Cancer Epidemiol. 2010;34:116–21.
39 Galit W, Green MS, Lital KB. Routine screening mammography in women older than 74 years: a review of the available data. Maturitas. 2007;57:109–19.
40 Nattinger AB. Older women, mammography, and mortality from breast cancer. Am J Med. 2000;108:174–5.
41 Schootman M, Jeffe DB, Lian M, Aft R, Gillanders WE. Surveillance mammography and the risk of death among elderly breast cancer patients. Breast Cancer Res Treat. 2008;111:489–96.
42 McCarthy EP, Burns RB, Freund KM, Ash AS, Shwartz M, Marwill SL, et al. Mammography use, breast cancer stage at diagnosis, and survival among older women. J Am Geriatr Soc. 2000;48:1226–33.
43 Schopper D, de Wolf C. How effective are breast cancer screening programmes by mammography? Review of the current evidence. Eur J Cancer. 2009;45:1916–23.
44 de Gelder R, Bulliard JL, de Wolf C, Fracheboud J, Draisma G, Schopper D, et al. Cost-effectiveness of opportunistic versus organised mammography screening in Switzerland. Eur J Cancer. 2009;45:127–38.
45 Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–92.
46 Hellquist BN, Duffy SW, Abdsaleh S, Björneld L, Bordás P, Tabár L, et al. Effectiveness of population-based service screening with mammography for women ages 40 to 49 years: evaluation of the Swedish Mammography Screening in Young Women (SCRY) cohort. Cancer. 2011;117:714–22.
47 Herzog TN, Scheuren FJ, Winkler WE. Data quality and record linkage techniques. NewYork, New York: Springer 2007.
48 Berrino F, Verdecchia A, Lutz JM, Lombardo C, Micheli A, Capocaccia R; EUROCARE Working Group. Comparative cancer survival information in Europe. Eur J Cancer. 2009;45:901–8.
Funding / potential competing interests: This work was supported by funding from the Swiss National Science Foundation (grant number 3347C0-108806) and Oncosuisse (grant number OCS-02288-08-2008). The sponsors had no role in the design, methods, subject recruitment, data collection, analysis, or paper preparation. None of the authors has a conflict of interest.